Abstract
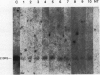
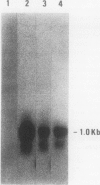
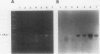
The transfer of genetic material into soybean tissue was accomplished by using an avirulent strain of Agrobacterium tumefaciens which contained the binary vector pGA482. The method used for transformation requires no tissue culture steps as it involves the inoculation of the plumule, cotyledonary node, and adjacent cotyledon tissues of germinating seeds. The identification of neomycin phosphotransferase (NPT) II enzyme activity in the tissues of 16 (R0) soybean plants indicated that the plant expressible Nos-NPT II gene, contained within the T-DNA region from pGA482, had been transferred at least into somatic tissues. Putative transformed R0 soybean plants were advanced to produce R1 plants which were also assayed for the presence of the transferred Nos-NPT II gene. The combined results of these assays indicated that about 0.7% of the surviving inoculated seeds yielded transformed tissues in the R0 plant, and that about 1/10 of these plants yielded transformed R1 plants. The presence of the Nos-NPT II gene in DNAs isolated from both R0 and R1 plant was demonstrated by using genomic blot hybridization and polymerase chain reaction methods. Integration of this gene into the soybean genome was demonstrated for three R1 soybean plants.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allmansberger R., Bräu B., Piepersberg W. Genes for gentamicin-(3)-N-acetyl-transferases III and IV. II. Nucleotide sequences of three AAC(3)-III genes and evolutionary aspects. Mol Gen Genet. 1985;198(3):514–520. doi: 10.1007/BF00332949. [DOI] [PubMed] [Google Scholar]
- An G. Development of plant promoter expression vectors and their use for analysis of differential activity of nopaline synthase promoter in transformed tobacco cells. Plant Physiol. 1986 May;81(1):86–91. doi: 10.1104/pp.81.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. D., Smith J. B. Nuclear dna amounts in angiosperms. Philos Trans R Soc Lond B Biol Sci. 1976 May 27;274(933):227–274. doi: 10.1098/rstb.1976.0044. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Feldmann K. A., Marks M. D., Christianson M. L., Quatrano R. S. A Dwarf Mutant of Arabidopsis Generated by T-DNA Insertion Mutagenesis. Science. 1989 Mar 10;243(4896):1351–1354. doi: 10.1126/science.243.4896.1351. [DOI] [PubMed] [Google Scholar]
- Fraley R. T., Rogers S. G., Horsch R. B., Sanders P. R., Flick J. S., Adams S. P., Bittner M. L., Brand L. A., Fink C. L., Fry J. S. Expression of bacterial genes in plant cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4803–4807. doi: 10.1073/pnas.80.15.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M. E., Taylor L. P., Walbot V. Stable transformation of maize after gene transfer by electroporation. 1986 Feb 27-Mar 5Nature. 319(6056):791–793. doi: 10.1038/319791a0. [DOI] [PubMed] [Google Scholar]
- Giardina S. L., Schroff R. W., Kipps T. J., Woodhouse C. S., Abrams P. G., Rager H. C., Morgan A. C., Jr, Foon K. A. The generation of monoclonal anti-idiotype antibodies to human B cell-derived leukemias and lymphomas. J Immunol. 1985 Jul;135(1):653–658. [PubMed] [Google Scholar]
- Hepburn A. G., White J., Pearson L., Maunders M. J., Clarke L. E., Prescott A. G., Blundy K. S. The use of pNJ5000 as an intermediate vector for the genetic manipulation of Agrobacterium Ti-plasmids. J Gen Microbiol. 1985 Nov;131(11):2961–2969. doi: 10.1099/00221287-131-11-2961. [DOI] [PubMed] [Google Scholar]
- Mazodier P., Cossart P., Giraud E., Gasser F. Completion of the nucleotide sequence of the central region of Tn5 confirms the presence of three resistance genes. Nucleic Acids Res. 1985 Jan 11;13(1):195–205. doi: 10.1093/nar/13.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai N., Kemp J. D., Sutton D. W., Murray M. G., Slightom J. L., Merlo D. J., Reichert N. A., Sengupta-Gopalan C., Stock C. A., Barker R. F., Hall T. C. Phaseolin gene from bean is expressed after transfer to sunflower via tumor-inducing plasmid vectors. Science. 1983 Nov 4;222(4623):476–482. doi: 10.1126/science.222.4623.476. [DOI] [PubMed] [Google Scholar]
- Owens L. D., Cress D. E. Genotypic variability of soybean response to agrobacterium strains harboring the ti or ri plasmids. Plant Physiol. 1985 Jan;77(1):87–94. doi: 10.1104/pp.77.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss B., Sprengel R., Will H., Schaller H. A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene. 1984 Oct;30(1-3):211–217. doi: 10.1016/0378-1119(84)90122-7. [DOI] [PubMed] [Google Scholar]
- Saghai-Maroof M. A., Soliman K. M., Jorgensen R. A., Allard R. W. Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci U S A. 1984 Dec;81(24):8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]