Abstract
Background/Aim
The prognostic outcome of the controlling nutritional status (CONUT) score in patients with colorectal liver metastases (CRLM) who underwent hepatectomy has not been investigated. The aim of this study was to investigate the prognostic value of preoperative CONUT score and other systemic inflammation-related biomarkers in patients who underwent hepatectomy for CRLM.
Patients and Methods
The subjects included 145 patients with CRLM who underwent hepatectomy and retrospectively investigated the association of preoperative CONUT score with disease-free survival (DFS), surgical failure-free survival (SFS), and overall survival (OS) using univariate and multivariate analyses.
Results
In this study, the cut-off of the CONUT score was 4. In the univariate analysis, the high CONUT score was associated with worse SFS and OS (p=0.01, 0.01). The multivariate analysis showed significant and independent predictors of OS were lymph node metastases (p=0.03) and a high CONUT score (p=0.04). In patients with a high CONUT score, postoperative complications due to infections were significantly more than in those with a low CONUT score (27% vs. 9%, p=0.04).
Conclusion
The CONUT score can be useful for predicting not only short-term but also long-term outcomes in patients with CRLM after hepatectomy.
Keywords: CONUT, CRLM, hepatectomy
Colorectal cancer is the third most common digestive gastrointestinal tract malignancy worldwide, with its incidence increasing yearly (1). Approximately 20-25% of patients with colorectal cancer present with colorectal liver metastases (CRLM) upon diagnosis and approximately 50% will develop metachronous CRLM after primary resection (2). Hepatectomy is the most promising treatment for CRLM; surgery is essential for local control and long-term survival, and 5-year survival rate of approximately 40% can be reached (3). However, since the prognosis is not yet satisfactory, further prognostic factor analysis and intervention is needed to improve prognosis.
Recently, there are numerous reports that nutrition and inflammation biomarkers can predict cancer-specific survival in patients with various cancers. Preoperative systemic inflammation-related biomarkers, such as Glasgow prognostic score (GPS), lymphocyte-to-C-reactive protein ratio, neutrophil-to-lymphocyte ratio (NLR), and prognostic nutritional index (PNI), are utilized as prognostic indicators in patients with CRLM (4-6).
Controlling nutritional status (CONUT) score, which is calculated using serum albumin, total cholesterol concentration, and lymphocyte count, was previously reported as a new biomarker for evaluating nutritional status (7). Several studies have shown that a high CONUT score is associated with poor long-term outcomes in patients with various tumors including hepatocellular carcinoma and colorectal cancer (8-10). However, the usefulness of the CONUT score for evaluating short and long-term outcomes for patients with CRLM who underwent hepatectomy has not been investigated.
The aim of this study was therefore to investigate the prognostic value including short-term outcome of preoperative CONUT score and compared its prognostic accuracy to that of other systemic inflammation-related biomarkers (GPS and PNI) in patients with CRLM who underwent hepatectomy.
Patients and Methods
Patients and patient management. The subjects were 145 patients who underwent hepatectomy for CRLM without extrahepatic tumor at our institution between May 2006 and October 2020. Surgical procedure and treatment for recurrence were performed according to our previous report (11). The retrospective study was approved by the human ethics committee of the Jikei University School of Medicine. Informed consent from patients was not required because this study was retrospective.
Calculation of the CONUT score and other systemic inflammation-related biomarkers. Serum samples were collected and assayed preoperatively. Preoperative CONUT score was calculated from serum albumin concentration, total peripheral lymphocyte counts, and serum total cholesterol concentration (7). Serum albumin concentration was categorized as: 0: ≥3.5 g/dl, 2: 3.0-3.49 g/dl, 4: 2.5-2.99 g/dl, and 6: <2.5 g/dl. Total peripheral total lymphocyte count was categorized as: 0: ≥1,600/mm3, 1: 1,200-1,599/mm3, 2: 800-1,199/mm3, and 3: <800/mm3. Serum total cholesterol concentration was categorized as: 0: ≥180 mg/dl, 1: 140-179 mg/dl, 2: 100-139 mg/dl, and 3: <100 mg/dl. According to our previous report, the cut-off of CONUT score was determined to be 4 (12). Based on the cut-off value, patients were classified into the low CONUT group (CONUT score <4) and the high CONUT group (CONUT score ≥4). In addition to the CONUT score, other nutrition and inflammation-based biomarkers including GPS and PNI were examined based on previous studies (13,14).
Analysis of oncological outcomes. We analyzed the relation between variables and disease-free, surgical failure-free, or overall survival after hepatectomy for CRLM. The variables were location of primary colorectal cancer, lymph node metastases, T factor according to the TNM staging system (15), timing of CRLM, presence of neoadjuvant chemotherapy, tumor number, tumor size, GPS, PNI, CONUT score, hepatectomy, curability, and infectious complication. For PNI, the cut-off values were determined using receiver operating characteristics curve. Infectious complications were defined based on our previous report (16).
Statistical analysis. The data are expressed as the median (inter quartile range). In univariate analysis, continuous variables were analyzed using the Mann-Whitney U-test, whereas categorical variables were analyzed using the chi-square test. All survival outcomes including disease-free, surgical failure-free, and overall survival were performed using the Kaplan–Meier method with the Log-rank test. To investigate risk factors of survival, univariate and multivariate analyses for disease-free, surgical failure-free, and overall survival were performed using the Cox proportional hazards regression models. p-values of less than 0.05 were considered to indicate statistical significance.
Results
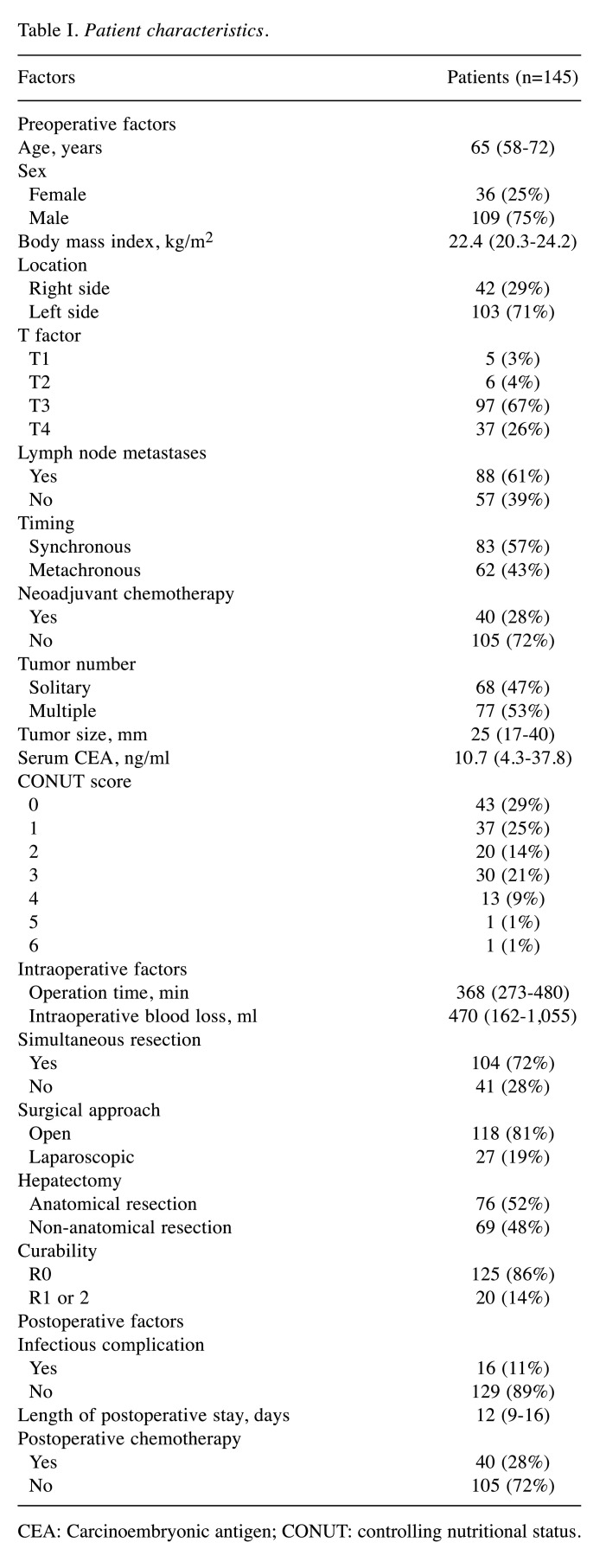
Patient characteristics. The patient characteristics are shown in Table I. The mean age was 65 years. The timing of CRLM was synchronous in 83 patients (57%) and metachronous in 62 patients (43%). Neoadjuvant chemotherapy was administered to 40 patients (28%). Among the 145 patients with CRLM, 43 patients (29%) had a preoperative CONUT score of 0, 37 patients (25%) had a score of 1, 20 patients (14%) had a score of 2, 30 patients (21%) had a score of 3, 13 patients (9%) had a score of 4, 1 (1%) patient had a score of 5, and 1 (1%) had a score of 6.
Table I. Patient characteristics.
CEA: Carcinoembryonic antigen; CONUT: controlling nutritional status.
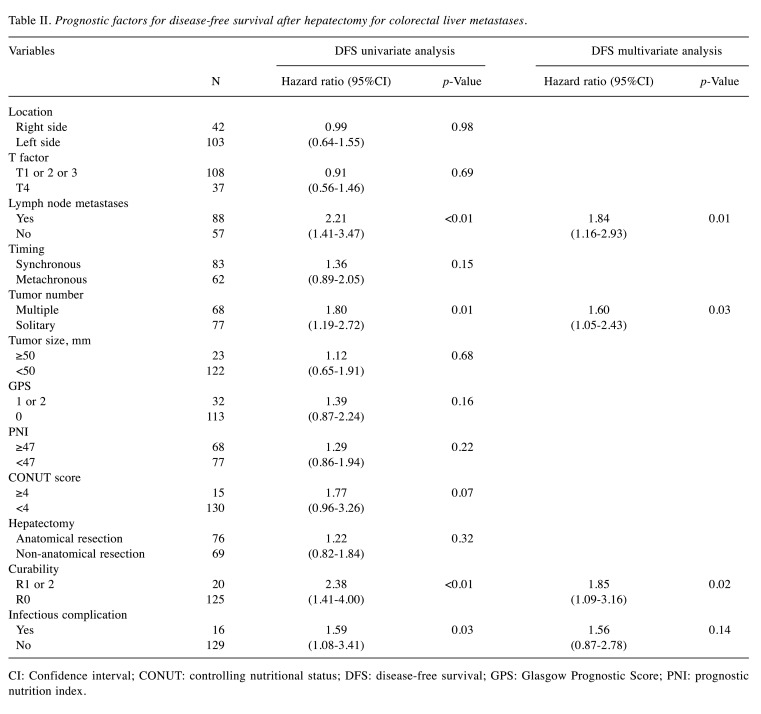
Association of disease-free survival with clinicopathological variables. Table II shows the association of disease-free survival with the clinicopathological variables after hepatectomy for CRLM. The univariate analysis showed that the disease-free survival was significantly worse in patients with lymph node metastases (p<0.01), multiple tumor (p=0.01), R1 or R2 (p<0.01), and infectious complication (p=0.03). The multi-variate analysis revealed that lymph node metastases [hazard ratio (HR)=1.84, 95%CI=1.16-2.93, p=0.01], multiple tumor (HR=1.60, 95%CI=1.05-2.43, p=0.03), and R1 or R2 (HR=1.85, 95%CI=1.09-3.16, p=0.02) were independent prognostic predictors for the disease-free survival.
Table II. Prognostic factors for disease-free survival after hepatectomy for colorectal liver metastases.
CI: Confidence interval; CONUT: controlling nutritional status; DFS: disease-free survival; GPS: Glasgow Prognostic Score; PNI: prognostic nutrition index.
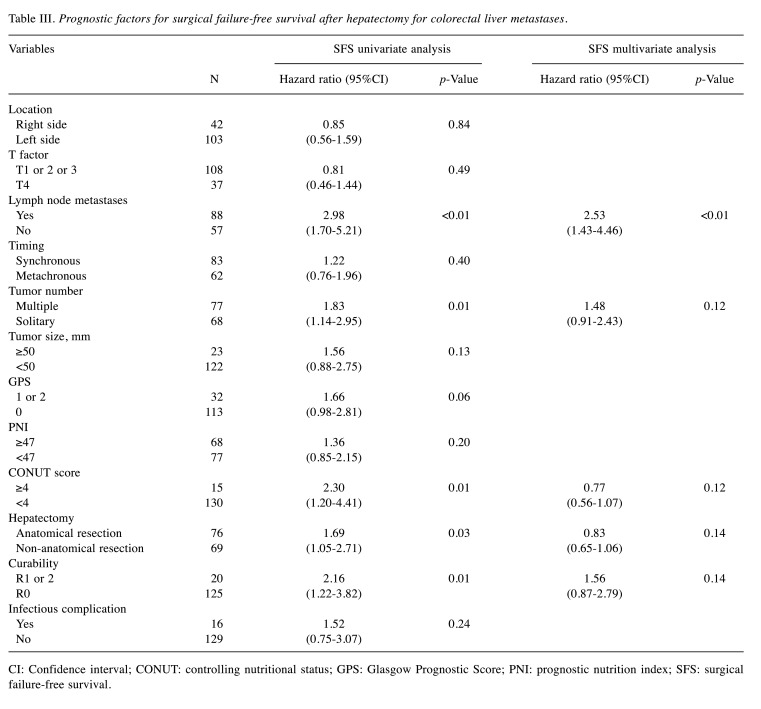
Association of surgical failure-free survival with clinicopathological variables. Table III shows the association of surgical failure-free survival with the clinicopathological variables after hepatectomy for CRLM. The univariate analysis showed that surgical failure-free survival was significantly worse in patients with lymph node metastases (p<0.01), multiple tumor (p=0.01), CONUT score ≥4 (p=0.01), anatomical resection (p=0.03), and R1 or 2 (p=0.01). The multivariate analysis revealed that lymph node metastases (HR=2.53, 95%CI=1.43-4.46, p<0.01) was an independent prognostic predictor for the surgical failure-free survival.
Table III. Prognostic factors for surgical failure-free survival after hepatectomy for colorectal liver metastases.
CI: Confidence interval; CONUT: controlling nutritional status; GPS: Glasgow Prognostic Score; PNI: prognostic nutrition index; SFS: surgical failure-free survival.
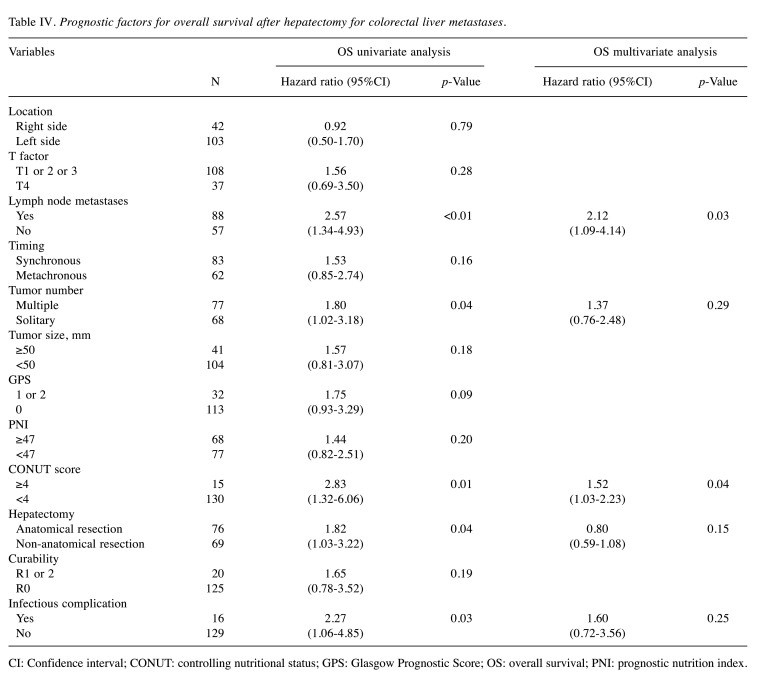
Association of overall survival with clinicopathological variables. Table IV shows the association of overall survival with the clinicopathological variables after hepatectomy for CRLM. The univariate analysis showed that the overall survival was significantly worse in patients with lymph node metastases (p<0.01), multiple tumor (p=0.04), CONUT score ≥4 (p=0.01), anatomical resection (p=0.04), and infectious complication (p=0.03). The multivariate analysis revealed that lymph node metastases (HR=2.12, 95%CI=1.09-4.14, p=0.03) and CONUT score ≥4 (HR=1.52, 95%CI=1.03-2.23, p=0.04) were independent prognostic predictors for the overall survival.
Table IV. Prognostic factors for overall survival after hepatectomy for colorectal liver metastases.
CI: Confidence interval; CONUT: controlling nutritional status; GPS: Glasgow Prognostic Score; OS: overall survival; PNI: prognostic nutrition index.
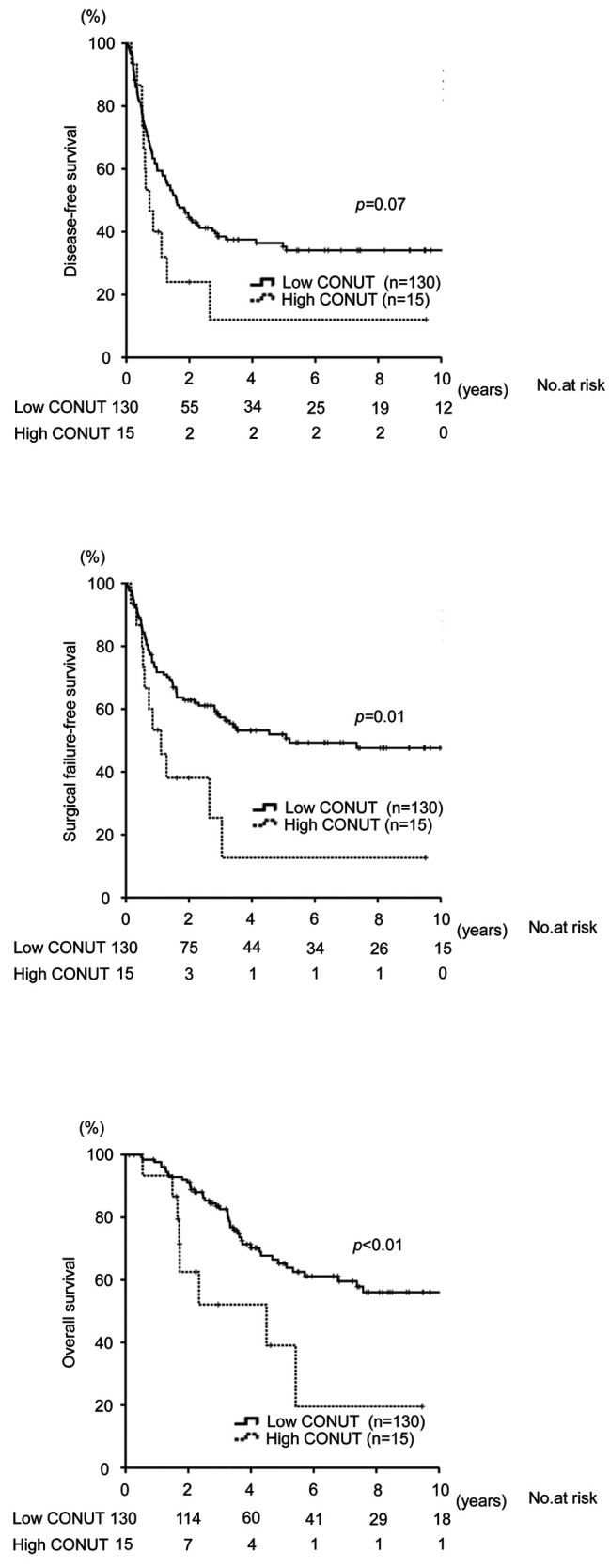
Impact of CONUT score on disease-free, surgical failure-free and overall survival. The surgical failure-free survival was significantly worse in patients with CONUT score ≥4 than in those with CONUT score <4 (p=0.01; 3-year survival, 25.4% vs. 57.3%). Similarly, the overall survival was significantly worse in patients with CONUT score ≥4 than in those with CONUT score <4 (p=0.01; 3-year survival, 52.1% vs. 83.6%) (Figure 1).
Figure 1. Kaplan–Meier curve for disease-free survival, surgical failure-free survival, and overall survival after hepatectomy for colorectal liver metastases according to the CONUT score.
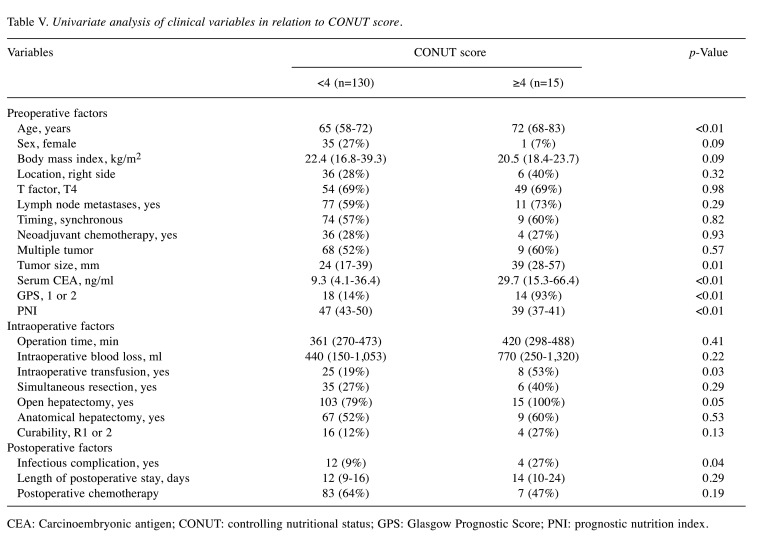
Association of clinical factors with CONUT score. Table V shows the association of clinical factors with CONUT score. Patients with CONUT ≥4 were significantly older, had larger tumor, higher carcinoembryonic antigen, worse GPS, lower PNI, more common intraoperative transfusion, and more common infectious complications than patients with CONUT <4 (p<0.01, 0.01, <0.01, <0.01, <0.01, 0.03, and 0.04, respectively). However, postoperative adjuvant chemotherapy was comparable between the two groups.
Table V. Univariate analysis of clinical variables in relation to CONUT score.
CEA: Carcinoembryonic antigen; CONUT: controlling nutritional status; GPS: Glasgow Prognostic Score; PNI: prognostic nutrition index.
Discussion
This study demonstrated that surgical failure-free and overall survival after hepatectomy for CRLM were significantly worse in patients with high CONUT score than in those with low CONUT score. Moreover, multivariate analysis demonstrated that the CONUT score was an independent prognostic predictor for overall survival. And a high CONUT score was significantly associated with a more common postoperative infectious complication. These findings show that the CONUT score is associated with both the short and long- term outcomes of patients who underwent hepatectomy for CRLM.
CONUT, a newly proposed immune-nutritional prognostic factor and comprehensive scoring system to assess patient’ nutritional and immune status, is measured using serum albumin concentration, total peripheral lymphocyte count, and serum total cholesterol concentration. It has been reported as a factor associated with the length of hospitalization (7).
The serum albumin concentration reflects hepatic function, nutrition, and systemic inflammation. Proinflammatory cytokines, such as interleukin-1 and 6, and tumor necrosis factor-alpha contribute to modulation of the synthesis of albumin in the liver resulting in decreased serum albumin concentration (17). Also, these cytokines play an important role in cancer proliferation, invasion, and metastasis because the cytokine-mediated inflammatory response can accelerate cancer cell growth and diminish tumor immunity (18).
Total peripheral lymphocyte count is an alternative and a surrogate biomarker of nutritional and immune status, which has been used for the calculation of immunological and nutrition-based biomarkers, such as PNI, NLR, lymphocyte-to-C-reactive protein ratio and platelet-to-lymphocyte ratio (6,19). It provides further insights into the immune response as lymphocytes are closely associated with host cancer-mediated immunity to inhibit tumor proliferation and migration resulting in cancer cell apoptosis (20).
Serum total cholesterol concentration, a unique factor in the CONUT score, is a marker of caloric storage (21). Total cholesterol level has been shown to be closely associated with tumor growth and prognosis of patients with various types of tumors (22-24). Although its role in cancer proliferation, migration, or metastasis has not been clarified, some studies have demonstrated several molecular mechanisms explaining the association of cancer progression with cholesterol. Some studies have suggested a negative association between increased risk of cancer and serum total cholesterol concentration. This might be due to tumor tissues containing higher levels of cholesterol than the normal tissues, resulting in reduction in plasma cholesterol levels and calorie intake (25,26). Moreover, gene mutations related to the pathways of cholesterol metabolism have been identified in cancer cells, which may contribute to promoting increased cholesterol levels in cancer cells to enhance cancer cell growth (27).
The combination of these three factors into the CONUT can enhance its ability to assess immune-nutritional status more accurately, which may explain why the CONUT score is a more useful prognostic predictor than other immune-nutritional biomarkers such as GPS and PNI. In addition, serum cholesterol levels can contribute to not only nutritional condition, but also liver function considering its lipid metabolic function. Therefore, the CONUT score might be an appropriate biomarker of the preoperative immunological and nutritional status for patients who underwent hepatectomy for CRLM.
The current study showed that patients with a high CONUT score were older, had more advanced tumor, worse other immune-nutritional biomarkers, and more common postoperative infectious complication than patients with a low CONUT score.
These findings may indicate that the CONUT score reflects cancer progression, immune-nutritional status, short-term outcomes, and long-term prognosis and will further help determine surgical indications for patients with CRLM.
In this study, there was no significant difference in disease-free survival between the patients based on the CONUT score. The new concept of time to surgical failure, and surgical failure-free survival was proposed as a novel surrogate endpoint in patients who underwent hepatectomy for CRLM because repeat hepatectomy provides a survival benefit (28). Our results also demonstrated that the CONUT score was a prognostic predictor for surgical failure-free survival.
This is the first report showing the prognostic significance of CONUT score in patients who underwent hepatectomy for CRLM. Daitoku et al. demonstrated that CONUT score was a reliable prognostic marker in patients who received first-line chemotherapy for metastatic colorectal cancer (29), which is consistent with our result.
This study has several limitations. It is a retrospective analysis from a single institution and the size of the subjects was relatively small. Also, its cohort was homogeneous, therefore the significance of the CONUT score needs to be validated using other cohorts. In the present study, the cut-off of the CONUT score was 4. Varying cutoff points have been used and we set cut-off in line with our previous report. Further studies are required to establish the optimal cut-off of the CONUT score.
In conclusion, this study demonstrated that preoperative CONUT score was more closely related to survival after surgery than GPS and PNI in patients who underwent hepatectomy for CRLM. CONUT is useful for not only estimating nutritional condition but also risk stratification predicting both short and long-term outcomes in CRLM patients undergoing hepatic resection. We believe interventions such as immune-nutritional treatment before surgery can improve outcomes in CRLM patients.
Conflicts of Interest
There are no conflicts of interest and funding to declare in relation to this study.
Authors’ Contributions
Atsuko Okamoto: research design, data analysis, writing of the paper; Kenei Furukawa: writing of the paper, performance of the research; Masahisa Ohkuma: data analysis; Takafumi Nakano: data analysis; Satoshi Yoshioka: performance of the research, data analysis; Yuta Imaizumi: performance of the research, data analysis; Hiroshi Sugano: data analysis; Yasuhiro Takeda: performance of the research; Makoto Kosuge: performance of the research; Ken Eto: final apporovement.
References
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam R, Kitano Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg. 2019;3(1):50–56. doi: 10.1002/ags3.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okimoto S, Kobayashi T, Tashiro H, Kuroda S, Ishiyama K, Ide K, Abe T, Hashimoto M, Iwako H, Hamaoka M, Honmyo N, Yamaguchi M, Ohdan H. Significance of the Glasgow Prognostic Score for patients with colorectal liver metastasis. Int J Surg. 2017;42:209–214. doi: 10.1016/j.ijsu.2017.04.068. [DOI] [PubMed] [Google Scholar]
- 5.Tang H, Li B, Zhang A, Lu W, Xiang C, Dong J. Prognostic significance of neutrophil-to-lymphocyte ratio in colorectal liver metastasis: a systematic review and meta-analysis. PLoS One. 2016;11(7):e0159447. doi: 10.1371/journal.pone.0159447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Utsumi M, Inagaki M, Kitada K, Tokunaga N, Kondo M, Yunoki K, Sakurai Y, Hamano R, Miyasou H, Tsunemitsu Y, Otsuka S. Lymphocyte-to-C-reactive protein ratio predicts prognosis in patients with colorectal liver metastases post-hepatic resection: a retrospective study. Anticancer Res. 2022;42(10):4963–4971. doi: 10.21873/anticanres.16003. [DOI] [PubMed] [Google Scholar]
- 7.Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, Rodríguez F, Fernández G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45. [PubMed] [Google Scholar]
- 8.Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, Kitano Y, Yamamura K, Ouchi M, Nakamura K, Baba Y, Sakamoto Y, Yamashita Y, Yoshida N, Chikamoto A, Baba H. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21(2):204–212. doi: 10.1007/s10120-017-0744-3. [DOI] [PubMed] [Google Scholar]
- 9.Harimoto N, Yoshizumi T, Inokuchi S, Itoh S, Adachi E, Ikeda Y, Uchiyama H, Utsunomiya T, Kajiyama K, Kimura K, Kishihara F, Sugimachi K, Tsujita E, Ninomiya M, Fukuzawa K, Maeda T, Shirabe K, Maehara Y. Prognostic significance of preoperative controlling nutritional status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma: a multi-institutional study. Ann Surg Oncol. 2018;25(11):3316–3323. doi: 10.1245/s10434-018-6672-6. [DOI] [PubMed] [Google Scholar]
- 10.Tokunaga R, Sakamoto Y, Nakagawa S, Ohuchi M, Izumi D, Kosumi K, Taki K, Higashi T, Miyamoto Y, Yoshida N, Oki E, Watanabe M, Baba H. CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int J Colorectal Dis. 2017;32(1):99–106. doi: 10.1007/s00384-016-2668. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa K, Haruki K, Taniai T, Hamura R, Shirai Y, Yasuda J, Shiozaki H, Onda S, Gocho T, Ikegami T. Osteosarcopenia is a potential predictor for the prognosis of patients who underwent hepatic resection for colorectal liver metastases. Ann Gastroenterol Surg. 2021;5(3):390–398. doi: 10.1002/ags3.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsunematsu M, Haruki K, Fujiwara Y, Furukawa K, Onda S, Matsumoto M, Gocho T, Shiba H, Yanaga K. Preoperative controlling nutritional status (CONUT) score predicts long-term outcomes in patients with non-B non-C hepatocellular carcinoma after curative hepatic resection. Langenbecks Arch Surg. 2021;406(1):99–107. doi: 10.1007/s00423-020-01987-9. [DOI] [PubMed] [Google Scholar]
- 13.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89(6):1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005. [PubMed] [Google Scholar]
- 15.Japanese Society for Cancer of the Colon and Rectum Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition [Secondary Publication] J Anus Rectum Colon. 2019;3(4):175–195. doi: 10.23922/jarc.2019-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furukawa K, Onda S, Taniai T, Hamura R, Yanagaki M, Tsunematsu M, Haruki K, Yasuda J, Sakamoto T, Gocho T, Ikegami T. Risk factors and overcoming strategies of surgical site infection after hepatectomy for colorectal liver metastases. Anticancer Res. 2021;41(11):5651–5656. doi: 10.21873/anticanres.15381. [DOI] [PubMed] [Google Scholar]
- 17.Peters SJ, Vanhaecke T, Papeleu P, Rogiers V, Haagsman HP, van Norren K. Co-culture of primary rat hepatocytes with rat liver epithelial cells enhances interleukin-6-induced acute-phase protein response. Cell Tissue Res. 2010;340(3):451–457. doi: 10.1007/s00441-010-0955-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 19.Kaida T, Nitta H, Kitano Y, Yamamura K, Arima K, Higashi T, Taki K, Nakagawa S, Okabe H, Hayashi H, Imai K, Hashimoto D, Yamashita Y, Chikamoto A, Ishiko T, Beppu T, Baba H. Preoperative platelet-to-lymphocyte ratio can predict recurrence beyond the Milan criteria after hepatectomy for patients with hepatocellular carcinoma. Hepatol Res. 2017;47(10):991–999. doi: 10.1111/hepr.12835. [DOI] [PubMed] [Google Scholar]
- 20.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Ulíbarri Pérez JI, Fernández G, Rodríguez Salvanés F, Díaz López AM. Nutritional screening; control of clinical undernutrition with analytical parameters. Nutr Hosp. 2014;29(4):797–811. doi: 10.3305/nh.2014.29.4.7275. [DOI] [PubMed] [Google Scholar]
- 22.Okuyama H, Ichikawa Y, Sun Y, Hamazaki T, Lands W. Cancer and all-cause mortalities are lower in the higher total cholesterol groups among general populations. World Rev Nutr Diet. 2006;96:37–54. doi: 10.1159/000097806. [DOI] [PubMed] [Google Scholar]
- 23.Lee YL, Li WC, Tsai TH, Chiang HY, Ting CT. Body mass index and cholesterol level predict surgical outcome in patients with hepatocellular carcinoma in Taiwan - a cohort study. Oncotarget. 2016;7(16):22948–22959. doi: 10.18632/oncotarget.8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang HW, The KORCC (KOrean Renal Cell Carcinoma) group, Seo SP, Kim WT, Yun SJ, Lee S, Kim W, Hwang EC, Kang SH, Hong S, Chung J, Kwon TG, Kim HH, Kwak C, Byun S, Kim Y. Low preoperative serum cholesterol level is associated with aggressive pathologic features and poor cancer-specific survival in patients with surgically treated renal cell carcinoma. Int J Clin Oncol. 2018;23(1):142–150. doi: 10.1007/s10147-017-1172-4. [DOI] [PubMed] [Google Scholar]
- 25.Cengiz O, Kocer B, Sürmeli S, Santicky MJ, Soran A. Are pretreatment serum albumin and cholesterol levels prognostic tools in patients with colorectal carcinoma. Med Sci Monit. 2006;12(6):CR240–CR247. [PubMed] [Google Scholar]
- 26.Dessì S, Batetta B, Pulisci D, Spano O, Anchisi C, Tessitore L, Costelli P, Baccino FM, Aroasio E, Pani P. Cholesterol content in tumor tissues is inversely associated with high-density lipoprotein cholesterol in serum in patients with gastrointestinal cancer. Cancer. 1994;73(2):253–258. doi: 10.1002/1097-0142(19940115)73:2<253::aid-cncr2820730204>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 27.Kuzu OF, Noory MA, Robertson GP. The role of cholesterol in cancer. Cancer Res. 2016;76(8):2063–2070. doi: 10.1158/0008-5472.CAN-15-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ninomiya M, Emi Y, Motomura T, Tomino T, Iguchi T, Kayashima H, Harada N, Uchiyama H, Nishizaki T, Higashi H, Kuwano H. Efficacy of neoadjuvant chemotherapy in patients with high-risk resectable colorectal liver metastases. Int J Clin Oncol. 2021;26(12):2255–2264. doi: 10.1007/s10147-021-02024-5. [DOI] [PubMed] [Google Scholar]
- 29.Daitoku N, Miyamoto Y, Tokunaga R, Sakamoto Y, Hiyoshi Y, Iwatsuki M, Baba Y, Iwagami S, Yoshida N, Baba H. Controlling Nutritional Status (CONUT) score is a prognostic marker in metastatic colorectal cancer patients receiving first-line chemotherapy. Anticancer Res. 2018;38(8):4883–4888. doi: 10.21873/anticanres.12802. [DOI] [PubMed] [Google Scholar]