Abstract
In the present study, oral supplementation of l-arginine in rats was evaluated for its anti-stress and adaptogenic activity using the cold (5°C)–hypoxia (428 mmHg)–restraint (C-H-R) animal model. A dose-dependent study of l-arginine was carried out at doses of 12.5, 25.0, 50.0, 100.0, 200.0 and 500.0 mg/kg body weight, administered orally 30 min prior to C-H-R exposure. The time taken by the rat to attain a rectal temperature of 23°C (Trec 23°C) during C-H-R exposure and its recovery to Trec 37°C at normal atmospheric pressure and 32 ± 1°C were used as biomarkers of anti-stress and adaptogenic activity. Biochemical parameters related to lipid peroxidation, anti-oxidants, cell membrane permeability, nitric oxide and stress, with and without administration of the least effective l-arginine dose, were measured in rats on attaining Trec 23°C and Trec 37°C. The least effective adaptogenic dose of l-arginine was 100.0 mg/kg body weight. The C-H-R exposure of control rats, on attaining Trec 23°C, resulted in a significant increase in plasma malondialdehyde (MDA), blood lactate dehydrogenase (LDH) and a decrease in blood catalase (CAT) and plasma testosterone levels. On recovery (Trec 37°C) of control rats, there was a further decrease in CAT and plasma testosterone, and an increase in LDH. l-Arginine supplementation resulted in a significant decrease in plasma MDA, an increase in blood superoxide dismutase (SOD), CAT levels maintained at control values and a lower increase in LDH compared with controls (45.3 versus 58.5% and 21.5 versus 105.2%) on attaining Trec 23°C during C-H-R exposure and on recovery to Trec 37°C. The results suggested that l-arginine possesses potent anti-stress activity during C-H-R exposure and recovery from C-H-R-induced hypothermia.
Keywords: hypoxia, cold, rectal temperature, oxidative stress
Introduction
Biological stress is a response to physical, chemical, biological and emotional changes, consisting of a pattern of metabolic and behavioral reactions that helps to strengthen the organism (1). During stressful situations, the energy requirement of the organism is increased, resulting in enhanced generation of free radicals (2–4). Free radicals cause oxidation of nucleic acids and proteins. Free radicals also damage biomembranes, reflected by increased lipid peroxidation, thereby compromising cell integrity and function. During this process, the ability of the body's defense system to combat the oxidative stress may diminish due to reduced anti-oxidants. If the stress level increases beyond the threshold limit of an individual, it results in decreased performance and stress-induced disorders. The management of unusual stress therefore has acquired enormous significance in day-to-day life. Such a management does not endeavor to eliminate stress but rather to raise the threshold level of the organism beyond which stress would start injuring and disturbing life processes. It is possible to support the body's adaptation by using food supplements, dietary elements, herbs and minerals for increasing physical and mental performance, described in various oriental systems of medicine including the ancient Indian medical system Ayurveda. Such substances have been described as ‘adaptogens’ (5). In strenuous conditions, the physical performance of the organism is dependent on the availability of appropriate macro- and micronutrients required in excess on account of their increased utilization during stressful situations (6). Supplementation with various macro- and micronutrient and herbal preparations has been evaluated for their adaptogenic activity during exposure to a stressful environment (7–10).
It has been suggested that amino acid supplementation might be able to increase human performance to a limited extent (11). An amino acid mixture supplementation was observed to enhance adrenocortical hormone, luteinizing hormone and follicle-stimulating hormone response to corticotropin-releasing hormone in athletes (12). Under certain metabolic, developmental or pathophysiological conditions, some of the non-essential amino acids become essential and are designated as ‘conditionally essential’. Arginine and glutamine are known to be conditionally essential amino acids (13). l-Arginine plays important roles in the urea cycle, protein synthesis, as a precursor of polyamines and creatine, and as a substrate for synthesis of nitric oxide (NO). NO was shown to be an endothelial-derived relaxation factor, a vasodilator, which acted as a modulator of vascular tone to regulate blood flow and blood pressure (14). NO is also involved in enhancement of the thermogenic function of brown adipose tissue in rats (15). It is interesting that herbs with adaptogenic activity, e.g. Panax ginseng, have been shown to contain large amounts of arginine (16).
It was shown that endogenous plasma arginine levels decreased significantly after 30 min immobilization stress and remain suppressed during a 3.5 h post-stress period (17). In burn patients, there was a higher rate of arginine loss from the body and supplementation of arginine was required to maintain homeostasis and promote recovery (18). In the present study, the anti-stress and adaptogenic effect, if any, of l-arginine supplementation was studied in rats subjected to a comprehensive and generalized stress of cold (5°C)–hypoxia (428 mmHg)– restraint (C-H-R) (19).
Materials and Methods
Rats and Maintenance
Randomly bred, healthy, adult albino rats of the Wistar strain, weighing 180 ± 20 g, from the animal colony of the Defence Institute of Physiology and Allied Sciences, Delhi, India, were used in this study. The experimental protocol was submitted to the animal ethics committee of the institute, and approval was obtained for conducting the experiments. The rats were kept in a room that was maintained at 25 ± 1°C with natural daylight. The room remained dark from 7 p.m. until 7 a.m. in the morning. The animals had free access to drinking water and food in pellet form (Lipton India Ltd., Calcutta, India). All the experiments were done on overnight fasted rats. The l-arginine used in the study was obtained from Sigma Chemical Company, St Louis, MO.
Experimental Design
In the present study, two experiments were carried out, one for dose-dependent study of l-arginine supplementation in rats using the C-H-R animal model to evaluate the least effective dose for adaptogenic activity and another for studying biochemical parameters in animals administered the least effective single oral dose of l-arginine.
Dose-dependent Studies
In total, 42 overnight fasted Wistar strain male rats were used in this experiment. The l-arginine was dissolved in distilled water in the appropriate concentration. A single dose of l-arginine was given orally in a 0.5 ml volume through a gastric cannula to overnight fasted rats, 30 min prior to C-H-R exposure (19). The l-arginine doses studied were 12.5, 25, 50, 100, 200 and 500 mg/kg body weight. Six rats were used for each dose. Six control rats were administered an equivalent amount of water orally 30 min prior to C-H-R exposure. The rats were exposed in a decompression chamber maintained at 5°C and a low atmospheric pressure of 428 mmHg pressure equivalent to an altitude of 4572 m. The rats were restrained and a rectal probe was inserted 2 cm past the rectum and kept there with the help of adhesive plaster. The rectal temperature (Trec) of the rats was monitored once per minute, by using a 16-channel Isothermex Temperature Recorder (Columbus Instrument, Columbus, OH). When the rats attained a rectal temperature (Trec) of 23°C, they were taken out of the chamber. The rats were allowed to recover to a normal Trec of 37°C at normal atmospheric pressure and ambient temperature 32 ± 1°C. The rats continued to be restrained during the recovery period. Although the comfortable temperature for housing the animals was 25 ± 1°C, the temperature for recovery of Trec (37°C) was selected as 32 ± 1°C because this was close to the ambient temperature of the outside environment. A constant room temperature was maintained in all experiments as recovery time also depended on the ambient temperature. The time taken to attain Trec 23°C and its recovery to 37°C were used as a measure of endurance.
Biochemical Analysis
In the second experiment, 36 separate overnight fasted rats were used. Rats were divided into two groups, containing 18 rats in each. One group was given the least effective dose of l-arginine and the other served as control. Both arginine-treated and control rats were subdivided further into three groups, six rats in each group: (i) rats not exposed to C-H-R; (ii) rats exposed to C-H-R to a fall of Trec to 23°C; and (iii) rats exposed to C-H-R and recovered to Trec 37°C.
A single oral dose of l-arginine was administered orally to rats in a 0.5 ml volume, 30 min prior to C-H-R exposure. In control rats, the equivalent volume of water was administered orally 30 min prior to C-H-R exposure. About 4 ml of blood was collected from the orbital plexus of the eye, in heparinized tubes, of different group of rats both control and arginine treated (exposed and unexposed) under mild ether anesthesia, on attaining both Trec 23°C and Trec 37°C. A portion of blood was used to separate the plasma. Malondialdehyde (MDA), a marker for lipid peroxidation, was measured in plasma by the method of Dousset et al. (20). Blood levels of lactate dehydrogenase (LDH), as a marker of cell membrane permeability, were estimated (21). In plasma, the levels of nitric oxide (NO), as a metabolite of nitrite and nitrate, were measured using diagnostic kits obtained from Oxis International Inc., Portland, OR. Plasma levels of testosterone, a stress marker (29), were estimated using the radioimmunoassay kits obtained from Immunotech, Marseille, Cedex, France. In blood, superoxide dismutase (SOD) was estimated by the method of Marklund (22), catalase (CAT) by the method of Aebi et al, (23) and reduced glutathione (GSH) by the method of Beutler et al. (24). In blood and plasma, protein was estimated by the method of Lowry et al. (25) for calculating the specific activity of the enzymes.
Statistics
The results were analyzed using one-way analysis of variance (ANOVA) for their statistical significance and expressed as the mean ± SEM. The results of l-arginine-treated animals were compared with the respective controls and P-values <0.05 were considered statistically significant.
Results
Dose-dependent Studies
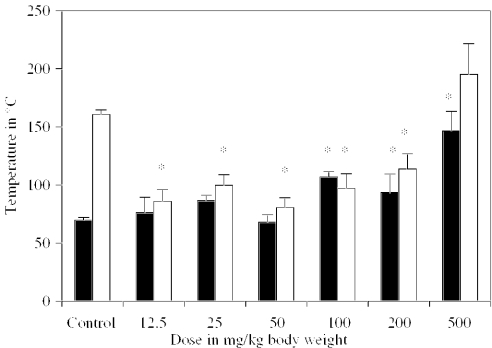
The orally administered doses of 12.5, 25 and 50 mg/kg body weight in rats were observed to have no significant effect on the time taken to attain Trec 23°C in comparison with controls. However, l-arginine doses of 100, 200 and 500 mg/kg body weight showed a significant increase in time taken to attain a fall of Trec to 23°C. The time taken by the rats to recover Trec 37°C from C-H-R-induced hypothermia was reduced significantly at all the doses of l-arginine used, except the 500 mg/kg body weight dose in comparison with control rats (Fig. 1). However, the oral administration of l-arginine at a dose of 100 mg/kg body weight was observed to possess an optimum adaptogenic activity as evidenced by a significant increase (53.5%) in the time taken to attain a fall of Trec to 23°C and significantly faster (39.6%) recovery (Trec 37°C) from C-H-R exposure-induced hypothermia, in comparison with control rats (Fig. 1).
Figure 1.
Dose-dependent study of l-arginine in rats on time (in minutes) taken to attain Trec 23°C during C-H-R exposure and recovery of rectal temperature to 37°C. ▪ = Trec 20°C □ = Trec 37°C. *Significant in comparison with their respective control values at P < 0.05.
Plasma MDA and Anti-oxidants
The results regarding the oxidative stress, i.e. TBA-reactive MDA levels and some of the circulating anti-oxidants (SOD, CAT and GSH), of rats exposed to C-H-R stress with and without prior intake of a single oral dose of l-arginine of 100 mg/kg body weight (least effective dose) are shown in Table 1. Plasma MDA showed a 19% increase in control rats on attaining Trec 23°C during C-H-R exposure, but supplementation of l-arginine resulted in MDA levels comparable with control values. In control rats exposed to C-H-R, blood SOD levels showed a non-significant decrease on attaining Trec 23°C, but increased significantly on recovery (Trec 37°C). l-Arginine administration resulted in increased SOD levels in rats on attaining Trec 23°C, in comparison with the respective unexposed rats. The blood CAT activity in control rats exposed to C-H-R decreased on attaining Trec 23°C and on recovery (Trec 37°C), in comparison with unexposed rats. However, in rats administered l-arginine, blood CAT activity was comparable with the respective unexposed animals, both on attaining Trec 23°C and on recovery (Trec 37°C). No significant change was observed in the GSH levels of control rats on attaining Trec 23°C during C-H-R exposure and on recovery of Trec 37°C. The l-arginine administration had no significant effect on blood GSH levels.
Table 1.
l-Arginine (100 mg/kg) supplementation and plasma MDA and blood SOD, GSH and CAT levels
| n | Control | l-Arginine-treated | |||||
|---|---|---|---|---|---|---|---|
| Unexposed | Exposed | Unexposed | Exposed | ||||
| Trec 23°C | Trec 37°C | Trec 23°C | Trec 37°C | ||||
| MDA (μmol/l) | 6 | 0.95 ± 0.1 | 1.13 ± 0.1* | 1.02 ± 0.06 | 0.93 ± 0.04 | 0.76 ± 0.08* | 0.73 ± 0.21 |
| SOD (U/mg protein) | 6 | 4.72 ± 0.44 | 4.27 ± 0.49 | 6.4 ± 0.45* | 4.88 ± 0.27 | 6.46 ± 0.32* | 5.72 ± 0.12* |
| CAT (U/mg protein) | 6 | 15.4 ± 0.9 | 11.2 ± 1.3* | 9.42 ± 0.8* | 12.4 ± 0.8 | 11.8 ± 0.9 | 11.4 ± 0.8 |
| GSH (mg %) | 6 | 21.67 ± 2.1 | 20.32 ± 1.9 | 21.46 ± 1.6 | 20.53 ± 1.4 | 24.13 ± 2.3 | 19.76 ± 1.2 |
*Significant in comparison with their respective unexposed groups at P < 0.05.
Blood LDH and Plasma NO and Testosterone
The results of the blood LDH and plasma NO and testosterone levels are shown in Table 2. In control rats, blood LDH levels increased significantly (58%) on attaining Trec 23°C during C-H-R exposure and on recovery (105%) of Trec 37°C, in comparison with unexposed control rats. In rats administered l-arginine, the increase in blood LDH levels was less compared with controls (45.3 versus 58.5%, and 21.5 versus 105.2%) both on attaining Trec 23°C and on recovery of Trec 37°C (Table 2). In control and l-arginine-treated rats, there was no change in the plasma NO levels on attaining Trec 23°C and recovery of Trec 37°C in comparison with the respective unexposed rats (Table 3). Plasma testosterone levels decreased significantly in both control and l-arginine-treated rats on attaining Trec 23°C and on recovery of Trec 37°C, in comparison with unexposed rats. The decrease was comparatively less in l-arginine-treated rats (Table 2).
Table 2.
l-Arginine supplementation and circulating level of blood LDH, plasma NO and testosterone levels
| n | Control | l-Arginine-treated | |||||
|---|---|---|---|---|---|---|---|
| Unexposed | Exposed | Unexposed | Exposed | ||||
| Trec 23°C | Trec 37°C | Trec 23°C | Trec 37°C | ||||
| LDH (nmol/mg protein) | 6 | 24.8 ± 3.7 | 39.3 ± 4.7* | 50.9 ± 4.9* | 32.5 ± 6.7 | 47.3 ± 3.9* | 39.5 ± 4.8 |
| NO (μM) | 6 | 3.47 ± 0.7 | 2.92 ± 0.5 | 2.74 ± 0.4 | 3.57 ± 0.5 | 3.82 ± 0.6 | 2.90 ± 0.5 |
| Testosterone (ng/ml) | 6 | 2.62 ± 0.80 | 0.43 ± 0.02* | 0.23 ± 0.01* | 2.58 ± 0.70 | 0.53 ± 0.02* | 0.42 ± 0.02* |
*Significant in comparison with their respective unexposed groups at P < 0.05.
Discussion
Dose-dependent studies were performed using a passive C-H-R animal model. In the C-H-R animal model, the time taken to attain Trec 23°C indicates exhaustion of the animal's energy resources or resistance to stress, and the time taken to recover Trec 37°C from C-H-R exposure-induced hypothermia (Trec 23°C), at normal atmospheric pressure and 32°C ambient temperature, indicates how fast the post-stress recovery of the animal is. The results of the dose-dependent study showed that for the two orally administered l-arginine doses (100 and 200 mg/kg body weight), the time taken to attain Trec 23°C and recovery (Trec 37°C) from C-H-R exposure-induced hypothermia was almost comparable. With the 100 mg dose, the stress resistance effect (i.e. the time taken to attain Trec 23°C) was slightly better than for the 200 mg/kg dose, while with the 200 mg/kg dose, the post-stress recovery effect was slightly better than with the 100 mg/kg body weight dose. This suggested that both the doses, i.e. 100 and 200 mg/kg, were effective. Hence out of these two doses of l-arginine, the lowest one, i.e. 100 mg/kg, was selected as the least effective anti-stress and adaptogenic dose. The l-arginine dose of 100 mg/kg body weight was used further to study the effect of l-arginine administration on some of the biochemical indices related to oxidative stress, NO levels and cell membrane permeability.
Hypoxia, cold and immobilization stressors present in the C-H-R animal model used are known to produce oxidative stress (2–4) and skeletal muscle fatigue (26). Free radicals cause oxidation of lipids, proteins and bases of nucleic acids. Free radicals also cause damage to biomembranes, reflected by lipid peroxidation, thereby compromising cell integrity and function. In the present study, plasma MDA levels also increased in control rats on attaining Trec 23°C during C-H-R exposure along with decreased blood CAT, suggesting oxidative stress. However, GSH and SOD levels showed no change on attaining Trec 23°C during C-H-R exposure; perhaps the stress was not sufficiently severe to cause changes in these parameters. On recovery of Trec 37°C from C-H-R exposure-induced hypothermia, the CAT values remained low but SOD showed an increase, which was reflected in normalized plasma MDA levels. The l-arginine (100 mg/kg body weight) administration resulted in decreased MDA levels, lower than in unexposed animals, both on attaining Trec 23°C during C-H-R exposure and on recovery of Trec 37°C. The observed decrease in oxidative stress was due to the better maintained CAT activity and increased SOD levels on attaining Trec 23°C and on recovery of Trec 37°C. This suggested an anti-oxidant effect of l-arginine administration. Lubec et al. (27) also reported similar findings of reduced lipid peroxidation product MDA in diabetic patients treated with l-arginine.
Exposure to stressful conditions increased cell membrane permeability, indicating cellular membrane damage. Herbert (28) observed altered cell membrane permeability as evidenced by a significant increase in circulating creatine phosphokinase (CPK) levels in rats restrained at 4°C for 2 h, and this increase correlated with the changes in rectal temperature. In the present study, blood LDH levels in control rats also increased, by 58 and 105% on attaining Trec 23°C and on recovery of Trec 37°C, respectively, in comparison with unexposed rats. In the rats treated with l-arginine (100 mg/kg body weight), the increase in LDH levels both on attaining Trec 23°C and on recovery of Trec 37°C were less than in control rats. This suggested that l-arginine supplementation was acting at a cellular level in maintaining the energy-dependent process of membrane permeability.
NO, a potent vasodilator, has also been shown to be involved in the enhancement of the thermogenic function of brown adipose tissue in rats (15). In the present study, no significant change was observed in blood NO levels in l-arginine-treated rats that were exposed to C-H-R stress.
Increased circulating testosterone levels have been reported in the organisms that are able to adapt successfully in stressful situations, while decreased testosterone levels are indicative of an organism's loss of control or a defeat reaction during stress (29). In the present study, a significant decrease in testosterone levels was also observed in control rats exposed to Trec 23°C, which decreased further on recovery to Trec 37°C. l-Arginine supplementation showed no significant effect on testosterone levels on Trec 23°C during C-H-R exposure and recovery to Trec 37°C in comparison with control rats.
The result of the present study suggested that in rats the least effective anti-stress and adaptogenic dose of l-arginine supplementation was 100 mg/kg body weight. The observed anti-stress activity of l-arginine was due to its anti-oxidative effects, i.e. better maintained anti-oxidants (SOD and catalase), reduced lipid peroxidation (MDA) and a lower increase in blood LDH levels.
References
- 1.Selye H. The Stress of Life. New York: McGraw Hill Books Co.; 1950. [Google Scholar]
- 2.Bhaumik G, Srivastava KK, Selvamurthy W, Purkayastha SS. The role of free radicals in cold injuries. Int J Biometeorol. 1995;38:171–75. doi: 10.1007/BF01245384. [DOI] [PubMed] [Google Scholar]
- 3.Kondo H, Miura M, Itokawa Y. Oxidative stress in skeletal muscle atrophied by immobilization. Acta Physiol Scand. 1991;142:527–8. doi: 10.1111/j.1748-1716.1991.tb09191.x. [DOI] [PubMed] [Google Scholar]
- 4.Simon SI. Oxidative stress at high altitude and effects of vitamin E. In: Marriot BM, Carlson SJ, editors. Nutritional Needs in Cold and in High Altitude Environments. Washington, DC: National Academy Press; 1996. pp. 393–418. [Google Scholar]
- 5.Brekhman II, Dardymov IV. New substances of plant origin which increase non-specific resistance. Annu Rev Pharmacol. 1969;9:419–30. doi: 10.1146/annurev.pa.09.040169.002223. [DOI] [PubMed] [Google Scholar]
- 6.Askew EW. Environmental and physical stress and nutrient requirement. Am J Clin Nutr. 1995;61(Suppl):631S–7S. doi: 10.1093/ajcn/61.3.631S. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Grover SK, Divekar HM, Gupta AK, Shyam R, Srivastava KK. Enhanced thermogenesis in rats by Panax ginseng, multivitamins and minerals. Int J Biometeorol. 1996;39:187–91. doi: 10.1007/BF01221390. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Grover SK, Shyam R, Divekar HM, Gupta AK, Srivastava KK. Enhanced thermogenesis in rats by a composite Indian herbal preparation I—its mechanism of action. J. Altern Complement Med. 1999;5:245–51. doi: 10.1089/acm.1999.5.245. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Shyam R, Divekar HM, Pahwa ML, Srivastava KK. Mechanism of increased tolerance to hypothermia after composite Indian herbal preparation II—administration. J. Altern Complement Med. 2000;6:509–17. doi: 10.1089/acm.2000.6.509. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Divekar HM, Gupta V, Srivastava KK. Antistress and adaptogenic activity of lecithin supplementation. J Altern Complement Med. 2002;8:487–92. doi: 10.1089/107555302760253685. [DOI] [PubMed] [Google Scholar]
- 11.Ushakov AS, Myasinikov VI, Shestkov BP, Agureev AN, Belakovsky MS, Rumyantseva MP. Effect of vitamin and amino acid supplementation on human performance and physical work. Aviat Space Environ Med. 1978;49:1185–7. [PubMed] [Google Scholar]
- 12.Liugi DI. Acute amino acid supplementation enhances pituitary responsiveness in athletes. Med Sci Sports Exerc. 1999;31:1748–54. doi: 10.1097/00005768-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Visek WJ. An update of concepts of essential amino acids. Annu Rev Nutr. 1984;4:137–55. doi: 10.1146/annurev.nu.04.070184.001033. [DOI] [PubMed] [Google Scholar]
- 14.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide accounts for the biological activity of EDRF. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 15.Saha SK, Kuroshima A. Nitric oxide and thermogenic function of brown adipose tissue in rats. Jpn J Physiol. 2000;50:337–342. doi: 10.2170/jjphysiol.50.337. [DOI] [PubMed] [Google Scholar]
- 16.Kuniyoshi K. The Efficacy and Proper Usage of Miracle Korean Ginseng. Republic of Korea: Korea Ginseng Research Institute; 1980. pp. 93–6. [Google Scholar]
- 17.Milakofsky L, Harris N, Vogel WH. Effects of repeated stress on plasma arginine levels in young and old rats. Physiol Behav. 1993;54:725–8. doi: 10.1016/0031-9384(93)90083-r. [DOI] [PubMed] [Google Scholar]
- 18.Yu YM, Ryan CM, Burke JF, Tompkins RG, Young VR. Relations among arginine, citrulline, ornithine and leucine kinetics in adult burn patients. Am J Clin Nutr. 1995;62:960–8. doi: 10.1093/ajcn/62.5.960. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran U, Divekar HM, Grover SK, Srivastava KK. New experimental model for the evaluation of adaptogenic products. J Ethnopharmacol. 1990;29:275–81. doi: 10.1016/0378-8741(90)90038-u. [DOI] [PubMed] [Google Scholar]
- 20.Dousset J, Trouilh M, Foglietti M. Plasma malonaldehyde levels during myocardial infarction. Clinica Chimica Acta. 1983;129:319–22. doi: 10.1016/0009-8981(83)90035-9. [DOI] [PubMed] [Google Scholar]
- 21.Kornberg A. Lactate dehydrogenase of muscle. Methods Enzymol. 1995:441–443. [Google Scholar]
- 22.Marklund A, Marklund G. Involvement of the superoxide anion radical in the auto-oxidation of pyrogallol and convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 23.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1984. pp. 673–84. [Google Scholar]
- 24.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 25.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 26.Barclay JK, Hansel M. Free radicals may contribute to oxidative skeletal muscle fatigue. Can J Physiol Pharmacol. 1991;69:279–284. doi: 10.1139/y91-043. [DOI] [PubMed] [Google Scholar]
- 27.Lubec B, Hayn M, Kitzmuller E, Vierhapper H, Lubeg G. l-Arginine reduces lipid peroxidation in patients with diabetes mellitus. Free Rad Biol Med. 1997;22:355–357. doi: 10.1016/s0891-5849(96)00386-3. [DOI] [PubMed] [Google Scholar]
- 28.Herbert YM. Plasma creatine phosphokinase activity increase and temperature changes in the rat. Fed Proc. 1970;29:747. (abstract) [Google Scholar]
- 29.Henry JP. Biological basis of the stress response. News Physiol Sci. 1993;8:69–73. doi: 10.1007/BF02691093. [DOI] [PubMed] [Google Scholar]