Abstract
Background/Aim
Advanced glycation end products (AGEs) accumulate in the body with increasing age. However, their excessive accumulation may lead to various inflammatory and chronic diseases. While it is common for older adults to experience various comorbidities, there is a scarcity of published literature documenting the specific impact of ageing and comorbidities on AGEs in this population. The present study aimed to retrospectively evaluate the correlation among AGEs in the skin, calendar age, and comorbidities in older adults.
Patients and Methods
Accumulated AGEs in the skin were assessed by non-invasive measurement of skin autofluorescence (SAF) inside the forearm. This retrospective study included individuals who underwent SAF measurements at Shujitsu University Community Pharmacy with or without a prescription from October 2019 to October 2021. Subsequently, the associations between SAF, calendar age, comorbidities, and blood test parameters were investigated.
Results
SAF showed a positive correlation with calendar age for all enrolled participants; the correlation weakened for participants aged ≥50 years and plateaued for those aged ≥60 years. Furthermore, we observed a significant increase in SAF among all participants with comorbidities compared to those without comorbidities. By contrast, among participants aged ≥50 years, SAF did not show a significant association with comorbidities. However, SAF was significantly positively correlated with white blood cell (WBC) counts in these aged populations.
Conclusion
The non-invasive assessment of SAF holds promise in evaluating changes in the physical condition associated with WBC counts among older adults.
Keywords: Skin autofluorescence, advanced glycation end products, comorbidity, white blood cells, ageing, non-invasiveness, older adults
Advanced glycation end products (AGEs) progressively accumulate in the skin as individuals age. These compounds are formed through non-enzymatic reactions involving carbonyl groups of reducing sugars and various biomolecules, such as proteins, lipids, or nucleic acids (1,2). Notably, AGEs have gained recognition as a biomarker for the ageing process (3,4). Recently, skin autofluorescence (SAF) intensity has been used to assess the accumulation of AGEs in the skin and has been reported to correlate significantly with age-dependent AGEs accumulation (5,6). Furthermore, previous studies have indicated that quantifying SAF in patients might be a valuable tool in evaluating the risk of AGEs-related diseases, including diabetes, within clinical settings (7).
The synthesis and accumulation of AGEs are accelerated under inflammatory and oxidative stress conditions (8). Moreover, the excessive accumulation of AGEs, as assessed by SAF, is associated with many adverse health conditions (9). For example, SAF has been reported as a potential marker for several inflammatory diseases, including lifestyle-related diseases, diabetes, cardiovascular disease, atherosclerosis, and chronic obstructive pulmonary disease (10-14). These studies suggest that SAF, as a measure of AGE deposition, could predict disease-related complications or all-cause mortality. Nevertheless, among older adults, the rising prevalence and severity of diverse comorbidities, including AGEs-related diseases and ageing, can potentially diminish the specificity of disease-specific indices. Nevertheless, research regarding the relationship between AGEs, ageing, and comorbidities, especially in older adults, is lacking. Additionally, the impact of various blood test parameters on the variability of SAF measurements in the elderly is not well comprehended. Therefore, investigating the relationship among SAF, age, and comorbidities in older adults, as well as exploring the association between SAF and the outcomes of individual blood test parameters, may lead to non-invasive assessment of physical health status using SAF in older adults.
The present study aimed to retrospectively evaluate changes in SAF and their correlation with age and comorbidity in older adults. Additionally, since blood test data are indicative of physical health status, we also investigated the correlation between SAF and blood test results to evaluate the impact of the physical condition of study participants on SAF.
Patients and Methods
Ethical standards. The present study was performed in accordance with the Ethical Guidelines for Medical and Biological Research Involving Human Subjects in Japan and with the Helsinki Declaration. This study was approved by the Ethics Committee of Shujitsu University (Approval Number: 244). As this study had a retrospective design and participant anonymity was ensured, the Shujitsu University Ethics Committee waived the requirement for written informed consent, and an opt-out method was applied by notifications displayed on Shujitsu University Community Pharmacy and the website of the pharmacy, as well as that of Shujitsu University.
Study participants. Data were extracted from study participants who underwent SAF measurement at Shujitsu University Community Pharmacy from October 2019 to October 2021. Participants with a calendar age <20 years, who failed SAF measurement attempts, or those who refused to consent to participate in this study were excluded. Information regarding calendar age and comorbidities related to the increase in AGEs was obtained through patient medical questionnaires, records of drug history, prescriptions, and data extracted from their medication registries. The identification of comorbidities that have the potential to relate to the increase of AGEs was conducted based on previously reported criteria (15,16). Subsequently, the association between SAF and calendar age or comorbidity was retrospectively investigated. In addition, 23 participants aged ≥50 years who supplied us with their prescriptions, including the information on blood test results, were evaluated for the association between SAF and blood tests.
Non-invasive skin autofluorescence and the assessment of AGEs. SAF was measured from inside the forearm using a non-invasive device (AGE Reader mu; DiagnOptics, Groningen, the Netherlands) (7,17). SAF was quantified as the ratio of average autofluorescence per nanometre (nm) within the 420-600 nm range to the average autofluorescence per nm within the 300-420 nm range, measured over a 1 cm2 skin area. SAF values were expressed in arbitrary units (AU). Furthermore, it has been reported that the error ratio in repeated SAF measurements in a single day is approximately 5.0%, while that of intra-individual seasonal variance is approximately 5.9% (4).
Blood tests. In Japanese outpatient practice, prescribing physicians can include blood test results on the prescription for patients, facilitating appropriate drug therapy from community pharmacists. Consequently, blood test data included in the prescriptions submitted to pharmacists, according to Japanese practise, were recorded and used for the analysis. Nine blood test parameters, including white blood cells (WBC), haemoglobin (Hb), platelets (PLT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum creatinine (Scr), urea nitrogen (UN), potassium (K), and C-reactive protein (CRP), were investigated as numerical indices indicative of the physical health status.
Statistical analysis. Continuous variables are expressed as median (interquartile range), based on the results of a Shapiro-Wilk normality test. The Kruskal-Wallis test followed by Dunn’s multiple comparisons test was utilised for comparing continuous variables, while the Mann-Whitney U-test was employed for specific pairwise comparisons. Fisher’s exact test was utilised for assessing associations between categorical variables. Furthermore, correlations between two continuous variables were evaluated using Spearman’s correlation coefficient. Correlations were evaluated based on the magnitude of the correlation coefficient (r). A significant correlation was observed for values of 0.4 ≤|r|<1.0, whereas a weak correlation was identified for values of 0.2≤|r|<0.4. To mitigate the influence of age and sex disparities on the statistical outcomes, propensity score matching analysis was conducted. Binomial logistic regression analysis incorporating age and sex as categorical variables was employed to estimate propensity scores. Subsequently, one-to-two propensity score matching was performed using nearest neighbour matching, with a calliper width set at 0.2 standard deviations of the propensity score. The statistical significance threshold was set at p<0.05 or p<0.01. EZR (Eazy R; Saitama Medical Center, Jichi Medical University, Saitama, Japan, version 4.0.3) was utilised for conducting the propensity score matching analysis (18). All other statistical analyses were performed using the GraphPad Prism 9.4.1 software (GraphPad Software Inc., San Diego, CA, USA).
Results
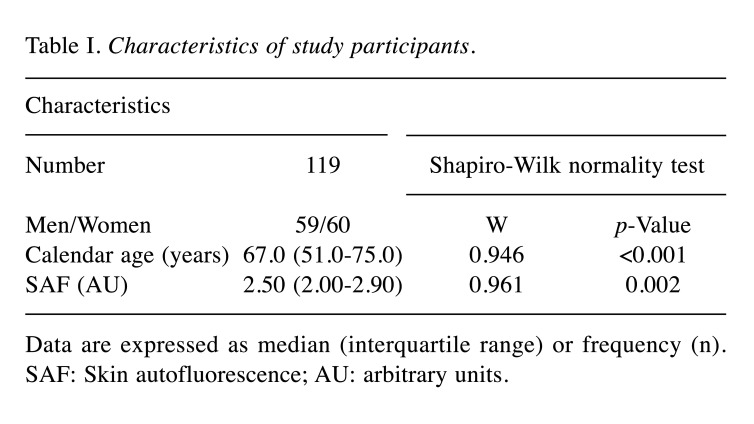
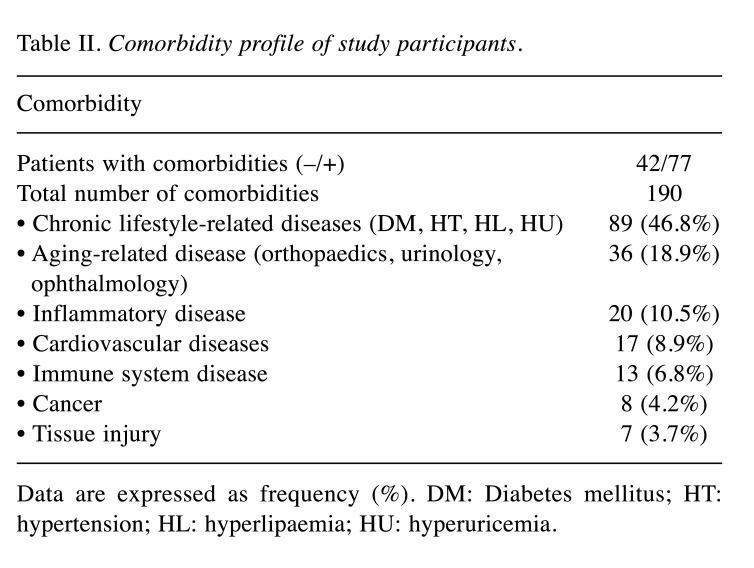
Background of the study participants. Of the 126 participants measured for SAF, 119 (59 men, 60 women) were enrolled in this study. The characteristics of the participants are listed in Table I. The study included participants with a calendar age ranging from 22 to 93 years, with a median age of 67.0 years (range=51.0-75.0 years). The median SAF value among the participants was 2.50 AU (range=2.00-2.90 AU) (Table I). Furthermore, 77 participants presented with a total of 190 comorbidities that could cause an increase in AGEs; these included chronic lifestyle-related diseases, aging-related diseases, inflammatory diseases, cardiovascular diseases, immune system diseases, cancer, and tissue injury (Table II).
Table I. Characteristics of study participants.
Data are expressed as median (interquartile range) or frequency (n). SAF: Skin autofluorescence; AU: arbitrary units.
Table II. Comorbidity profile of study participants.
Data are expressed as frequency (%). DM: Diabetes mellitus; HT: hypertension; HL: hyperlipaemia; HU: hyperuricemia.
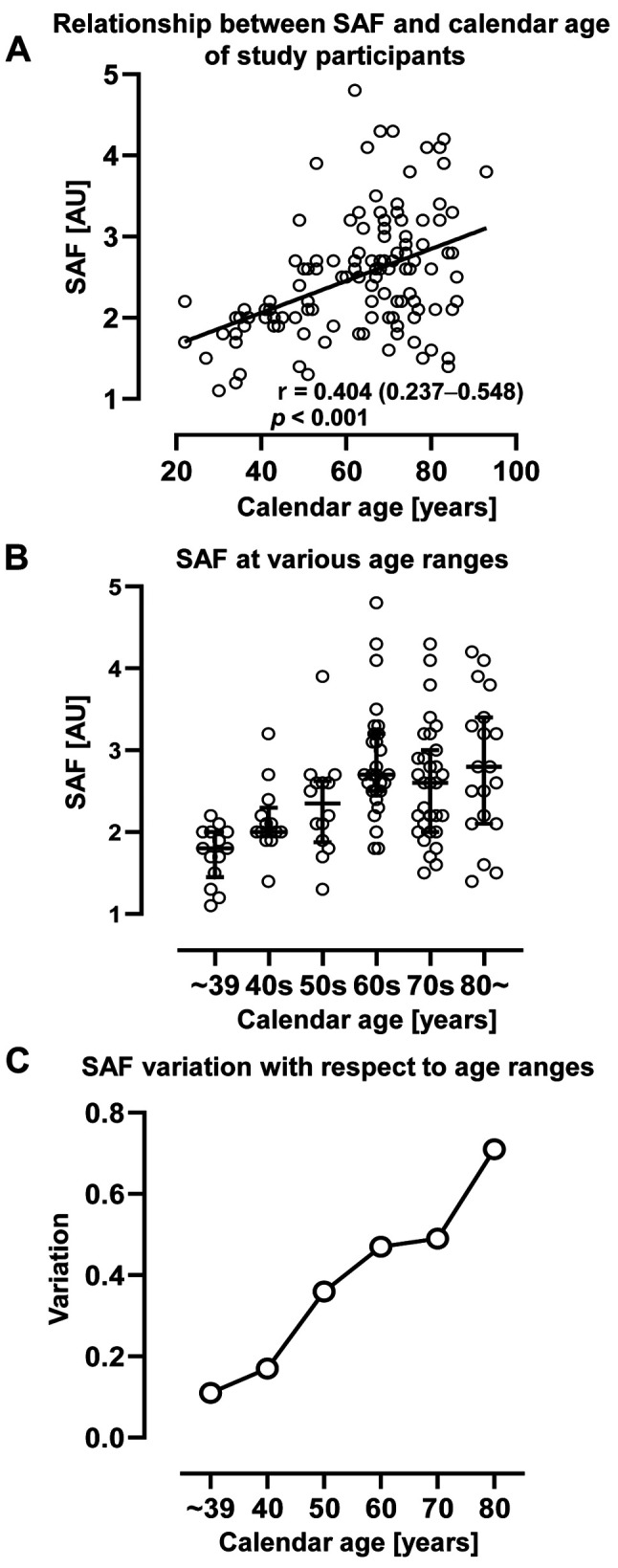
SAF values and variability increased significantly with increasing calendar age. We examined the correlation between SAF values and calendar age in all participants. SAF was significantly correlated with calendar age (Figure 1A). However, the median SAF value plateaued after 60 years of age (Figure 1B), and the variability in SAF values increased remarkably with age (Figure 1C).
Figure 1. Correlation between skin autofluorescence (SAF) and calendar age. SAF significantly correlates with calendar age (A). Median SAF plateaus at 60 years of age (B). The variation in SAF in each age group increases with age (C). Correlation analysis performed using the Spearman’s correlation coefficient. The bars represent the median and interquartile range. AU: Arbitrary unit; r: correlation coefficient. p-Value and r (95%CI) are shown in the figure.
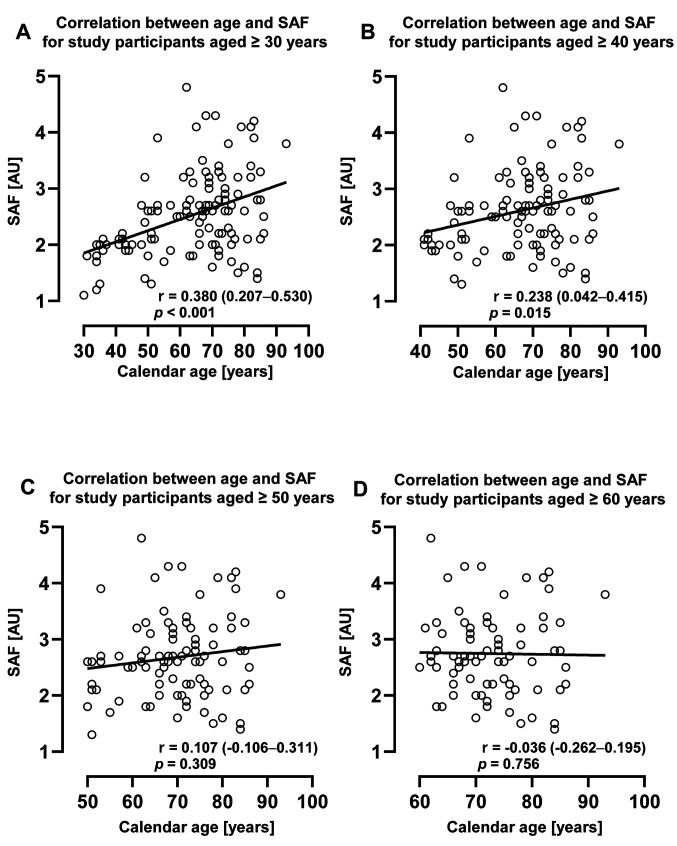
The correlation of SAF with ageing in older adults. As shown in Figure 2, the correlation between SAF and calendar age tended to weaken with age. For study participants aged <50 years, there was a significant correlation between SAF and calendar age (Figure 2A and B). However, this correlation weakened with increasing calendar age, ceased to be significant for participants ≥50 years of age (Figure 2C), and was completely abolished in participants >60 years of age (Figure 2D).
Figure 2. Change in the correlation between SAF and calendar age with changing age. The figure shows the correlation between SAF and calendar age of participants aged ≥30 years (A), ≥40 years (B), ≥50 years (C), and ≥60 years (D). Correlation analysis was performed using Spearman’s correlation coefficient. SAF: Skin autofluorescence; AU: arbitrary unit; r: correlation coefficient. p-Value and r (95%CI) are shown in the figure.
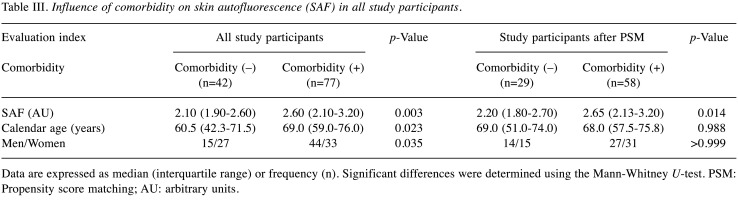
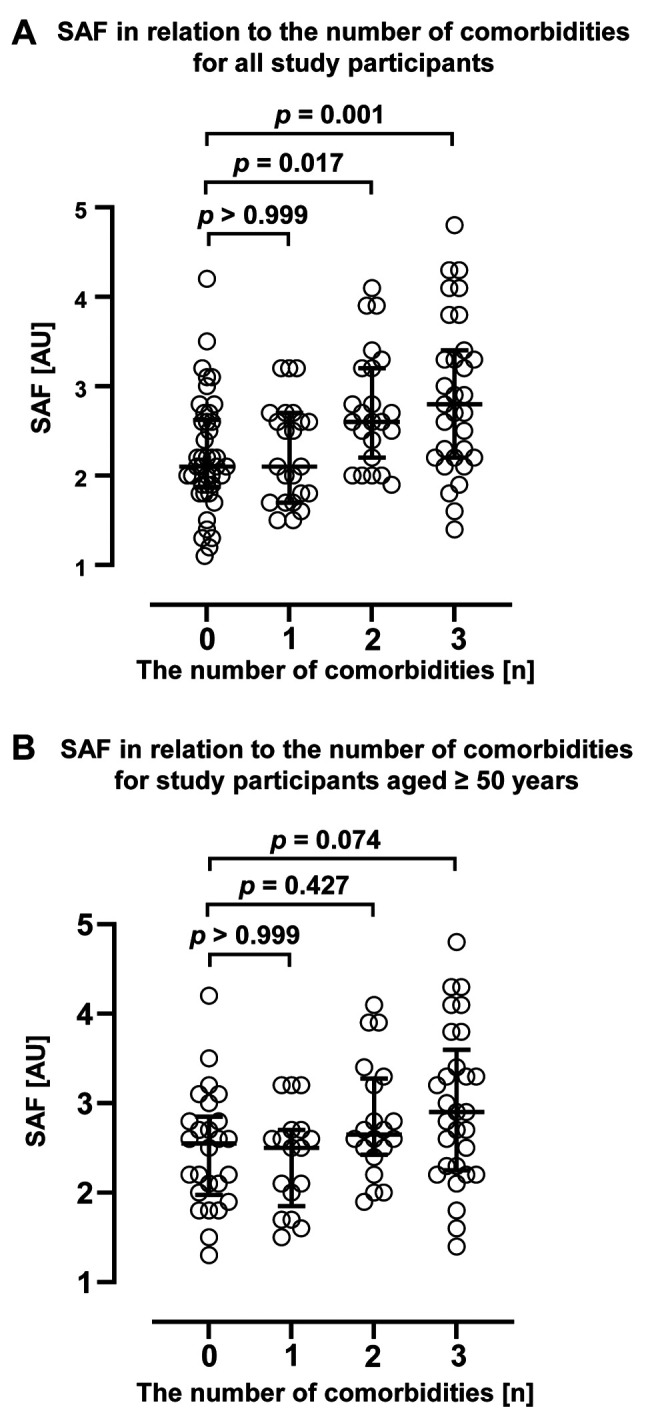
Effect of comorbidity on the correlation between SAF and calendar age. Next, changes in SAF due to comorbidities with a potential to increase AGEs are presented in Table III, Table IV, and Figure 3. The results showed that SAF was significantly higher in study participants with comorbidities (Table III). However, calendar age and men ratio were also significantly higher in the participants with comorbidities. Therefore, to mitigate the impact of age and sex on SAF measurements, we employed the propensity score matching method to assess the variation in SAF both with and without disease after adjusting for age and sex. The results showed that the SAF was significantly higher in subjects with comorbidities, even when the effect of age and sex were not considered. Furthermore, a significant increase in SAF was observed when multiple comorbidities were present (Figure 3A). Nonetheless, in individuals aged 50 years and above, SAF did not exhibit a significant change in the presence of comorbidities, even after minimising the influence of age and sex on SAF measurements (Table IV). Furthermore, SAF did not demonstrate a significant increase with an increasing number of diseases (Figure 3B). These findings indicate that while comorbidities may have impacted the association between age and SAF across all cases, additional factors beyond age and comorbidities may have influenced SAF variations among individuals in the middle and upper age strata (above 50 years).
Table III. Influence of comorbidity on skin autofluorescence (SAF) in all study participants.
Data are expressed as median (interquartile range) or frequency (n). Significant differences were determined using the Mann-Whitney U-test. PSM: Propensity score matching; AU: arbitrary units.
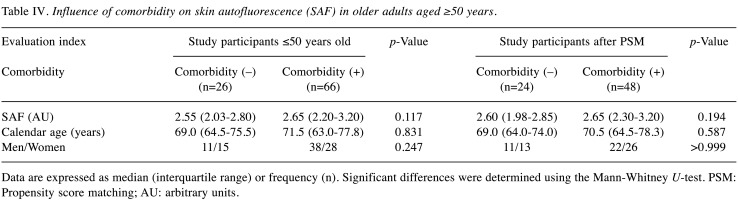
Table IV. Influence of comorbidity on skin autofluorescence (SAF) in older adults aged ≥50 years.
Data are expressed as median (interquartile range) or frequency (n). Significant differences were determined using the Mann-Whitney U-test. PSM: Propensity score matching; AU: arbitrary units.
Figure 3. Variation in skin autofluorescence (SAF) with changes in the number of comorbidities. The figure shows the SAF variations according to the number of comorbidities in all patients (A) and in patients aged ≥50 years (B). Comparisons for study participants without comorbidities were performed using the Kruskal-Wallis test followed by Dunn’s multiple comparisons test. The bars represent the median and interquartile range. AU: Arbitrary unit; p-value is shown in each figure.
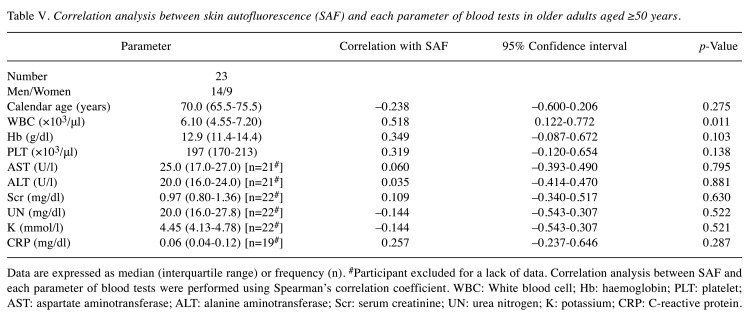
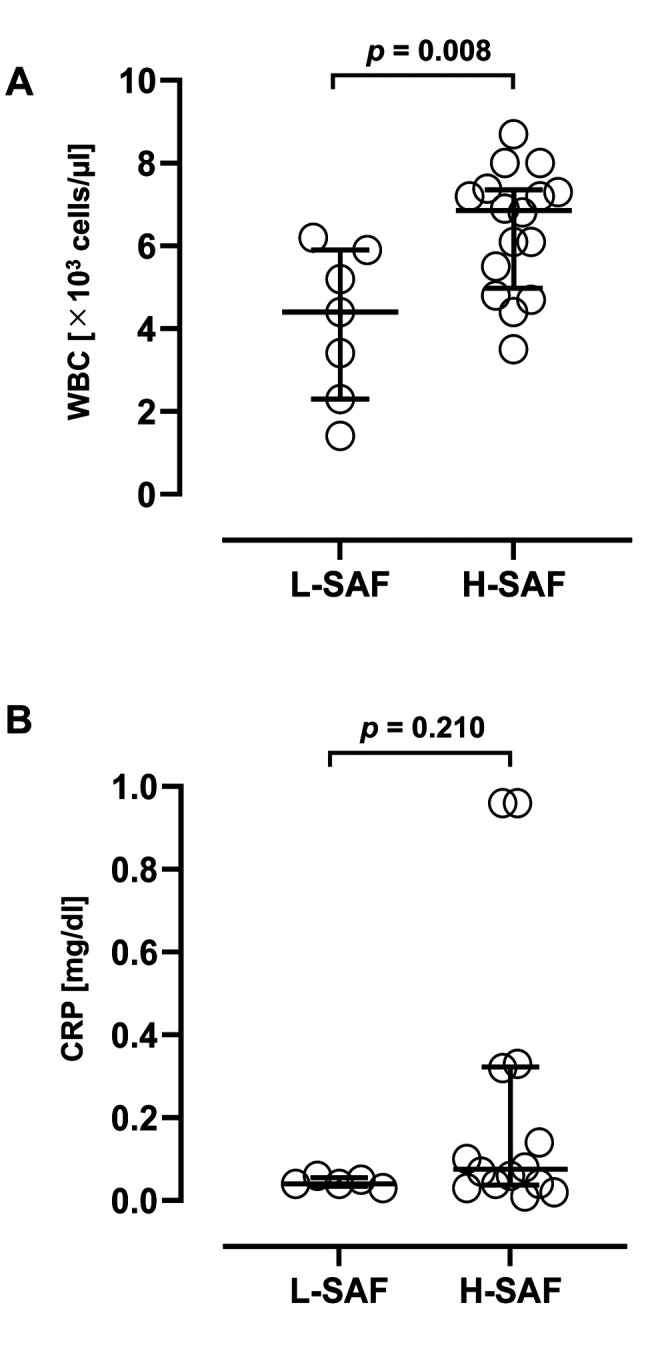
SAF correlated significantly with WBC counts in participants >50 years old. To confirm the factors influencing SAF changes in the study participants aged ≥50, we investigated the relationship between blood test data and SAF in 23 participants aged ≥50 years who provided us with their prescriptions (Table V). Remarkably, among the blood test parameters examined, only WBC counts exhibited a significant correlation with SAF (r=0.518, p=0.011). However, no significant correlation was observed between SAF and other inflammatory markers, such as CRP levels (r=0.257, p=0.287). Furthermore, calendar age and other blood test parameters did not significantly correlate with liver function, kidney function, degree of anaemia, or electrolyte balance. Subsequently, we divided study participants into two groups: the High-SAF (H-SAF) group, consisting of individuals with SAF values higher than the standard value for their respective calendar age as described in the product documentation and a previous report, and the Low-SAF (L-SAF) group, comprising individuals with SAF values lower than the standard value (19). WBC counts and CRP levels were compared between the L– and H– SAF groups (Figure 4). The WBC counts in the H–SAF group were significantly higher than those in the L–SAF group (p=0.008). However, the CRP levels were not significantly different between the two groups (p=0.210).
Table V. Correlation analysis between skin autofluorescence (SAF) and each parameter of blood tests in older adults aged ≥50 years.
Data are expressed as median (interquartile range) or frequency (n). #Participant excluded for a lack of data. Correlation analysis between SAF and each parameter of blood tests were performed using Spearman’s correlation coefficient. WBC: White blood cell; Hb: haemoglobin; PLT: platelet; AST: aspartate aminotransferase; ALT: alanine aminotransferase; Scr: serum creatinine; UN: urea nitrogen; K: potassium; CRP: C-reactive protein.
Figure 4. Comparison of inflammatory marker levels between the high and low skin autofluorescence (SAF) groups. The WBC counts are significantly higher in the high SAF group than in the low SAF group (A). However, the C-reactive protein (CRP) levels are not significantly different between the two groups (B). Significant differences were determined using the Mann-Whitney U-test. The bar shows the median and interquartile range. WBC: White blood cell; p-values are shown in each figure.
Discussion
Although a positive correlation between SAF and the calendar age of a person is well established in the medical literature, our study shows for the first time that this SAF-calendar age association diminishes in middle age and reaches a plateau among older individuals. Furthermore, there was no significant increase in SAF due to comorbidity in subjects over 50 years of age. Conversely, among individuals aged 50 years and older, there was a positive correlation between SAF and WBC counts. According to previous reports, SAF has the potential to be a predictor of physical ageing owing to its significant correlation with calendar age (4,6). Furthermore, SAF has been reported to be a potential predictor of several inflammatory diseases (20,21). However, our data indicate that SAF might not predict ageing and comorbidity reliably in older adults. Therefore, it may be necessary to consider additional attributes in addition to SAF to assess the deteriorating health status of older adults.
The SAF values of the study participants aged ≥50 showed a significant correlation with WBC count among the various blood test parameters examined; however, no significant correlation was observed between SAF values and the levels of CRP, which is another marker of inflammation. Nonetheless, some studies have reported a strong correlation between SAF and CRP ≥0.3 mg/dl in older patients with diabetes and mild cognitive impairment (22). In this study, the CRP levels of almost all participants were below the normal upper limit (≤0.3 mg/dl). The low CRP levels of the study population might be one of the reasons that the association between SAF and CRP was not detected. However, the SAF-WBC count correlation was significant despite WBC count was lower than the upper limit of the normal range. These results suggest that SAF values in older adults might specifically correlate with WBC count. Furthermore, AGEs may play a key role in the induction of oxidative stress because of the increased production of ROS by circulating polymorphonuclear neutrophils in vitro (23). Although the detailed mechanism of the positive correlation between SAF and WBC count has not yet been fully investigated, we hypothesize that SAF values may be associated with neutrophil-derived oxidative stress, since they comprise the are the main cell population of WBC.
In clinical practice, it is well-known that WBC counts typically increase in response to cancer and infections caused by bacteria and other pathogens. Conversely, WBC counts may decrease in severe infections, aplastic anaemia, and as a side effect of certain medications like anticancer drugs and immunosuppressive drugs. Hence, detecting changes in WBC counts is crucial in assessing overall health and identifying underlying diseases, making it one of the most clinically significant blood tests in general practice. Additionally, there has been a global increase in the proportion of individuals aged over 65 years (24). In particular, Japan has witnessed the most rapid increase in the ageing population, with a high ratio of older adults over the age of 65 (25). Given this demographic shift, older adults must focus on disease prevention and health maintenance as early as possible to prolong their life expectancy. Monitoring WBC counts in the elderly may enhance healthy life expectancy, prevent diseases, facilitate early disease detection, and mitigate disease exacerbation. Additionally, understanding changes in WBC counts during drug treatment can aid in monitoring and identifying adverse drug reactions.
The strength of this study is our finding that SAF is significantly correlated with WBC counts, which are mostly within the Japanese normal range (3,300-8,600 cells/mm3) (26) in older adults. The standard methods of WBC count assessment involve a blood test in clinical settings. However, blood collection is a medical procedure that can be performed in medical institutions and individuals with medical qualifications. In addition, the sample needs to be processed after collection, and it takes time to obtain the results; finally, consumables and labour costs are also required. Moreover, patients undergoing blood tests may experience adverse events, such as pain, nerve damage, bleeding, and infection due to needle punctures. By contrast, SAF measurement is a non-invasive, straightforward, and cost-effective method that imposes no restrictions on the measurement location or person. Furthermore, the risk of adverse events associated with SAF measurement is extremely low. Additionally, SAF measurement is a quick procedure, and the results can be immediately obtained on-site, typically within approximately 12 seconds.
Therefore, this study showed, for the first time, that the change in WBC counts in older adults can be assessed noninvasively by simply measuring SAF. Additionally, SAF-based assessment of health status or recommendations of medical checks have the potential to improve the health status of people before the occurrence or exacerbation of the disease.
The present study has several limitations. First, this retrospective study was performed at a single centre. Therefore, the study design did not allow for randomisation, missing data were excluded from the analysis, and the sample size was small and not controlled. Second, the information on comorbidities was based on the voluntary reports by the study participants, based on interviews and the materials they provided. These reports were not verified medical data reflecting confirmed diagnoses from medical institutions. Although future research with larger sample sizes of older adults are required, this study indicated that using the non-invasive measurement of SAF in older adults can be useful in evaluating and following diseases that can impact WBC counts. Furthermore, SAF measurement may prove useful in monitoring reductions in WBC counts caused by anticancer or immunosuppressive drugs.
Conclusion
Using non-invasive SAF assessment, skin AGEs showed no significant association with calendar age and comorbidities in older adults. However, SAF was significantly correlated with the WBC count in older adults. This correlation can potentially contribute to healthcare management, such as diseases and drugs that affect WBC- counts.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Study conceptualisation: Y.I., K.Y., K.M., and K.S. Study design: Y.I., K.Y., Y.T., K.M., Y.K., and K.S. Data Collection: Y.I., K.Y., and K.M. Data acquisition, analysis, and interpretation: Y.I., K.Y., Y.T., K.M., Y.K., and K.S. Manuscript draft: Y.I., Y.T., Y.K. Critical revision of the draft for important intellectual content: Y.I., Y.T., Y. K., and K.S. All co-authors have revised and approved the manuscript.
Acknowledgements
The Authors thank Professor Hiroyuki Kataoka for providing the device for non-invasive SAF measurements (AGE reader mu). The Authors also thank Professor Emeritus Takashi Shibata, Ms. Noriko Nishiyama, Ms. Tomoko Kawasaki, and Ms. Saki Takeuchi for their helpful support with data collection and analyses. The Authors would also like to thank Editage (www.editage.com) for English language editing.
References
- 1.Luevano-Contreras C, Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2(12):1247–1265. doi: 10.3390/nu2121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N, Gugliucci A, Kapahi P. The role of advanced glycation end products in aging and metabolic diseases: Bridging association and causality. Cell Metab. 2018;28(3):337–352. doi: 10.1016/j.cmet.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corstjens H, Dicanio D, Muizzuddin N, Neven A, Sparacio R, Declercq L, Maes D. Glycation associated skin autofluorescence and skin elasticity are related to chronological age and body mass index of healthy subjects. Exp Gerontol. 2008;43(7):663–667. doi: 10.1016/j.exger.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Băbţan AM, Ilea A, Boşca BA, Crişan M, Petrescu NB, Collino M, Sainz RM, Gerlach JQ, Câmpian RS. Advanced glycation end products as biomarkers in systemic diseases: premises and perspectives of salivary advanced glycation end products. Biomark Med. 2019;13(6):479–495. doi: 10.2217/bmm-2018-0448. [DOI] [PubMed] [Google Scholar]
- 5.Isami F, West BJ, Nakajima S, Yamagishi SI. Association of advanced glycation end products, evaluated by skin autofluorescence, with lifestyle habits in a general Japanese population. J Int Med Res. 2018;46(3):1043–1051. doi: 10.1177/0300060517736914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita Y, Yagi M, Ishizaki K, Takabe W, Komatsu T, Nakazawa M, Matsushima M, Urata T, Yonei Y. Evaluation of the glycative stress by non-invasive skin AGEs measurement devices. Glycative Stress Res. 2019;6(2):92–102. doi: 10.24659/gsr.6.2_92. [DOI] [Google Scholar]
- 7.Meerwaldt R, Graaff R, Oomen PHN, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gans ROB, Smit AJ. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47(7):1324–1330. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- 8.Yamagishi S. Potential clinical utility of advanced glycation end product cross-link breakers in age- and diabetes-associated disorders. Rejuvenation Res. 2012;15(6):564–572. doi: 10.1089/rej.2012.1335. [DOI] [PubMed] [Google Scholar]
- 9.Demirer B, Samur G. Possible effects of dietary advanced glycation end products on maternal and fetal health: a review. Nutr Rev. 2023;81(7):844–856. doi: 10.1093/nutrit/nuac090. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka M, Matsumura T, Ohno R, Fujiwara Y, Shinagawa M, Sugawa H, Hatano K, Shirakawa J, Kinoshita H, Ito K, Sakata N, Araki E, Nagai R. Non-invasive measurement of skin autofluorescence to evaluate diabetic complications. J Clin Biochem Nutr. 2016;58(2):135–140. doi: 10.3164/jcbn.15-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerrits EG, Lutgers HL, Kleefstra N, Graaff R, Groenier KH, Smit AJ, Gans RO, Bilo HJ. Skin autofluorescence. Diabetes Care. 2008;31(3):517–521. doi: 10.2337/dc07-1755. [DOI] [PubMed] [Google Scholar]
- 12.Cavero-Redondo I, Soriano-Cano A, Álvarez-Bueno C, Cunha PG, Martínez-Hortelano JA, Garrido-Miguel M, Berlanga-Macías C, Martínez-Vizcaíno V. Skin autofluorescence-indicated advanced glycation end products as predictors of cardiovascular and all-cause mortality in high-risk subjects: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(18):e009833. doi: 10.1161/JAHA.118.009833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vos LC, Mulder DJ, Smit AJ, Dullaart RP, Kleefstra N, Lijfering WM, Kamphuisen PW, Zeebregts CJ, Lefrandt JD. Skin autofluorescence is associated with 5-year mortality and cardiovascular events in patients with peripheral artery disease. Arterioscler Thromb Vasc Biol. 2014;34(4):933–938. doi: 10.1161/ATVBAHA.113.302731. [DOI] [PubMed] [Google Scholar]
- 14.Zaigham S, Persson M, Jujic A, Frantz S, Borné Y, Malinovschi A, Wollmer P, Engström G. Measures of lung function and their relationship with advanced glycation end-products. ERJ Open Res. 2020;6(2):00356–2019. doi: 10.1183/23120541.00356-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin J, Wu C, Yen G. Perspective of advanced glycation end products on human health. J Agric Food Chem. 2018;66(9):2065–2070. doi: 10.1021/acs.jafc.7b05943. [DOI] [PubMed] [Google Scholar]
- 16.Ashraf JM, Ahmad S, Choi I, Ahmad N, Farhan M, Tatyana G, Shahab U. Recent advances in detection of AGEs: Immunochemical, bioanalytical and biochemical approaches. IUBMB Life. 2015;67(12):897–913. doi: 10.1002/iub.1450. [DOI] [PubMed] [Google Scholar]
- 17.Xin C, Wang Y, Liu M, Zhang B, Yang S. Correlation analysis between advanced glycation end products detected noninvasively and skin aging factors. J Cosmet Dermatol. 2021;20(1):243–248. doi: 10.1111/jocd.13452. [DOI] [PubMed] [Google Scholar]
- 18.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koetsier M, Lutgers H, De Jonge C, Links T, Smit A, Graaff R. Reference values of skin autofluorescence. Diabetes Technol Ther. 2010;12(5):399–403. doi: 10.1089/dia.2009.0113. [DOI] [PubMed] [Google Scholar]
- 20.Paolillo FR, Mattos VS, Borghi-Silva A, Bagnato VS, De Castro Neto JC. Advanced glycation endproducts as biomarkers for risk of diabetes and cardiovascular diseases by skin autofluorescence: a noninvasive optical screening. Photobiomodul Photomed Laser Surg. 2019;37(3):168–174. doi: 10.1089/photob.2018.4563. [DOI] [PubMed] [Google Scholar]
- 21.Viramontes Hörner D, Taal MW. Skin autofluorescence. Curr Opin Nephrol Hypertens. 2019;28(6):507–512. doi: 10.1097/MNH.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 22.Gorska-Ciebiada M, Saryusz-Wolska M, Borkowska A, Ciebiada M, Loba J. C-reactive protein, advanced glycation end products, and their receptor in type 2 diabetic, elderly patients with mild cognitive impairment. Front Aging Neurosci. 2015;7:209. doi: 10.3389/fnagi.2015.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal S, Siddarth M, Chawla D, Banerjee BD, Madhu SV, Tripathi AK. Advanced glycation end products enhance reactive oxygen and nitrogen species generation in neutrophils in vitro. Mol Cell Biochem. 2012;361(1-2):289–296. doi: 10.1007/s11010-011-1114-9. [DOI] [PubMed] [Google Scholar]
- 24.World Population Prospects 2022: Summary of Results: United Nations Department of Economic and Social Affairs, Population Division 2022.UN DESA/POP/2022/TR/NO. 3. Available at: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf. [Last accessed on June 15, 2023]
- 25.Top 50 Countries With the Largest Percentage of Older Adults, Countries With the Oldest Populations in the World: PRB, 2019. Available at: https://www.prb.org/resources/countries-with-theoldest-populations-in-the-world/ [Last accessed on December 23, 2022]
- 26.Ichihara K, Yomamoto Y, Hotta T, Hosogaya S, Miyachi H, Itoh Y, Ishibashi M, Kang D, on behalf of the Committee on Common Reference Intervals, Japan Society of Clinical Chemistry. Collaborative derivation of reference intervals for major clinical laboratory tests in Japan. Ann Clin Biochem. 2016;53(3):347–356. doi: 10.1177/0004563215608875. [DOI] [PubMed] [Google Scholar]