Abstract
Propolis, a honeybee product, has gained popularity as a food and alternative medicine. Its constituents have been shown to exert pharmacological effects, such as anti-microbial, anti-inflammatory and anticancer. Shoot apices of Baccharis dracunculifolia (alecrim plant, Asteraceae) have been pointed out as sources of resin for green propolis. The present work aimed (i) to observe the collecting behavior of bees, (ii) to test the efficacy of histological analysis in studies of propolis botanical origin and (iii) to compare the chemistries of alecrim apices, resin masses and green propolis. Bee behavior was observed, and resin and propolis were microscopically analyzed by inclusion in methacrylate. Ethanol extracts of shoot apices, resin and propolis were analyzed by gas chromatography/mass spectroscopy. Bees cut small fragments from alecrim apices, manipulate and place the resulting mass in the corbiculae. Fragments were detected in propolis and identified as alecrim vestiges by detection of alecrim structures. Prenylated and non-prenylated phenylpropanoids, terpenoids and compounds from other classes were identified. Compounds so far unreported for propolis were identified, including anthracene derivatives. Some compounds were found in propolis and resin mass, but not in shoot apices. Differences were detected between male and female apices and, among apices, resin and propolis. Alecrim apices are resin sources for green propolis. Chemical composition of alecrim apices seems to vary independently of season and phenology. Probably, green propolis composition is more complex and unpredictable than previously assumed.
Keywords: africanized Apis mellifera, anthracene derivatives, Baccharis dracunculifolia, dehydrocostus lactone, prenylated phenylpropanoids
Introduction
Propolis is a chemically complex resinous bee product, containing material collected from buds or exudates of plants (resin), volatile substances and beeswax (1–3). Propolis has gained popularity as an alternative medicine or food for health amelioration and disease prevention in various parts of the world, including the United States of America, the European Union and Japan.
Elsewhere in this volume (4) information is given about uses and properties of propolis and an account about difficulties dealing with aspects of origin and variation in chemical composition of green propolis, namely Brazilian propolis derived mainly or exclusively from alecrim plants (Baccharis dracunculifolia).
The present study aims to raise new data about collection of resin by bees visiting apices of alecrim plants and to verify the anatomical characteristics of alecrim vestiges in resin and propolis, as a means to search propolis botanical origin. It aims also to compare (i) the chemical composition of alecrim apices, (ii) freshly collected resin mass and (iii) propolis of nearby hives, with the purpose of evaluating the degree of chemical congruence among the three materials.
Materials and Methods
Plant Material
Shoot apices of Baccharis dracuncufolia DC (alecrim) were collected in the rainy season (January), from shrubs in Paula Cândido municipality (state of Minas Gerais, southeast Brazil, S20°49′, W42°54′) in a period of high availability of plant material. Fragments of mountainous semi-deciduous seasonal forest characterize the local predominant vegetation, with areas altered by anthropic influence at their borders, which undergo a process of early succession. Plants were aluminum tagged as female and male during the previous flowering season. Apices were collected separately from female and male individuals. Voucher specimens are deposited in the Herbarium of the Viçosa Federal University (UFV).
Bee Behavior and Collection of Material for Histology and Chemical Analysis
Observations of bee behavior were visually monitored, recorded with a Panasonic NV/M30 video camera and photographed with a digital Mavica/MVC FD88 and Leica MZ6 stereomicroscope. The behavior of the insects was registered from arrival at the plant and along work on the apex, to flight back to the hive. The process of resin collection and time required was also registered. Along the observations, samples of plant material collected by the bees were fixed in FAA50 for histological analysis. Soon after introducing the masses of resin into the corbiculae, some bees were caught with a pincer and placed in a vial containing FAA50 (formaldehyde, alcohol, 50% acetic acid) for histological analysis of the resin mass. From some bees, resin masses were taken out of the corbiculae and introduced in Eppendorf tubes for chemical analysis.
Propolis was collected for analysis from hives growing in the same site. The wooden box housing the hives had slits 3 cm wide on both lateral sides, to stimulate propolis production (4). Only propolis recently produced was collected for the present study. A sample pooled from five hives was used for chemical analysis. Masses of propolis were also used for histological study. A sample of the propolis used in this investigation is maintained for reference in a freezer at Viçosa Federal University.
The whole process of collection of shoot apices, resin masses and propolis was carried out over a 7-day period.
Histology
Slides were prepared for histological analysis from plants, resin masses and propolis. Resin masses and propolis were included in methacrylate, according to an unpublished procedure (patent PI 0306421-2, Brazil). The methacrylate inclusion was sliced with a Leica RM2155 rotatory microtome with automatic advance and glass razor. Serial slices 12 μm thick were placed on histological slides and stained with toluidine pH 4.0 for 18 min at room temperature. The dried slides were mounted with Permount. Anatomical observations were made with an Olympus AX-70 photomicroscope, equipped with U-Photo system, filming camera and video. The software Image Pro Plus was used in the process of collecting images. Anatomical characteristics of fragments detected in the slides were compared with anatomical structures of alecrim histological preparations from a reference slide collection made from material of the same area.
Extraction, Purification and Isolation of Compounds
Propolis sample (5 g), 10 male apices (0.0138 g dry weight), 10 female apices (0.0119 dry weight) and 18 resin masses (0.0332 g dry weight) were treated with hexane for 3 h in Soxhlet, the extract having not been used in this investigation. A second extraction in Soxhlet followed, with methanol, for 3 h. Waxes from the propolis extract were eliminated by three consecutive steps of cooling in freezer, filtration, concentration of the filtrate and dissolution of the residue in 5 ml of methanol. The methanol extracts of apices and resin masses were concentrated under reduced pressure and transferred to glass vials with a small volume of methanol. The solvent was evaporated and the residue weighed. The residues were dissolved in ethyl ether at concentrations of 1000 p.p.m. (resin mass and propolis) and 2000 p.p.m. (apices). The final ether solutions were kept in freezer.
Gas Chromatography (GC)/Electron Ionization Mass Spectrometry (EIMS) and Identification of Compounds
Part of the wax-free methanol extracts was treated with diazomethane for methylation of carboxylic acids. Ether solutions (1 μl) of diazomethane treated and non treated extracts were injected into a Shimadzu GCMS-QP5050A 17A ChemStation System Mass Spectrometer operating with the EI mode at 70 eV, equipped with an auto injector AOC-5000 and mass selective detector. A DBS fused silica capillary column (30 m × 0.25 mm internal diameter, 0.25 μm film thickness), helium as carrier gas with flux 1.5 ml/min and splitless mode were used. Oven temperatures ranged from 100 to 310°C at 10°C/min, followed by an isothermal period of 30 min. The mass limit was 40–500 m/z. Injector and detector temperature was 300°C.
Identification of the substances followed computer searches over library Wiley 229L, and solutions of some reference compounds were injected in order to assist in the identification. Only known compounds are here reported.
Results
Bee Behavior
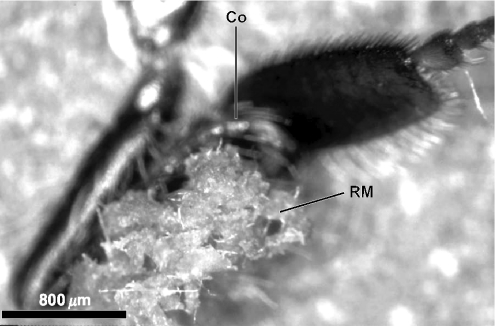
Numerous honeybees were seen on alecrim plants fragmenting vegetative apices (bud, leaf primordia and young leaves). The fragments in the mandibles have sticky aspect from the beginning of the collecting process, indicating liberation of resinous substances from trichomes and ducts. The bees manipulate the fragments, a mass of resinous material being the final product. Using the first pair of legs, the bees move the resin mass to the median legs and then to the opposite corbicula (Fig. 1). The resin masses may be transferred to the corbicula of the same side. Very rarely the material is transferred directly from the first pair of legs to the corbiculae.
Figure 1.
Resin mass adhered to corbicula of an Africanized Apis mellifera. RM, resin mass; Co, corbicula.
The time spent from the beginning of the collecting process to the deposit of the resin mass in the corbiculae was, on average, 7 min. The frequency of visits varied, depending on several factors, among which was the hive's demand for propolis.
Histology of Resin Masses and Propolis
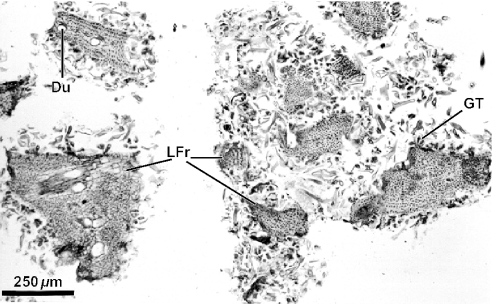
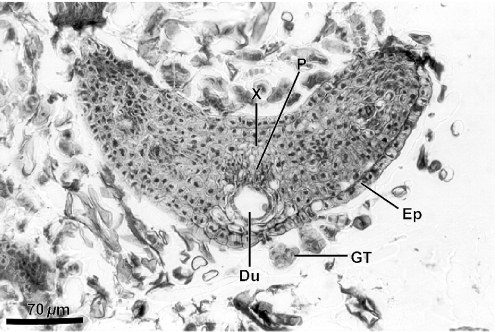
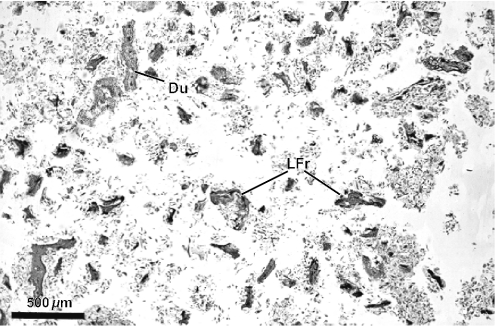
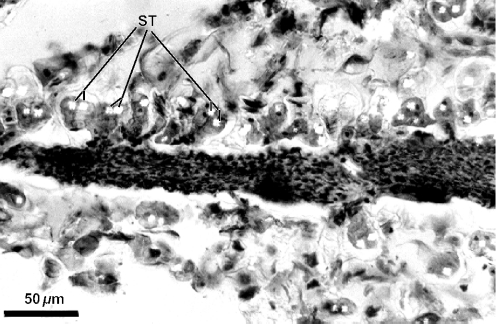
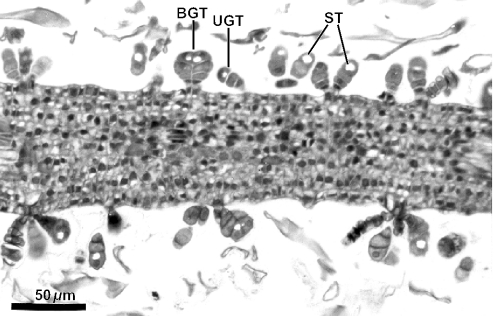
Figure 2 evidences the high degree of fragmentation of the material in the resin mass. Some details are relevant as diagnostic of the resin plant source. Resiniferous ducts are conspicuous in histological sections of leaves of Baccharis. Several ducts are apparent in Fig. 2, which shows a fragment of a young alecrim leaf from a resin mass. Another leaf fragment from a resin mass with a resiniferous duct in cross section is shown in Fig. 3; glandular and non-glandular trichomes are also visible. In Fig. 4, several leaf fragments in the residue of the propolis sample are seen, most with conspicuous ducts. Glandular and non-glandular trichomes of alecrim leaves were commonly seen. Viewed with polarized light, alecrim glandular trichomes show brilliant contents, which correspond to starch grains (Fig. 5) and were detected in histological preparations of green propolis (Fig. 6). Figures 2–5 evidence that the fragments correspond to young plant leaves, because the chlorenchyma in all cases is still undifferentiated, no palisade and spongy parenchyma being apparent.
Figure 2.
View of histological methacrylate preparation of resin mass collected from a corbicula of Africanized Apis mellifera on shoot apex of Baccharis dracunculifolia, showing leaf fragments in cross section. Du, resiniferous duct; GT, glandular trichome; LFr, leaf fragments.
Figure 3.
Leaf fragment in cross section in histological methacrylate preparation of resin mass collected from a corbicula of Africanized Apis mellifera on shoot apex of Baccharis dracunculifolia. Du, resiniferous duct; Ep, epiderm; GT, glandular trichome; P, phloem; X, xylem.
Figure 4.
View of histological methacrylate preparation of a green propolis sample, showing leaf fragments of Baccharis dracunculifolia. Du, resiniferous duct; LFr, leaf fragments.
Figure 5.
Cross section of leaf fragment of Baccharis dracunculifolia in methacrylate histological preparation of a green propolis sample viewed with polarized light, showing glandular trichomes with brilliant starch inclusions. St, starch inclusion.
Figure 6.
Cross section of a young leaf from shoot apex of Baccharis dracunculifolia in a histological methacrylate preparation, showing glandular trichomes with brilliant starch inclusions. BGT, biseriate glandular trichome; St, starch inclusion; UGT, uniseriate glandular trichome.
Slides prepared from the propolis samples showed no fragments of plants other than alecrim. Hence the strategy of choosing a time of intense vegetative alecrim growth, the use of hive boxes with slits to promote propolis production and the restriction of such production to a short time was successful to attain a product from a single plant source.
Chemical Analyses
Tables 1–4 list the substances identified in shoot apices of female and male alecrim plants, in the resin masses and in the pooled propolis sample. Table 1 refers to phenolic compounds, Table 2 to terpenoids (sesqui, di and pentacyclic triterpenoids), Table 3 to waxy substances, and Table 4 to compounds from other classes. A total of 64 substances were identified, but other compounds (probably unknown) were also detected. Prenylated (compounds 10–17) and non-prenylated (compounds 1–9) cinnamic acid derivatives were often detected.
Table 1.
Phenolic compounds detected in shoot apices of Baccharis dracunculifolia DC. (Asteraceae), resin masses from bee corbiculae and a sample of green propolis
| Compounds | Apices | Resin | Propolis | Pharmacological activities | Ref. | |
|---|---|---|---|---|---|---|
| Female | Male | |||||
| Cinnamic acid derivatives | ||||||
| 1. Hydrocinnamic acid | + | + | + | + | ||
| 2. p-Hydroxyhydrocinnamic acid | − | − | − | + | ||
| 3. p-Hydroxycinnamic acid | + | + | + | + | Anti-inflammatory | 22 |
| 4. p-Methoxycinnamic acid | + | + | + | + | ||
| 5. cis-3-Methoxy-4-hydroxy-cinnamic acid | − | + | − | + | ||
| 6. cis-3,4-Dimethoxycinnamic acid | + | + | − | − | ||
| 7. trans-3-methoxy-4-hydroxy-cinnamic acid | + | + | + | + | Antimicrobial | 1 |
| Anti-inflammatory | 22 | |||||
| 8. trans-3,4-Dimethoxycinnamic acid | + | + | + | + | Anti-inflammatory | 22 |
| 9. p-Coumaric acid | − | − | + | + | ||
| Prenylated cinnamic acid derivatives | ||||||
| 10. Allyl-3-prenylcinnamate | + | + | + | + | ||
| 11. 4-Hydroxy-3-prenylcinnamic acid | − | − | + | + | Hepatoprotection | 14 |
| 12. 4-Hydroxy-3,5-diprenylcinnamic acid (artepillin C) | − | − | + | + | Antitrypanocidal | 23 |
| Cytotoxicity | 16 | |||||
| Chromane derivatives | ||||||
| 13. 2,2-Dimethylchromene-6-propenoic acid | + | + | + | + | Antioxidant | 24 |
| 14. 2,2-Dimethyl-8-prenylchromene-6-propenoic acid | + | + | + | + | Hepatoprotection | 23 |
| Cytotoxicity | 25 | |||||
| 15. 8-(methyl-butanechromane)-6-propenoic acid | − | − | − | + | ||
| 16. 3-Hydroxy-2,2-dimethyl-8-prenylchromane-6-propenoic acid | − | − | + | + | ||
| Naphtalene and anthracene derivatives | ||||||
| 17. 2-t-Butylnaphto-[2,3-b]-furan-4,9-dione | − | − | + | + | ||
| 18. 2-Hydroxy-7,12-dimethyl-benzanthracene (A) | + | + | + | + | ||
| 19. 1-Hydroxy-2-(1-methoxyethyl)-3-methoxyanthraquinone (B) | − | − | + | + | ||
| Simple benzene and phenol derivatives | ||||||
| 20. Benzene-3,3-dimethyl-4-pentenyl | + | + | − | − | ||
| 21. p-Vinylphenol | − | − | − | + | ||
| 22. p-Vinyl-o-prenylphenol | + | + | + | + | ||
Compound names in bold refer to occurrences so far unreported in propolis; letters in parenthesis correspond to structures in Fig. 7.
−, Not detected.
Table 4.
Compounds representative of classes other than phenolics, terpenoids and waxes, detected in shoot apices of Baccharis dracunculifolia DC. (Asteraceae), resin masses from bee corbiculae and a sample of green propolis
| Compound | Apices | Resin | Propolis | |
|---|---|---|---|---|
| Female | Male | |||
| 60. Quinic acid | + | + | − | − |
| 61. 1,7,7-Trimethyl-3-phenyl-2-oxabicyclo-(4.4.0)-deca-3,5-diene (F) | − | − | − | + |
| 62. Allyl-n-butanoate | − | + | + | − |
| 63. Vinyl-butyrate | − | + | + | − |
| 64. 4,8-Dimethyl-5-hydrindacene (G) | + | + | + | + |
No pharmacological properties have as yet been assigned to these compounds Compound names in bold refer to occurrences so far unreported in propolis; letters in parenthesis correspond to structures in Fig. 7.
−, Not detected.
Table 2.
Terpenoids detected in shoot apices of Baccharis dracunculifolia DC. (Asteraceae), resin masses from bee corbiculae and a sample of green propolis
| Compound | Apices | Resin | Propolis | Pharmacological activity | Ref. | |
|---|---|---|---|---|---|---|
| Female | Male | |||||
| Sesquiterpenes | ||||||
| 23. Farnesol | − | − | − | + | Antimicrobial | 26 |
| 24. Dehydrocostus lactone (C) | − | − | + | + | Trypanocidal | 15 |
| Antimycobacterial | 27 | |||||
| 25. Viridiflorol | − | − | + | − | ||
| 26. Isomaturnin (D) | − | − | + | + | ||
| 27. 1H-Cyclopropazulene-1a,2,3,4,4a,5,-6,7b-octahydro-1,1,4,7-tetramethyl | + | + | − | − | ||
| Diterpenes | ||||||
| 28. Isocupressic acid derivative | − | − | + | + | Antibacterial | 17 |
| Cytotoxicity | 28 | |||||
| 29. 1,4aβ-Dimethyl-7-isopropyl-2,3,4,-4a,9,10-hexahydrophenanthrene (E) | − | − | + | + | ||
| 30. Tα-Hydroxysandaracopimar-8(14)-15-diene | − | − | + | − | ||
| Triterpenes and steroids | ||||||
| 31. Squalene | + | + | − | − | ||
| 32. Bauer-7-en-3β-yl acetate | − | − | − | + | ||
| 33. Stigmasta-3,5-dien-7-one | + | + | − | − | ||
| 34. Cholest-5-en-3β-ol | + | + | + | − | ||
| 35. Clionasterol | + | + | + | − | ||
Compound names in bold refer to occurrences so far unreported in propolis; letters in parenthesis correspond to structures in Fig. 7.
−, Not detected.
Table 3.
Wax constituents detected in shoot apices of Baccharis dracunculifolia DC. (Asteraceae), resin masses from bee corbiculae and a sample of green propolis
| Compound | Apices | Resin | Propolis | |
|---|---|---|---|---|
| Female | Male | |||
| Wax constituents | ||||
| 36. Methyl-dodecanoate | + | + | − | − |
| 37. Methyl-tetradecanoate | − | + | − | − |
| 38. Hexadecanoic acid | + | + | − | − |
| 39. Octadecanoic acid | − | + | + | − |
| 40. Hexacosanoic acid | − | + | − | − |
| 41. Docosanoic acid | + | + | − | − |
| 42. Tetracosanoic acid | − | + | − | − |
| 43. Octacosanoic acid | − | + | − | − |
| 44. Hexacosanoic acid | − | + | − | − |
| 45. Methyl-4-methoxyoctadecanoic acid | + | + | − | − |
| 46. Hexanedioic acid dioctyl ester | + | + | + | + |
| 47. n-Dodecanal | + | + | − | − |
| 48. n-Hexadecanal | + | + | − | − |
| 49. 9,12-Octadecadienal | + | + | − | − |
| 50. (Z,Z)-9,12-octadecadienoic acid | − | − | − | + |
| 51. Methyl-(Z)-9-Octadecenoate | − | − | − | + |
| 52. n-Docosanol | + | + | − | − |
| 53. n-Tetracosanol | + | + | − | − |
| 54. n-Hexacosanol | + | + | − | − |
| 55. 4-Ethyloctane | − | − | − | + |
| 56. 7-n-Hexyleicosane | + | + | − | − |
| 57. n-Hentriacontane | + | + | − | − |
| 58. 17-Tritriacontene | + | + | − | − |
| 59. 17-Pentatriacontene | − | − | − | + |
No pharmacological properties have as yet been assigned to these compounds Compound names in bold refer to occurrences so far unreported in propolis.
−, Not detected.
Among the identified substances, 42 were detected in male and 33 in female apices, 29 in the resin masses and 34 in the propolis sample. No exact match is observed in the distribution of compounds among the four resin sources. A higher score of coincidences is observed comparing resin masses and propolis (23 matches), most substances corresponding to phenolics, some of them common in propolis, such as derivatives of cinnamic acids. There are 17 matches comparing resin masses either with male, female or both apices, 13 comparing propolis/apices and 12 comparing all three sources.
Discussion
Our results support previous observations that Africanized bees in Brazil have a preference for alecrim plants as sources of resin propolis. The observed collecting behavior is similar to literature reports regarding visits of bees to apices of Populus (5,6) and B.dracunculifolia. Park et al. (7) observed that Africanized honeybees collect alecrim leaf-buds and unexpanded leaves, but rarely adult leaves, which is in agreement with our observations.
Oliveira and Bastos (8) found glandular and non-glandular trichomes, but no foliar fragments with resiniferous ducts in the residue of green propolis, concluding that bees collect resin stemming from surface trichomes but not from internal ducts. The present investigation gives no support to this conclusion. In fact, the bees destroy shoot apices of alecrim plants. The ramification of alecrim plants after a process of intense resin collecting activity is very common and results from breakage of the apical dominance due to destruction of vegetative buds. In previous observations, we verified a significant preference for female (55.9%) over male (44.1%) plants (unpublished results). Preferences for plants independently of sex were also noted in the present study. Integer and robust apices were often rejected for similar apices of other plants. The bees were seen to probe the apices with their antenna for a few seconds and then move to another plant. It is known that bee antennas have high olfactory capacity and that Baccharis plants produce volatile oils (9). Liberation of such substances probably triggers bee attraction.
Observation of bees in the field is not easy, because of the small percentage of individuals collecting propolis, although bee visits may become numerous, as in the present investigation. In tropical regions, observing bees is even more difficult, because of the greater plant diversity.
A procedure for assigning propolis botanical origin is the anatomical study to detect fragments of plants for comparison with reference to histological slides of likely plant sources (10,11). Identification of fragments of plant material is a powerful means for assignment of propolis origin. Baccharis is a huge (more than 500 species) and cosmopolite genus distributed in Oceania, South, Central and North America. Dense populations of Baccharis species are often found in field vegetation in Brazil. Volatile substances, either from resiniferous ducts or glandular trichomes, are probably effective at attracting bees for resin collection.
Absence of differentiated chlorenchyma is coherent with behavioral observations of this work and that of Park et al. (7). Absence of plant vestiges in propolis samples is sometimes verified. This fact can be indicative that, in such cases, the resin source corresponds to exudates, not to plant parts.
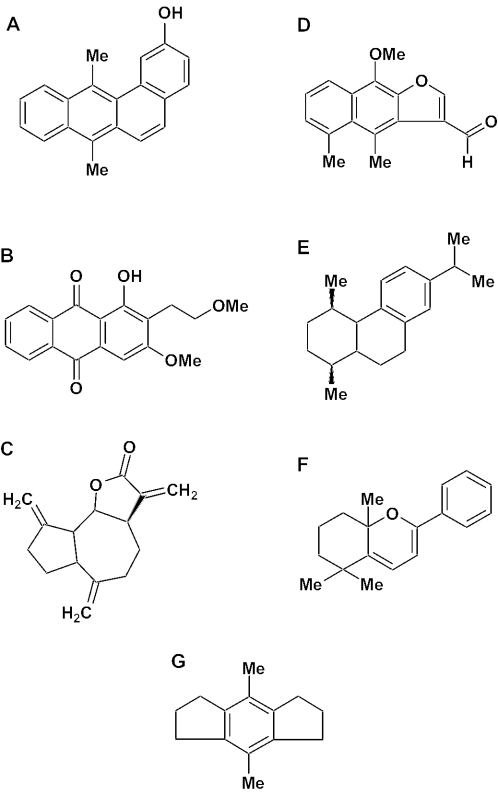
Prenylated phenylpropanoids are characteristic of B.dracunculifolia and green propolis (4). A prenylated phenylpropanoid recently reported as a novel compound, allyl-3-prenylcinnamate (12), was found again as a relevant constituent, not only in propolis but also in alecrim apices (Table 1, compound 10). Dehydrocostus lactone (Table 2, compound 24; Fig. 7C), a sesquiterpene reported from an unusual sample of propolis (13), but never in a typical green propolis, was detected in the propolis sample and resin mass, although not in the shoot apices. This guaianolide lactone has been shown to exert several activities, among them antimycobacterial (14) and against Trypanosoma cruzi (15), an important cardiac parasite in rural areas of Brazil. Artepillin C (4-hydroxy-3,5-diprenyl cinnamic acid), a compound from Brazilian propolis with anti-tumor activity (16), was not found in apices and resin mass, but was detected in propolis (Table 1, compound 12). Other phenolics from Brazilian propolis possess not only cytotoxic but also hepatoprotective activity (14). Labdane diterpenes identical or similar to compounds 28–30 (Table 2; Fig. 7E) were reported from Brazilian propolis (17) and B.dracunculifolia (18). In addition, alecrim apices are probably also sources of other classes of terpenoids (Table 2), such as sesquiterpenes (farnesol, compound 23; dehydrocostus lactone, compound 24, Fig. 7C; viridiflorol, compound 25; isomaturnin, compound 26, Fig. 7D; 1H-cyclopropazulene-1a,2,3,4,4a,5,6,7b-octahydro-1,1,4,7-tetramethyl, compound 27), triterpenes (squalene, compound 31; bauer-7-en-3β-yl acetate, compound 32) and steroids (stigmasta-3,5-dien-7-one, compound 33; cholest-5-en-3β-ol, compound 34; clionasterol, compound 35).
Figure 7.
Structures of some compounds identified in a sample of green propolis. (A) 2-hydroxy-7,12-dimethylbenzanthracene. (B) 1-Hydroxy-2-(1-methoxyethyl)-3-methoxyanthraquinone; (C) Dehydrocostus lactone. (D) Isomaturnin. (E) 1,4aβ-dimethyl-7-isopropyl-2,3,4,4a,9,10-hexahydrophenanthrene. (F) 1,7,7-Trimethyl-3-phenyl-2-oxabixyclo-(4.4.0)-deca-3,5-diene. (G) 4,8-Dimethyl-5-hydrindacene.
Some compounds detected in the present investigation have so far not been reported in propolis. Such is the case for cis-3-methoxy-4-hydroxycinnamic acid (Table 1, compound 5), trans-3-methoxy-4-hydroxycinnamic acid (Table 1, compound 7), 2-hydroxy-7,12-dimethylanthracene (Table 1, compound 18; Fig. 7A), 1-hydroxy-2-(1-methoxyethyl)-3-methoxyanthraquinone (Table 1, compound 19; Fig. 7B), farnesol (Table 2, compound 23), isomaturnin (Table 2, compound 26; Fig. 7D), 1,4aβ-dimethyl-7-isopropyl-2,3,4,4a,9,10-hexahydrophenanthrene (Table 2, compound 29; Fig. 7E), (2,2)-9,12-octadecadienoid acid (Table 3, compound 50), 4-ethyloctane (Table 3, compound 55), 17-pentatriacontene (Table 3, compound 59), 1,7,7-trimethyl-3-phenyl-2-oxabicyclo-(4.4.0)-deca-3, 5-diene (Table 4, compound 61; Fig. 7F) and 4,8-dimethyl-5-hydrindacene (Table 4, compound 64; Fig. 7G). Anthracenes and anthraquinones are probably rare, possibly so far unreported, in propolis.
In a study by HPLC (high performance liquid chromatography), Kumazawa et al. (19) reported the same composition of alecrim shoot apices and a sample of green propolis. The main compounds were prenylated and non-prenylated cinnamic acids. However, the list of substances they reported is distinct from that of Table 1. For example, among several substances not detected in the present work, they detected chlorogenic acid and other caffeoylquinic acids, whereas terpenoids, chromene derivatives and allyl-3-prenylcinnamate are not listed in their paper. Also Park et al. (7) found identical composition in apices of alecrim plants and propolis from associated hives. Artepillin C, simple phenylpropanoids and flavonoids were the reported compounds; no mention was made of prenylated compounds and terpenoids.
In addition to flora, season and phenology (factors often assumed to affect propolis chemistry), Tables 1–4 suggest that other factors probably influence propolis composition. Our results agree with the papers of Kumazawa et al. (19) and Park et al. (7), insofar as they indicate alecrim as main source of green propolis resin. On the other hand, they disagree from those papers because our results indicate differences between compositions of apices and propolis. Compounds 2, 9, 11, 15–17, 19, 21 (Table 1), 23, 24, 26, 28, 29, 32 (Table 2), 50, 51, 55, 59 (Table 3) and 61 (Table 4) were detected in propolis and not in apices. Even artepillin C, a green propolis substance with high notoriety for its cytotoxicity (16), was not detected in apices (Table 1, compound 12). On the other hand, the reverse holds, for example, for compounds 6, 20 (Table 1), 27, 31 (Table 2), 38, 45 (Table 3), 60 and 63 (Table 4). The higher score of matches in the comparison resin masses/propolis (Tables 1–4) is not surprising, since material from resin is undergoing incorporation into the propolis mass. Possibly the integer apices collected for chemical analyses were chemically different from the apices visited by the bees and then converted into resin masses. Coherently, the latter had compositions closer to the propolis sample. Chemical differences between individuals of the same species, growing side by side (as is probably the case in this study), have long been known. For example, Dannel et al. (20) claimed that herbivores make distinction among individuals of different sex and age in the same taxon, probably because of differences in chemical composition. The differential preference of bees observed in this study suggests different chemical contents of plants of the same species, at the same phenologic status, growing side by side. In fact, it was commented above that bees were noticed probing the apex of a plant and then moving to another, from which they collected material. Different compositions occur between apices of female and male plants (Tables 1–4). Compounds 60 and 62 are volatile and hence probably contribute to the plant odor. Compound 5, undetected in female plants, is an interesting substance, with the unusual cis-geometry of the extra-ring double bond. It seems that apices of male alecrim plants are chemically more complex than those of female plants.
With so many factors affecting its composition, it is no wonder that green propolis may co-exist with other propolis types (13). As new chemical data accumulate, a conviction grows that green propolis composition is more complex and unpredictable than previously assumed. Under such circumstance, attempts to establish patterns of Brazilian propolis according to geographic areas and chemical composition (21) may be frustrated. On the other hand, such recognition strengthens the relevance of propolis chemistry and pharmacology in the process of raising inventories of active natural products, without which interesting pharmacologically active substances would hardly be uncovered.
References
- 1.Ghisalberti EL. Propolis – review. Bee World. 1979;60:59–84. [Google Scholar]
- 2.Marcucci MC. Propolis – chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. [Google Scholar]
- 3.Bankova VS, de Castro SL, Marcucci MC. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31:3–15. [Google Scholar]
- 4.Salatino A, Teixeira EW, Negri G, Message D. Origin and chemical variation of Brazilian propolis. eCAM. 2004 doi: 10.1093/ecam/neh060. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer W. Propolis bees and their activities. Bee World. 1956;37:25–36. [Google Scholar]
- 6.Gary NE. Activities and behavior of honey bees. In: Graham JM, editor. The hive and the honey bee. Michigan: Chelsea; 1993. pp. 269–361. [Google Scholar]
- 7.Park YK, Paredes-Guzman JF, Aguiar CL, Alencar SM, Fujiwara FY. Chemical constituents in Baccharis dracunculifolia as the main botanical origin of southeastern Brazilian propolis. J Agric Food Chem. 2004;52:1100–3. doi: 10.1021/jf021060m. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira VC, Bastos EM. Aspectos morfo-anatômicos da folha de Baccharis dracunculifolia DC. (Asteraceae) visando a identificação da origem botânica da própolis. Acta Bot Bras. 1998;12(Supplement):431–9. (in Portuguese) [Google Scholar]
- 9.Loayza I, Abujder D, Aranda R, et al. Essential oils of Baccharis salicifolia, B. latifolia and B. dracunculifolia. Phytochemistry. 1995;38:381–9. [Google Scholar]
- 10.Warakomska Z, Maciejevisk W. Microscopic analysis of propolis from Polish regions. Apidologie. 1992;23:277–83. [Google Scholar]
- 11.Montenegro G, Peña R, Avila G, Timmermann BN. Botanical origin and seasonal production of propolis in hive of Central Chile. Bolm Bot Univ S. Paulo. 2001;19:1–6. [Google Scholar]
- 12.Negri G, Salatino MLF, Salatino A. ‘Green propolis’: unreported constituents and a novel compound from chloroform extracts. J Apicult Res. 2003;42:39–41. [Google Scholar]
- 13.Negri G, Salatino MLF, Salatino A. Unusual chemical composition of a sample of Brazilian propolis as assessed by analysis of a chloroform extract. J Apicult Res. 2003;42:53–6. [Google Scholar]
- 14.Banskota AH, Tezuka Y, Adnyana IK, et al. Hepatoprotective and anti-Helicobacter pylori activities of constituents from Brazilian propolis. Phytomedicine. 2001;8:16–23. doi: 10.1078/0944-7113-00004. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama N, Matsunaga K, Kiuchi F, et al. Trypanocidal terpenoids from Laurus nobilis L. Chem Pharm Bull. 2002;50:1514–6. doi: 10.1248/cpb.50.1514. [DOI] [PubMed] [Google Scholar]
- 16.Matsuno T, Jung S-K, Matsumoto Y, Saito M, Morikawa J. Preferential cytotoxicity to tumor cells of 3,5-diprenyl-4-hydroxycinnamic acid (artepillin C) isolated from propolis. Anticancer Res. 1997;17:3565–8. [PubMed] [Google Scholar]
- 17.Bankova V, Marcucci MC, Simova S, Nikolova N, Kujumgiev A, Popov S. Antibacterial diterpenic acids from Brazilian propolis. Z Naturforsch. 1996;51C:277–80. doi: 10.1515/znc-1996-5-602. [DOI] [PubMed] [Google Scholar]
- 18.Midorikawa K, Banskota AH, Tezuka Y, et al. Liquid chromatography–mass spectrometry analysis of propolis. Phytochem Anal. 2001;12(6):366–73. doi: 10.1002/pca.605. [DOI] [PubMed] [Google Scholar]
- 19.Kumazawa S, Yoneda M, Shibata I, Kanaeda J, Hamasaka T, Nakayama T. Direct evidence for the plant origin of Brazilian propolis by the observation of honeybee behavior and phytochemical analysis. Chem Pharm Bull. 2003;51:740–2. doi: 10.1248/cpb.51.740. [DOI] [PubMed] [Google Scholar]
- 20.Dannel K, Elmqvist T, Ericson L, Salomonson A. Sexuality in willows and preference by barkeating voles: defense or not? Oikos. 1985;44:82–90. [Google Scholar]
- 21.Park YK, Alencar SM, Aguiar CL. Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem. 2002;50:2502–2506. doi: 10.1021/jf011432b. [DOI] [PubMed] [Google Scholar]
- 22.Krol W, Scheller S, Czuba Z, et al. Inhibition of neutrophils chemiluminescence by ethanol extract of propolis (EEP) and phenolic components. J Ethnopharmacol. 1996;45:19–25. doi: 10.1016/s0378-8741(96)01466-3. [DOI] [PubMed] [Google Scholar]
- 23.Marcucci MC, Ferreres F, Garcia-Viguera C, et al. Phenolic compounds from Brazilian propolis with pharmacological activities. J Ethnopharmacol. 2001;74:105–12. doi: 10.1016/s0378-8741(00)00326-3. [DOI] [PubMed] [Google Scholar]
- 24.Orhan H, Marol S, Hepsen IF, Sahin G. Effects of some probable antioxidants on selenite-induced cataract formation and oxidative stress-related parameters in rats. Toxicology. 1999;139:219–32. doi: 10.1016/s0300-483x(99)00128-6. [DOI] [PubMed] [Google Scholar]
- 25.Hirota M, Matsuno T, Fujiwara T, Sugiyama H, Mineshita S. Enhanced cytotocity in a Z-photoisomer of a benzopyran derivative of propolis. J Nat Prod. 2000;63:366–70. doi: 10.1021/np990463m. [DOI] [PubMed] [Google Scholar]
- 26.Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother. 2002;46:1302–9. doi: 10.1128/AAC.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantrell CL, Nunez IS, Castañeda-Acosta J, et al. Antimycobacterial activities of dehydrocostus lactone and its oxidation products. J Nat Prod. 1998;61:1181–6. doi: 10.1021/np970333i. [DOI] [PubMed] [Google Scholar]
- 28.Matsuno T, Matsumoto Y, Saito M, Morikawa J. Isolation and characterization of cytotoxic diterpenoid isomers from propolis. Z Naturforsch. 1997;52C:702–4. doi: 10.1515/znc-1997-9-1020. [DOI] [PubMed] [Google Scholar]