Abstract
We evaluated the cancer chemopreventive efficacy of the Withania somnifera root, which has been used in the Indian traditional medicine system for many centuries for the treatment of various ailments. Since, studies showing its mechanism-based cancer chemopreventive efficacy are limited, this was investigated in the present study. We studied the effect of dietary administration of Withania root on hepatic phase I, phase II and antioxidant enzymes by estimation of its level/activity, as well as in attenuating carcinogen-induced forestomach and skin tumorigenesis in the Swiss albino mouse model. Our findings showed that roots of W.somnifera inhibit phase I, and activates phase II and antioxidant enzymes in the liver. Further, in a long-term tumorigenesis study, Withania inhibited benzo(a)pyrene-induced forestomach papillomagenesis, showing up to 60 and 92% inhibition in tumor incidence and multiplicity, respectively. Similarly, Withania inhibited 7,12-dimethylbenzanthracene-induced skin papillomagenesis, showing up to 45 and 71% inhibition in tumor incidence and multiplicity. In both studies, Withania showed no apparent toxic effects in mice as monitored by the body weight gain profile. Together, these findings suggest that W.somnifera root has chemopreventive efficacy against forestomach and skin carcinogenesis and warrants the identification and isolation of active compounds responsible for its anticancer effects, which may provide the lead for the development of antitumor agents.
Keywords: chemoprevention, drug metabolizing enzymes, antioxidant enzymes, papillomagenesis, Withania somnifera
Introduction
Ayurveda, the Indian traditional system of medicine, which dates back many centuries, uses many herbal extracts to cure a variety of diseases including cancer. One such popularly used plant that is reported to have antitumor, radiosensitizer, antistressor, immunomodulatory, anti-inflammatory and antibacterial effects is Withania somnifera Dunal, which is commonly known as ‘Ashwagandha’ (1–5). Withania holds a position of importance similar to ginseng in China, having also a rejuvenativing effect on the body; however, this plant is little known in the West. Withania somnifera belongs to the family Solanaceae, and is an evergreen tomentose shrub, grown wild and also cultivated for medicinal use in many parts of India. The roots of W.somnifera contain several alkaloids, withanolides, a few flavanoids and reducing sugars (5,6). The active compounds reported in W.somnifera include withaferin A, sitoindosides VII–X, 5-dehydroxywithanolide-R, withasomniferin-A, 1-oxo-5β,6β-epoxy-witha-2-ene-27-ethoxy-olide, 4-(1-hydroxy-2,2-dimethylcyclpropanone)-2,3-dihydrowithaferin A, 2,3-dihydrowithaferin A, 24,25-dihydro-27-desoxywithaferin A, physagulin D (1→6)-β-d-glucopyranosyl-(1→4)-β-d-glucopyranoside, 27-O-β-d-glucopyranosylphysagulin D, physagulin D, withanoside I–VII, 27-O-β-d-glucopyranosylviscosalactone B, 4,16-dihydroxy-5β,6β-epoxyphysagulin D, viscosalactone B and diacetylwithaferin A (5–9). These reports suggest that Withania is a rich source of bioactive compounds.
The overall metabolism of exogenous xenobiotic compounds is directed towards producing chemical species that can be excreted from the body. Two classes of enzyme systems have been shown for the metabolism of xenobiotic compounds. They are phase I and phase II enzymes. The microsomal cytochrome P450 system that is a product of the CYP superfamily of genes constitutes a major electron transport chain in the endoplasmic reticulum of mammalian cells. It plays a pivotal role in oxidative activation of several toxic xenobiotic compounds, including carcinogens, followed by their detoxification by phase II enzymes and subsequent excretion (10). Carcinogen-induced skin and forestomach papillomagenesis in animal models have been widely used to study the chemopreventive potential of various agents. The 7,12-dimethylbenzanthracene (DMBA)–12-O-tetradecanoylphorbol-13-acetate (TPA) model of skin carcinogenesis involves initiation, promotion and progression, and provides ample scope for studying the stage-specific effect of a chemopreventive compound (11). Several phytochemicals have been tested in this model, and found to have substantial potential to prevent cancer (11,12). Forestomach papillomagenesis is initiated by benzopyrene and involves an endogenous promoter for tumor growth and progression (13). There are few studies showing the anticancer potential of Withania in the mouse skin carcinogenesis model (14,15). To the best of our knowledge, this is the first study reporting the chemopreventive efficacy of Withania root in carcinogen-induced forestomach papillomagenesis in a mouse model. There are two studies reporting the chemopreventive potential of Withania against skin carcinogenesis; one study used oral administration (14), while the other used intraperitoneal injection of Withania root extract (15); however, in the present study, we used dietary administration of Withania root. Dietary administration of the test agent is the preferred method in the study of cancer chemoprevention, as it is practical for preventive measures and provides better absorption and physiological distribution of the test agent (16). Moreover, our findings in forestomach carcinogenesis are novel, whereas in skin carcinogenesis support earlier findings but with a different form and mode of administration of Withania than those reported earlier.
Methods
Mice
Swiss albino mice (6 weeks old) used for this study were maintained in the animal house facility of Jawaharlal Nehru University, New Delhi with controlled temperature and a 12 h light/dark cycle. Animal handling and care were in accord with NIH guidelines. Mice were provided with standard food pellets (Hindustan Lever Ltd, India) and regular drinking water ad libitum. Standard rodent diet was composed of (w/w) wheat (60.3%), roasted bengal gram (28.2%), refined peanut oil (5%), skimmed milk powder (1%), casein (1%), vitamin mixture (0.5%) and mineral mixture (4%), as recommended by the National Institute of Nutrition, Hyderabad, India. In skin tumor studies, the hairs on the back of mice were clipped before the first treatment and thereafter were shaved when needed during the experiment.
Plant Material and Preparation of Test Diet
The dried roots of W.somnifera were purchased from an authorized dealer in New Delhi, India, and were authenticated by a competent botanist, Dr Raveesha, at the Department of Botany, Mysore University, Mysore, India, and a voucher specimen was preserved in our laboratory (Fig. 1). The major active compounds in roots are reported to be withanolides, glycosides and many different alkaloids. To date, up to 19 withanolide derivatives have been isolated from Withania roots (17). The roots were thoroughly washed with 95% ethanol and with sterile water, and air-dried and powdered. Next, 125 g (2.5%, w/w) and 250 g (5%, w/w) of the powdered root were mixed uniformly with 5 kg of standard laboratory diet each and made into pellets. Mice were subjected to control diet and these experimental diets during the experiment as mentioned. We observed that 2 weeks of dietary feeding of Withania at these doses did not show any loss in body weight or reduction in diet consumption in mice; therefore, the same test diets were used for the study of tumorigenesis.
Figure 1.
Roots of Withania somnifera.
Study of Xenobiotic Metabolizing Enzymes in Liver
For the study of xenobiotic metabolizing enzymes in liver, mice were divided into three groups (six mice/group): group I, control group; group II, low dose; and group III, high dose, with 0, 2.5 and 5% (w/w) of Withania in the diet, respectively, and fed for 14 days. Mice were sacrificed by cervical dislocation and the entire liver was then perfused immediately with ice-cold 0.9% normal saline and thereafter carefully removed, trimmed free of extraneous tissue and rinsed in chilled 0.15 M Tris–KCl buffer (pH 7.4). The liver was then blotted dry, weighed quickly and homogenized in ice-cold 0.15 M Tris–KCl buffer (pH 7.4) to yield a 10% (w/v) homogenate. An aliquot of this homogenate (0.5 ml) was used for assaying the acid-soluble sulfhydryl group (-SH) as published recently (18). The remainder of the homogenate was processed for microsome and cytosolic fractions (18). The microsome fraction was used for the estimation of cytochrome P450 (cyt P450) and cytochrome b5 enzymes (cyt b5). The cytosolic fraction was used for assaying total cytosolic glutathione S-transferase (GST), DT-diaphorase (DTD), superoxide dismutase (SOD), catalase (CAT) and lactate dehydrogenase (LDH) activities.
Assays for Cytochrome P450 and Cytochrome b5
Cyt P450 was determined by using the carbon monoxide difference spectra. Both cyt P450 and cyt b5 contents were assayed in the microsomal suspension by the method of Omura and Sato (19), using absorption coefficients of 91 and 185 cm2/mmol, respectively, as reported earlier (20).
Assays for GST, DTD, SOD, CAT and LDH Enzyme Activities
The cytosolic GST activity was determined according to the standard procedure of Habig et al. (21). The specific activity of GST is expressed as μmol of GSH–CDNB conjugate formed/min/mg protein using an extinction coefficient of 9.6/mM/cm. DTD activity was measured by following the procedure of Ernster et al. (22) with nicotinamide adenine dinucleotide (NADH) as the electron donor and 2,6-dichlorophenol-indophenol (DCPIP) as the electron acceptor, at 600 nm. The activity was calculated using an extinction coefficient of 21/mM/cm. One unit of enzyme activity was defined as the amount of enzyme required to reduce 1 μmol of DCPIP/min.
SOD was assayed by the method of Marklund and Marklund (23), which involves inhibition of pyrogallol autoxidation at pH 8.0, as reported earlier (20,24). A single unit of enzyme activity is defined as the quantity of SOD required to produce 50% inhibition of autoxidation. CAT activity was monitored by the decomposition of H2O2 as reported recently (20,24). The specific activity of catalase has been expressed as mol of H2O2 reduced/min/mg of protein. LDH was assayed by measuring the rate of oxidation of NADH at 340 nm according to the method of Bergmeyer and Bernt as reported recently (20,24). The enzyme activity was calculated using an extinction coefficient of 6.22/mM/cm. One unit of enzyme activity is defined as that causing oxidation of 1 μmol of NADH/min. Protein was estimated by the Lowry method as reported earlier (20,24).
Assays for B(a)P-induced Forestomach Papillomagenesis
The effect of Withania diets on benzo(a)pyrene [B(a)P]-induced forestomach tumors was studied as described by Wattenberg et al. (13) and modified by Azuine and Bhide (25). Female Swiss albino mice (6 weeks old) were kept on 2.5 and 5% test diets and normal diet for 2 weeks (n = 15–20/group). From the third week onwards, the mice received B(a)P (1 mg in 0.1 ml peanut oil/mouse) twice a week for 4 weeks by oral gavage. Test/normal diets were continued during as well as 2 weeks after the carcinogen treatment. The experimental groups were: group I, mice kept on the normal diet and who received only B(a)P treatment twice a week for 4 weeks; group II, mice kept on the 2.5% test diet for 2 weeks before, during and 2 weeks after B(a)P treatment; and group III, mice fed with the 5% test diet 2 weeks before, during and 2 weeks after the carcinogen treatment. The body weights of the mice were noted at regular intervals and the mice were sacrificed at the end of 180 days. The forestomach was cut open longitudinally and the number of papillomas was counted under a dissecting microscope.
Analysis of DMBA-induced Skin Papillomagenesis
The effect of 2.5 and 5% Withania in the diet was evaluated on DMBA-induced skin tumorigenesis in female Swiss albino mice as described by Yasukawa et al. (26) with some modifications. The control group, i.e. group I (control diet), received a single topical application of 50 μg of DMBA in 50 μl of acetone per mouse on the dorsal skin. Group II (2.5% test diet) and group III (5% test diet) also received a single topical application of 50 μg of DMBA in 50 μl of acetone per animal. Mice in groups II (LD) and III (HD) were kept on the test diets for 2 weeks before and 2 weeks after carcinogen administration. Croton oil twice/week (0.2 ml/mouse) was used as a skin tumor promoter in all the three groups, the treatment of which continued until the end of the experiment (12 weeks after DMBA treatment). Each group comprised 15 mice.
Histopathology of Tumor Samples
Tissue samples collected from both the forestomach and skin tumorigenesis study were processed conventionally using paraffin-embedded sections. These sections were deparaffinized in xylene and rehydrated in descending concentrations of ethanol, and stained with hematoxylin and eosin. Sections were then dehydrated in ascending concentrations of ethanol followed by xylene and mounted for microscopic observation.
Statistical Analysis
Statistical analysis was performed using analysis of variance (ANOVA) following the Mann–Whitney U-test to determine the statistical difference in the enzyme studies. Student's t-test was used to compare the tumor burden between control and treated groups. P < 0.05 was considered significant.
Results
Withania Modulates the Activities of Xenobiotic Metabolizing and Antioxidant Enzymes in Mouse Liver
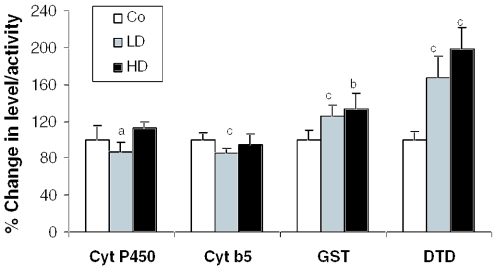
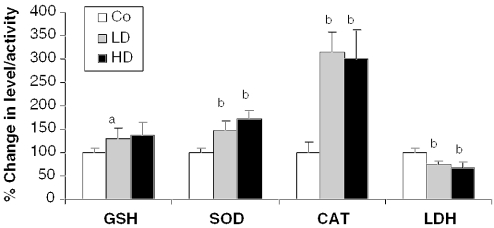
The effect of 2 weeks of dietary feeding of Withania roots was evaluated on the activities of the cyt P450 system and antioxidant enzymes in mouse liver. There was a moderate reduction in the activity of the cyt P450 monoxygenase system. Both cyt P450 and cyt b5 showed a slight but significant reduction (13%, P < 0.05; and 14%, P < 0.005, respectively) in only the LD group compared with controls (Fig. 2). Further, a significant dose-dependent increase in the activity of the phase II enzymes, GST (1.26-fold, P < 0.001 in LD; and 1.33-fold, P < 0.01 in HD) and DTD (1.67-fold, P < 0.005 in LD; and 1.98-fold, P < 0.005 in HD) was observed compared with controls (Fig. 2). Antioxidant enzymes, SOD (1.48-fold, P < 0.01 in LD; and 1.73-fold, P < 0.01 in HD), and catalase (3.15 fold, P < 0.01 in LD; and 2.99 fold, P < 0.01 in HD) also showed a significant increase in the Withania root-fed groups compared with the controls (Fig. 3). A significant increase in GSH content was observed in the LD group (1.31-fold, P < 0.05). In the case of LDH, the activity was reduced by 25–32% (P < 0.01) in the Withania root-fed-groups, indicating no cell damage/toxicity due to the treatment (Fig. 3). Overall, these findings suggested that Withania roots increase phase II enzyme activity as well as cellular antioxidant potential in mouse liver, which could be helpful in detoxification of xenobiotic compounds including carcinogens.
Figure 2.
Effect of the roots of Withania on hepatic phase I and phase II xenobiotic metabolizing enzymes. Mice were exposed to control and test diets for 14 days, and enzyme activity assays were performed as described in Methods. Data are presented as mean ± SD. Error bars represent the SD. Co, control; LD, 2.5% Withania in the diet; HD, 5% Withania in the diet. a (P < 0.05), b (P < 0.01) and c (P < 0.005) indicate significant changes compared with control.
Figure 3.
Roots of Withania induce hepatic reduced glutathione and antioxidant enzymes, and inhibit lactate dehydrogenase. Mice were treated as detailed in Fig. 1 and described in Methods. Data are presented as mean ± SD. Error bars represent the SD. Co, control; LD, 2.5% Withania in the diet; HD, 5% Withania in the diet. a (P < 0.05) and b (P < 0.01) indicate significant compared with control.
Withania Inhibits Carcinogen-induced Forestomach Papillomagenesis in Mice
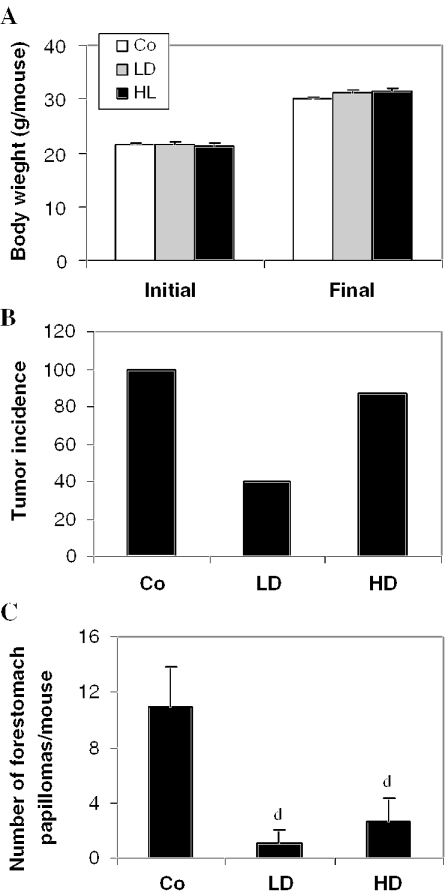
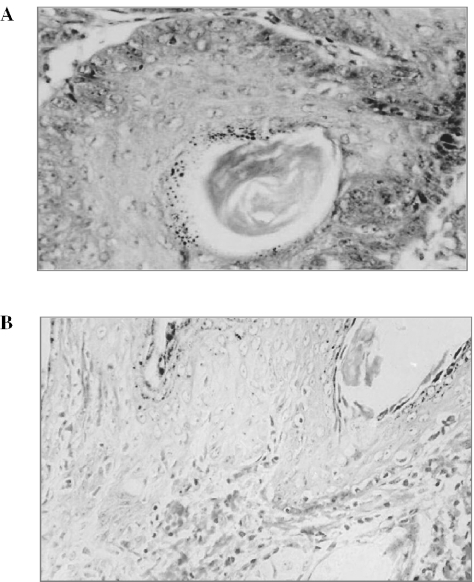
During forestomach papillomagenesis, we observed that dietary feeding of Withania root did not produce any decease in body weight gain (Fig. 4A) or diet consumption. The control group treated with B(a)P alone showed a 100% incidence of forestomach papillomas (% of mice bearing tumors) at the end of 180 days (Fig. 4B). Estimation of the number of papillomas showed 11.1 ± 2.68 papillomas/animal in the control group (Fig. 4C). The mice fed with the 2.5% Withania diet for 6 weeks [2 weeks before, 4 weeks during and 2 weeks after B(a)P treatment] showed only 40% tumor incidence (Fig. 4B), and a strong and significant (P < 0.001) decrease in mean number of papillomas/animal, which was only 0.89 ± 1.08 (Fig. 4C). Though the tumor incidence was 87% in the HD group, the mean number of papillomas/animal was significantly (P < 0.001) reduced to only 2.61 ± 1.72 (Fig. 4C). We also observed a slight increase in the body weight of the mice fed with Withania diets compared with the control group, indicating no apparent side effects or toxicity in the mice (Fig. 4A). Further, forestomach tumors (Fig. 7A) were processed for histopathological evaluation by hematoxylin and eosin staining, showing that keratinized pearl was more prevalent in tumors of the control group of mice, representing an advanced stage in tumor development of epithelial origin. Together, these results showed that Withania root has preventive efficacy against carcinogen-induced forestomach papillomagenesis, and the lower dose used in the present study showed slightly more preventive efficacy as compared with a higher dose in this regard.
Figure 4.
Roots of Withania inhibit B(a)P-induced forestomach papillomagenesis in mice. Mice were treated with B(a)P, and exposed to control and test diets as described in Methods. Co, B(a)P + control diet; LD, B(a)P + 2.5% Withania in the diet; HD, B(a)P + 5% Withania in the diet. (A) Initial and final body weight, (B) Tumor incidence and (C) number of tumors (papillomas)/animal. Data in (C) are presented as the mean ± SD of 15–20 mice in each group. d (P < 0.01) indicates a significant change compared with control.
Figure 7.
Histopathology of tumor samples from forestomach and skin papillomagenesis studies. Tissues samples were harvested at the end of each experiment and processed for hematoxylin and eosin staining as mentioned in Methods. A representative picture is shown for (A) forestomach tumor and (B) skin tumor at 200× magnification in each case.
Withania Inhibits Carcinogen-induced Skin Papillomagenesis in Mice
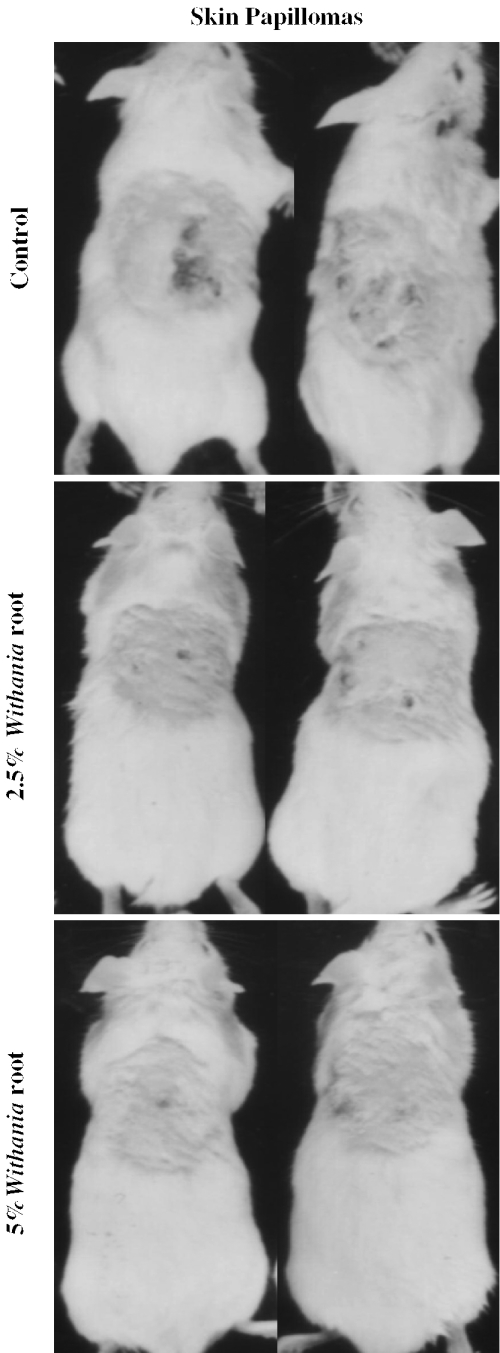
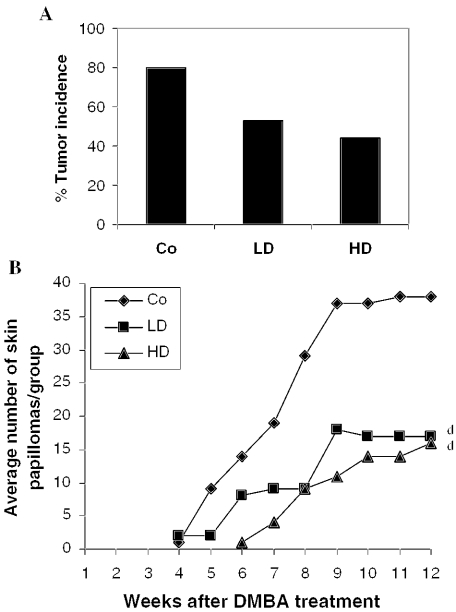
A representative picture of mice bearing skin papillomas in each group is shown in Fig. 5. A single topical application of 50 μg of DMBA/animal in acetone to the dorsal skin followed by croton oil treatment induced tumors in 80% of the control group of mice (12 out of 15) after 12 weeks of DMBA treatment (Fig. 6A). The mean number of papillomas in the control group was 3.17 ± 2.4 papillomas/animal (Fig. 6B). The experimental group fed with the lower dose (2.5%, w/w) Withania diet (2 weeks before and 2 weeks after the DMBA treatment) showed a 53% tumor incidence (Fig. 6A) and 1.1 ± 1.6 papillomas/animal at the end of the experiment (Fig. 6B). However, we observed no increase in tumor multiplicity after 9 weeks of DMBA treatment. Mice fed with the higher dose (5%, w/w) Withania diet showed only a 44% tumor incidence (Fig. 6A) and 0.93 ± 1.58 papillomas/animal (Fig. 6B). We also observed that the high dose of Withania diet delayed tumor appearance by 2 weeks (Fig. 6B). Further, skin tumors (Fig. 7B) were processed for histopathological evaluation by hematoxylin and eosin staining, in which tumors of the control group of mice showed a relatively progressive stage of skin tumor development as characterized by their cellular and nuclear morphology as well as dermal invasiveness. These results demonstrate the preventive efficacy of Withania root against carcinogen-induced skin tumorigenesis in mice.
Figure 5.
Effect of roots of Withania on DMBA-induced papillomas in mouse skin. Pictures were taken at the end of the experiment and are representative for each group. Treatments were as indicated in the figure.
Figure 6.
Roots of Withania inhibit DMBA-induced skin papillomagenesis in mice. Mice were treated with DMBA and croton oil, and exposed to control and test diets. Co, DMBA + croton oil + control diet; LD, DMBA + croton oil + 2.5% Withania in the diet; HD, DMBA + croton oil + 5% Withania in the diet. (A) Tumor incidence and (B) tumor number/group. Data in (B) are presented as the mean of 15 mice in each group. d (P < 0.01) indicate significant change compared with control.
Discussion
Cancer chemoprevention involves pharmacological intervention with synthetic or naturally occurring chemicals or substances to prevent, inhibit or reverse the process of carcinogenesis or to prevent the development of invasive cancer (27,28). In the present study, the modulatory influence of roots of Withania on xenobiotic drug metabolizing enzymes along with organ-specific carcinogenesis was assessed. Our results suggest that Withania root is basically a monofunctional inducer that increases the activity of mainly phase II enzymes and has little effect on phase I monofunctional oxygenases, which is also supported by earlier studies (15). Our data show that Withania root has little effect on the cyt P450 system. We further studied the effect of Withania on phase II enzymes DTD and GST, as well as other antioxidant enzymes SOD, CAT and reduced glutathione (GSH) levels. They assist in conjugating eletrophilic metabolites and carcinogens with endogenous ligands, thus facilitating their removal from the cell (29). A number of promising chemopreventive agents have been proved to be potent inducers of GSH and GST, for example allylic sulfides, oltipraz, N-acetyl cysteine, etc. (30). GST is a critical detoxification enzyme that primarily functions in conjugating the cyt P450 metabolites to endogenous ligands (GSH) favoring their elimination from the body of the organisms (31). Induction of DTD has been evaluated as a means of determining the potency of many chemopreventive substances (32). Consistent with these facts, we observed a significant induction in both GST and DTD in Withania root-fed mice. In addition, we also observed an increase in SOD and CAT activities, and increased levels of GSH in Withania root-fed mice. These effects could have a potential role in the detoxification and elimination of potential carcinogens from the body, leading to the cancer preventive efficacy of Withania roots as we observed in carcinogen-induced forestomach and skin papillomagenesis. However, more studies are needed to show the direct involvement of the antioxidative potential of Withania roots in detoxification of carcinogens.
In the case of B(a)P-induced forestomach papillomagenesis, tumor incidence as well as tumor burden were decreased in Withania-fed mice. B(a)P is a polycyclic aromatic hydrocarbon and a potent inducer of forestomach carcinogenesis (33). B(a)P is metabolized to its oxidized derivatives (epoxides, phenols, diols, tetrols and quinones) by monooxygenases and epoxide hydrolases. The moderately polar metabolites are conjugated with sulfate, glucuronic acid and glutathione by cytosolic enzymes to produce their water-soluble and excretable forms. Since Withania moderately inhibited the specific activities of cyt P450 and cyt b5, the conversion of B(a)P to its epoxide would have been reduced, and at the same time, an increase in the activity of phase II and antioxidant enzymes could have facilitated the detoxification and excretion of oxidized metabolites of B(a)P.
Withania roots were effective in reducing the tumor incidence and tumor burden in DMBA-induced skin papillomagenesis. We observed a dose-dependent decrease in skin tumor incidence; however, the dose-dependent inhibitory effect of Withania roots on tumor burden was lost after 12 weeks of DMBA treatment. Reactive intermediates of skin tumor initiators such as DMBA are mutagenic and bind covalently to DNA of epidermal stem cells leading to transformation and irreversible alteration in the differentiation capacity of these cells (27). TPA, one of the active principles of croton oil that was used as a promoter in the present study, stimulates the generation of superoxide radicals, hydrogen peroxide and lipid peroxidation that may be involved in the process of tumor promotion and can be interfered with by GST, DTD and CAT (11,12,34). Since Withania significantly induces the specific activity of GST, DTD and CAT, we speculate that it can intervene in the formation of active carcinogen as well as the processes involved in tumor promotion. However, more studies are needed to confirm this. We also anticipate that an immunomodulatory as well as an anti-inflammatory potential of Withania roots might have contributed to its overall efficacy against chemical carcinogenesis in forestomach and skin that needs further investigation.
Withania is already popularly used as a home remedy for several diseases such as arthritis, geriatric problems, fever, tuberculosis, asthma, etc., and is an official drug mentioned in the Indian Pharmacopoeia. The findings of the present investigation support Withania root to be an effective cancer chemopreventive agent in a murine model system. Therefore, a pre-clinical cancer chemoprevention study with Withania root might help in generating valuable data to determine its usefulness in cancer chemoprevention in humans. Further studies are needed to evaluate its pharmacokinetics and toxicity profile to determine its clinical doses. No apparent toxicity of Withania root (2.5 and 5%) in the diet in the present study is supportive for advocating it as an effective and safe chemopreventive agent in a mouse model; however, further studies are needed to assess its effect with chronic administration. Isolation and identification of bioactive components from Withania have attracted many investigators in Asian countries. This suggests that W.somnifera root should be explored for cancer chemopreventive drug development, in addition to its existing utility as a drug in the traditional medicine system of India, Ayurveda.
References
- 1.Chandrashekar A, Sharada P, Solomon E, Umadevi P, Nayanabhirama U, Srinivasan K. Antitumor and radiosensitizing effects of withaferin A on mouse Erhlich ascites carcinoma in vivo. Acta Oncol. 1996;35:95–100. doi: 10.3109/02841869609098486. [DOI] [PubMed] [Google Scholar]
- 2.Devi PU, Akagi K, Ostapenko V, Tanaka Y, Sugahara T. Withaferin A: a new radiosensitizer from the Indian medicinal plant Withania somnifera. Int J Radiat Biol. 1996;69:193–7. doi: 10.1080/095530096146020. [DOI] [PubMed] [Google Scholar]
- 3.Archana R, Namasivayam A. Antistressor effect of Withania somnifera. J Ethnopharmocol. 1999;64:91–3. doi: 10.1016/s0378-8741(98)00107-x. [DOI] [PubMed] [Google Scholar]
- 4.Ziauddin M, Phansalkar N, Patki P, Diwanay S, Patwardhan B. Studies on the immunomodulatory effects of Ashwagandha. J Ethnopharmacol. 1996;50:69–76. doi: 10.1016/0378-8741(95)01318-0. [DOI] [PubMed] [Google Scholar]
- 5.Umadevi P. Withania somnifera dunal (Ashwagandha): potential plant source of promising drug for cancer chemotherapy and radiosensitization. Indian J Exp Biol. 1996;34:927–32. [PubMed] [Google Scholar]
- 6.Ganzera M, Choudhary MI, Khan IA. Quantitative HPLC analysis of withanolides in Withania somnifera. Fitoterapia. 2003;74:68–76. doi: 10.1016/s0367-326x(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 7.Jayaprakasam B, Zhang Y, Seeram NP, Nair MG. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003;74:125–32. doi: 10.1016/j.lfs.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Kaur P, Sharma M, Mathur S, et al. Effect of 1-oxo-5beta, 6beta-epoxy-witha-2-ene-27-ethoxy-olide isolated from the roots of Withania somnifera on stress indices in Wistar rats. J Altern Complement Med. 2003;9:897–907. doi: 10.1089/107555303771952244. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda H, Murakami T, Kishi A, Yoshikawa M. Structures of withanosides I, II, III, IV, V, VI, and VII, new withanolide glycosides, from the roots of Indian Withania somnifera DUNAL and inhibitory activity for tachyphylaxis to clonidine in isolated guinea-pig ileum. Bioorg Med Chem. 2001;9:1499–507. doi: 10.1016/s0968-0896(01)00024-4. [DOI] [PubMed] [Google Scholar]
- 10.Guengerich FP. Roles of cytochrome P450 enzymes in chemical carcinogenesis and cancer chemotherapy. Cancer Res. 1988;48:2946–54. [PubMed] [Google Scholar]
- 11.Slaga TJ. Multistage skin carcinogenesis: a useful model for the study of the chemoprevention of cancer. Acta Pharmaco Toxicol (Copenh) 1984;55(Suppl 2):107–24. doi: 10.1111/j.1600-0773.1984.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 12.Singh RP, Tyagi AK, Zhao J, Agarwal R. Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis. 2002;23:499–510. doi: 10.1093/carcin/23.3.499. [DOI] [PubMed] [Google Scholar]
- 13.Wattenberg LW, Coccia JB, Lam LKT. Inhibitory effects of phenolic compunds on benzo(a)pyrene-induced neoplasia. Cancer Res. 1980;40:2820–3. [PubMed] [Google Scholar]
- 14.Prakash J, Gupta SK, Dinda AK. Withania somnifera root extract prevents DMBA-induced squamous cell carcinoma of skin in Swiss albino mice. Nutr Cancer. 2002;42:91–7. doi: 10.1207/S15327914NC421_12. [DOI] [PubMed] [Google Scholar]
- 15.Davis L, Kuttan G. Effect of Withania somnifera on DMBA-induced carcinogenesis. J Ethnopharmacol. 2001;75:165–8. doi: 10.1016/s0378-8741(00)00404-9. [DOI] [PubMed] [Google Scholar]
- 16.Singh RP, Dhanalakshmi S, Tyagi AK, Chan DCF, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice, and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002;62:3063–9. [PubMed] [Google Scholar]
- 17.Zhao J, Nakamura N, Hattori M, Kuboyama T, Tohda C, Komatsu K. Withanolide derivatives from the roots of Withania somnifera and their neurite outgrowth activities. Chem Pharm Bull (Tokyo) 2002;50:760–5. doi: 10.1248/cpb.50.760. [DOI] [PubMed] [Google Scholar]
- 18.Singh RP, Banerjee S, Rao AR. Effect of Aegle marmelos on biotransformation enzymes system and protection against free radicals mediated damage in mice. J Pharm Pharmacol. 2000;52:991–1000. doi: 10.1211/0022357001774714. [DOI] [PubMed] [Google Scholar]
- 19.Omura T, Sato R. The carbon monoxide binding pigment of liver. J Biol Chem. 1964;239:2370–8. [PubMed] [Google Scholar]
- 20.Singh RP, Padmavathi B, Rao AR. Chemomodulatory influence of Adhatoda vesica (Justicia adhatoda) on the enzymes of xenobiotic metabolism, antioxidant status and lipid peroxidation in mice. Mol Cell Biochem. 2000;213:99–109. doi: 10.1023/a:1007182913931. [DOI] [PubMed] [Google Scholar]
- 21.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases—the first step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 22.Ernster L, Danielson L, Ljunggren M. DT-diaphorase—purification from the soluble fraction of rat liver cytoplasm. Biochim Biophys Acta. 1962;58:171–88. doi: 10.1016/0006-3002(62)90997-6. [DOI] [PubMed] [Google Scholar]
- 23.Marklund S, Marklund G. Involvement of superoxide anion radical in autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 24.Singh RP, Banerjee S, Rao AR. Modulatory influence of Andrographis paniculata on mouse hepatic and extrahepatic carcinogen metabolizing enzymes and antioxidant status. Phytother Res. 2001;15:382–90. doi: 10.1002/ptr.730. [DOI] [PubMed] [Google Scholar]
- 25.Azuine MA, Bhide SV. Chemopreventive effect of turmeric against stomach and skin tumors induced by chemical carcinogens in Swiss mice. Nutr Cancer. 1992;17:77–83. doi: 10.1080/01635589209514174. [DOI] [PubMed] [Google Scholar]
- 26.Yasukawa K, Yu SY, Yamanouchi S, Takido M, Akihisa T, Tamura T. Some lupene-type triterpenes inhibit tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Phytomedicine. 1995;4:309–13. doi: 10.1016/S0944-7113(11)80008-5. [DOI] [PubMed] [Google Scholar]
- 27.Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 2000;78:199–334. doi: 10.1016/s0065-230x(08)61026-x. [DOI] [PubMed] [Google Scholar]
- 28.Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–7. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 29.Prestera T, Holtzelaw WD, Zhang Y, Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci USA. 1993;90:2956–69. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelloff GJ, Boone CW, Steele VE, et al. Mechanistic consideration in the evaluation of chemopreventive data. Principles of chemoprevention. 1995. IARC Publication No: 139. [PubMed]
- 31.Hartman PE, Shankel DW. Antimutagens and anticarcinogens; a survey of putative interceptor molecules. Environ Mol Mutagen. 1990;15:145–82. doi: 10.1002/em.2850150305. [DOI] [PubMed] [Google Scholar]
- 32.DeLong MJ, Prochaska HJ, Talalay P. Inhibition of NAD(P)H: quinone reductase in murine hepatoma cells by phenolic antioxidants, azo dyes, and other chemoprotectors: a model for the study of anticarcinogens. Proc Natl Acad Sci USA. 1986;85:787–91. doi: 10.1073/pnas.83.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estensen RD, Wattenberg LW. Studies of chemopreventive effects of myo-inositol on benzo(a)pyrene-induced neoplasia of the lung and forestomach of female A/J mice. Carcinogenesis. 1993;14:1975–7. doi: 10.1093/carcin/14.9.1975. [DOI] [PubMed] [Google Scholar]
- 34.Awasti YC, Singhal SS, Awasti S. Mechanisms of anticarcinogenic effects of antioxidant nutrients. In: Watson RR, Mufti SI, editors. Nutrition and Cancer Prevention. CRC Press, Inc.; 1996. pp. 139–72. [Google Scholar]