The majority of veno-venous extra corporeal membrane oxygenation (VV-ECMO) patients are unable to participate in awake rehabilitation, a proven treatment for minimising the negative sequalae of prolonged bedrest. 1 Neuromuscular electrical stimulation (NMES) is commonly used with ICU patients unable to participate in active rehabilitation. However, its utilisation in this population is unclear, with concerns raised for the potential of vascular “steal”, whereby the dilation of blood vessels in the thigh during exercise in poorly perfused limbs further reduces perfusion pressure to the distal vascular beds. A relationship between VV-ECMO and significant pedal vascular complications has previously been described. 2
We examined the effects of NMES applied to the quadriceps muscle on the pedal perfusion of patients receiving VV-ECMO via bifemoral cannulation in a randomised crossover feasibility trial conducted in a single centre between January 2016 and December 2017. Institutional ethics committee approval and written next of kin informed consent was obtained prior to study commencement.
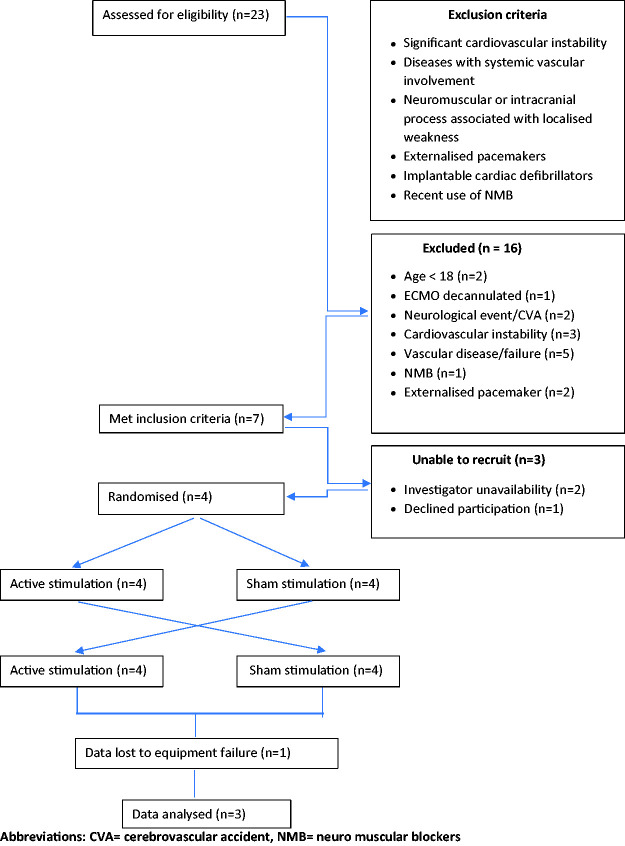
Adult patients admitted to ICU for VV-ECMO via bifemoral cannulation were screened for possible inclusion (Figure 1). Participants received, in a randomised order, both a 30-minute active and sham NMES session delivered to the quadriceps following previously published protocols. 3 Randomisation also determined the limb imaged, and the order of the initial application. Amplitude was adjusted in each active NMES session to an intensity sufficient to obtain a visible contraction of the quadriceps muscle and a palpable tensioning of the patellar ligament. 4
Figure 1.
Exclusion criteria and derivation of the study sample for neuromuscular electrical stimulation in veno-venous extra corporeal membrane oxygenation (VV ECMO) patients.
Abbreviations: CVA = cerebrovascular accident, NMB = neuro muscular blockers.
Pedal perfusion was assessed via a combination of laser speckle contrast imaging (LSCI), non-imaging laser doppler (NILD) flowmetry, and transcutaneous oximetry (PtcO2). 5 Data interpretation was undertaken via a blinded assessor.
Three participants were included in the study. Absolute data values obtained during active NMES, and change between measurements, are shown in Table 1.
Table 1.
Absolute values and change (Δ) in laser speckled contrast imaging, non-imaging laser Doppler and transcutaneous oximetry during ACTIVE neuromuscular electrical stimulation.
| Participant number and time points | LSCI (Δ) | NILD (Δ) | PtcO2 (Δ) |
|---|---|---|---|
| P1 Baseline | 73.9 | 1.2 | 166 |
| P1 15 minutes | 71.2 (–2.7) | 1.6 (+0.4) | 167 (+1) |
| P1 30 minutes | |||
| P1 5 minutes post | |||
| P2 Baseline | 61.4 | –12.9 | 162 |
| P2 15 minutes | 64.2 (+2.8) | –3.8 (+9.1) | 168 (+6) |
| P2 30 minutes | 59.1 (–5.1) | –3.2 (+0.7) | 168 (0) |
| P2 5 minutes post | 51.2 (–7.9) | –10.6 (–7.5) | 170 (+2) |
| P3 Baseline | 135 | 60.7 | 168 |
| P3 15 minutes | 176.4 (+41.4) | 67.5 (+6.8) | 166 (–2) |
| P3 30 minutes | 213.2 (+36.8) | 56.1 (–11.4) | 161(–5) |
| P3 5 minutes post | 124.4 (–88.8) | 83.6(+27.5) | 160 (–1) |
Data expressed as mean values; Blank space = no data available; LSCI = Laser speckled contrast imaging perfusion units; NILD= Non-imaging laser doppler perfusion units; P1 = participant one; P2 = participant two; P3 = participant three; PtcO2 = transcutaneous oximetry in mmHg.
Participants 1 and 2 showed minimal change in pedal perfusion during NMES. Participant 3 demonstrated increased pedal perfusion during NMES on the LSCI data, which was maintained for the duration of the stimulation session (see supplementary video).
All participants demonstrated recruitable muscle with NMES and its application was well tolerated, with no alteration to ECMO flows, sedation requirements, or measured cardiovascular parameters. Increases in pedal blood flow appeared to be most marked when a very strong quadriceps contraction was obtained. However, small participant numbers mean that no definitive results can be drawn from our study. The practical difficulties of prolonged imaging on critically ill patients also contributed towards two sessions being prematurely terminated to allow for necessary medical interventions, resulting in limited data sets.
NMES appeared to offer a viable alternative to volitional exercise in this small cohort of bifemorally cannulated VV-ECMO patients. Strong or very strong muscle contractions could be obtained, during which pedal perfusion was maintained, or improved.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received financial support for the research, authorship, and/or publication of this article: This research was supported by a grant from the Prince Charles Hospital Foundation (NI 2015-209).
ORCID iD
Paul F McCormack https://orcid.org/0000-0002-5802-2662
References
- 1.Abrams D, Javidfar J, Farrand E, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care 2014; 18: R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin HV, Lazzarini PA, Kinnear EM, et al. Dying feet in ICU: why might extracorporeal membrane oxygenation machines cause necrotic feet? J Foot Ankle Res 2013; 6: 1. [Google Scholar]
- 3.Patsaki I, Gerovasili V, Sidiras G, et al. Effect of neuromuscular stimulation and individualized rehabilitation on muscle strength in intensive care unit survivors: a randomized trial. J Crit Care 2017; 40: 76–82. [DOI] [PubMed] [Google Scholar]
- 4.Fischer A, Spiegl M, Altmann K, et al. Muscle mass, strength and functional outcomes in critically ill patients after cardiothoracic surgery: does neuromuscular electrical stimulation help? The catastim 2 randomized controlled trial. Crit Care 2016; 20: 30–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perimed A. Introduction to microcirculation, www.perimed-instruments.com/introduction-to-microcirculation (accessed 27 November 2018).