Abstract
Salvia columbariae (chia) was examined and found to contain miltionone II, cryptotanshinone and tanshinone IIA. These compounds may be of interest in the treatment of stroke and heart attack.
Keywords: chia, Salvia columbariae, tanshinones
Introduction
Chia (Salvia columbariae, Benth., Lamiaceae) grows in the west from California to Utah and south to Northern Mexico (Fig. 1). It is usually found growing on decomposing granite and grows best in shade. Chia grows at many elevations, from coastal scrub up to pine woodlands at 1200 m. The seeds have been and continue to be used as food. The seeds are available in stores and are sold by the kg. The seeds were especially used by the Chumash messengers (ksen) who ran perhaps 30 km or more in a day delivering messages between villages. Eating the seeds was supposed to maintain their energy during the run.
Figure 1.
Salvia columbariae photographed by J. Adams.
Chumash people historically inhabited the Californian coastal region from Malibu to San Luis Obispo and inland for about 160 km. There are many Chumash people living currently in California and other locations. The Chumash culture and religion are still practiced in California. Chumash legends tell of a plant called ‘ilepesh (pronounced gheelaypaysh) that was used to ‘wake the dead, or the nearly dead’ (1). Apparently, ‘ilepesh’ is chia (2). How the plant was used to ‘wake the dead’ is unknown. However, it may have been the root that was used. Probably the people who were treated with this plant had suffered from strokes or heart attacks and appeared to be nearly dead.
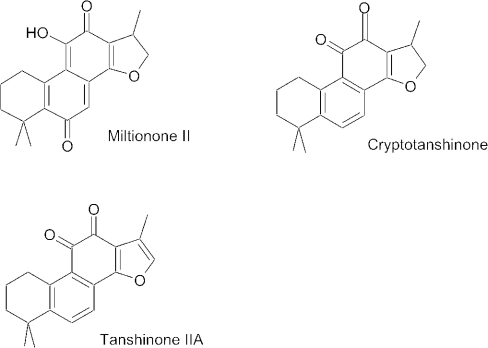
Salvia miltiorrhiza (dan shen) is a related species from China that is used in the treatment of stroke (3). Dan shen is reported to be very effective at preventing death from stroke (3). The roots of dan shen are used in this treatment. The roots have been shown to contain tanshinones (Fig. 2) , cryptotanshinone and miltionones (4,5). These compounds apparently are the active medicines in the plant and are able to prevent clotting (6) and restore blood flow (7) in stroke.
Figure 2.
Structures of miltionone II, cryptotanshinone and tanshinone IIA.
The current work examined the roots of chia to see if tanshinones and similar compounds are present. The presence of tanshinones may explain the legendary ability of the plant to wake the dead. This is the first report of the chemistry of chia. Experiments are planned for the future examination of the effects of chia on infarction in a stroke model.
Materials and Methods
Extract Preparation
The roots were separated from the remainder of the plants. The roots were woody, about 15 cm long and 1 cm in diameter at the widest point. From four large plants, 11.4 g of root material was collected and finely chopped with a cleaver. To this was added 50 ml of 90% ethanol. The compounds in the roots were extracted by the microwave technique (8). The ethanol extracts were filtered through filter paper.
Analytical Methods
HPLC and UV spectra of extracts. The extracts were injected (50–100 μl) onto an HPLC system with a Supelcosil LC-18T column. The mobile phase was 80% methanol, 20% water flowing at 1 ml/min. UV spectra were collected with a photodiode array detector.
HPLC–Mass Spectrometry of extracts. The extracts were submitted to the California Institute of Technology, Regional Mass Spectrometry Facility (Dr Mona Shagholi). The extracts were injected onto an HPLC–MS system with an Eclipse XDB-C18 column and were developed at 1 ml/min in 80/20 methanol/water containing 1% formic acid.
Identification of Tanshinones
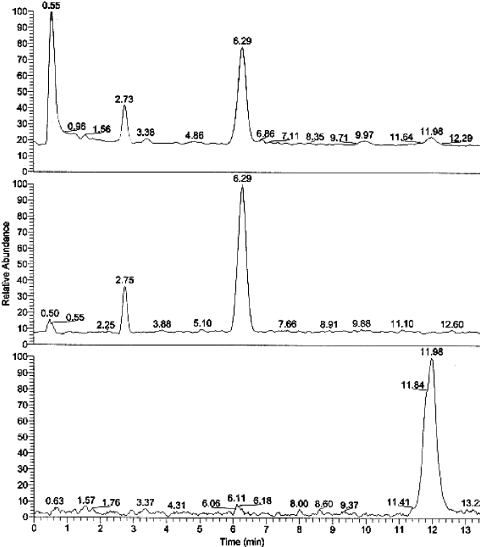
The root extracts were found to contain three major peaks on the HPLC system as visualized at 254 nm. The retention times were 4.2, 6.9 and 10.2 min. The UV spectra of each peak were similar with maxima at about 250 and 300 nm. The HPLC conditions were chosen based on the chromatography of tanshinones (5). The retention times were similar to published retention times for tanshinones (5). The UV spectra were similar to published spectra for miltionones, cryptotanshinone and related compounds (4). The extinction coefficients of tanshinone IIA are lambamaxMeoH nm (log epsilon): 220 (4.46), 250 (4.40) and 269 (4.44), (9). Based on the similar UV spectra and similar chromophores of the three compounds, the extinction coefficients are probably similar for each. The HPLC peaks for the three compounds integrated as follows: miltionone II (see below), 4.2 min 25.2%, cryptotanshinone, 6.9 min 69% and tanshinone IIA, 10.2 min 5.8%. The amounts of each compound in the 50 ml extract were: miltionone II, 0.7 μmol, cryptotanshinone, 2 μmol, and tanshinone IIA, 0.2 μmol. An HPLC–MS chromatogram is shown in Figure 3.
Figure 3.
HPLC–MS total ion current, base peak current and 283 m/z ion current of a chia root extract. Peaks are: miltionone II (2.7 min), cryptotanshinone (6.3 min) and tanshinone IIA (12 min).
Miltionone II. HPLC retention time 4.2 min; UV (MeOH, H2O) lambamax 254 and 300 nm; HPLC–MS (electrospray) retention time 2.73 min, [MH]+ m/z 313, [M2Na]+ m/z 647, [M3Na]+ m/z 959.
Cryptotanshinone. HPLC retention time 6.9 min; UV (MeOH, H2O) lambamax 254 and 300 nm; HPLC–MS (electrospray) retention time 6.29 min, [MH]+ m/z 297, [MNa]+ m/z 342, [M2Na]+ m/z 615, [M2Na2]+ m/z 638, [M3Na]+ m/z 911.
Tanshinone IIA. HPLC retention time 10.2 min; UV (MeOH, H2O) lambamax 246 and 294 nm; HPLC–MS (electrospray) retention time 11.98 min, [MH]+ m/z 295, [MH-C]+ m/z 283, [M2Na-CO]+ m/z 583, [M2Na-H2O]+ m/z 595, [M2Na-CH2]+ m/z 599, [M2Na]+ m/z 613.
Results
Chia was grown at the Rancho Santa Ana Botanic Garden, Claremont, CA. When the plants were about 1 m tall, the entire plants were harvested. At this time, they were in flower with large seed clusters. The plants were put into plastic bags and stored in a freezer. Chia can be difficult to grow to maturity. The seeds sprout sometimes abundantly, but die quickly if not in the correct environment. The seeds were found to grow best in full shade, with plenty of water, good drainage and the application of lime (a few grams) when the plants are about 2 cm tall. Transplanting the seedlings into lime-containing soil resulted in the loss of most plants.
Chia was found to contain 17.5 μmol of tanshinone IIA per kg of root material (Table 1). This is three-fold less than is found in dan shen (5). However, chia contains nearly five-fold more cryptotanshinone than is found in dan shen (5). Cryptotanshinone is a precursor for tanshinone IIA and is converted into tanshinone IIA in the liver (10). This implies that chia contains 192.5 μmol/kg of active tanshinones. Dan shen contains 91 μmol/kg of active tanshinones.
Table 1.
Amounts of tanshinones present in chia (Salvia columbariae) and dan shen (Salvia miltiorrhiza)
| Compound | S.columbariae (μmol/kg) | S.miltiorrhiza (μmol/kg) |
|---|---|---|
| Tanshinone IIA | 17.5 | 54 |
| Cryptotanshinone | 175 | 37 |
Discussion
The presence of tanshinone IIA and similar compounds in chia could explain the historical use of this plant, to ‘wake the dead, or the nearly dead’ such as with stroke and heart attack patients. Tanshinones have a range of pharmacological activities including inhibition of clotting (6), vasodilatation (7) and inhibition of NO synthase (11). All of these activities are potentially beneficial in stroke. Stroke is frequently caused by blood clots that dislodge from one location and travel in the blood system until they lodge in small cerebral arteries. This causes brain ischemia and usually stimulates more clotting in the area. Vasodilatation and inhibition of clotting may help dislodge and dissolve the clot. NO synthase is known to become activated in ischemia and can generate NO that damages DNA leading to cell death. Inhibition of NO synthase may protect neurons from DNA damage and cell death.
Chia contains some of the same compounds found in dan shen, including tanshinone IIA. In China, tanshinone IIA is available as a purified sulfonate salt for use in stroke, heart attack and angina patients (3,12). Although, tanshinone IIA is regarded as the active agent in chia, it is also recognized that cryptotanshinone is a precursor to tanshinone IIA in the body (10). While tanshinone IIA is very rapidly cleared from the body by hepatic metabolism (10), cryptotanshinone is oxidized in the liver to make tanshinone IIA. Therefore, tanshinone IIA levels may be higher and stay higher for a longer time period after cryptotanshinone than after tanshinone IIA administration. Chia contains more cryptotanshinone and less tanshinone IIA than dan shen (Table 1). Chia contains two times more active tanshinones than does dan shen. This implies that chia may be superior to dan shen for use as a delivery agent or precursor for tanshinone IIA. It may be of interest to test dan shen and chia extracts to see which plant extract produces higher plasma levels of tanshinone IIA and better protection from infarction.
References
- 1.Blackburn TC. December's child: A book of Chumash oral narratives. Berkeley, CA: University of California Press; 1975. [Google Scholar]
- 2.Timbrook J. Ethnobotany of Chumash Indians, California, based on collections by John P. Harrington. Econ Botany. 1990;44:236–53. [Google Scholar]
- 3.Ji XY, Tan BK, Zhu YZ. Salvia miltiorrhiza and ischemic diseases. Acta Pharm Sin. 2000;21:1089–94. [PubMed] [Google Scholar]
- 4.Ikeshiro Y, Mase I, Tomita Y. Abietane type diterpenoids from Salvia miltiorrhiza. Phytochemistry. 1989;28:3139–41. [Google Scholar]
- 5.Tian G, Zhang Y, Zhang T, Yang F, Ito Y. Separation of tanshinones from Salvia miltiorrhiza Bunge with high speed counter current chromatography using stepwise elution. J Chromatog. 2000;904:107–11. doi: 10.1016/s0021-9673(00)00916-x. [DOI] [PubMed] [Google Scholar]
- 6.Chan TY. Interaction between warfarin and danshen (Salvia miltiorrhiza) Ann Pharmacol. 2001;35:501–4. doi: 10.1345/aph.19029. [DOI] [PubMed] [Google Scholar]
- 7.Lei XL, Chiou GC. Cardiovascular pharmacology of Panax notoginseng (Burk) and Salvia miltiorrhiza. Am J Chin Med. 1986;14:145–52. doi: 10.1142/S0192415X86000235. [DOI] [PubMed] [Google Scholar]
- 8.Pan X, Niu G, Liu H. Microwave assisted extraction of tanshinones from Salvia miltiorrhiza Bunge with analysis by high performance liquid chromatography. J Chromatog. 2001;922:371–5. doi: 10.1016/s0021-9673(01)00949-9. [DOI] [PubMed] [Google Scholar]
- 9.Danheiser RL, Casebier DS, Loebach JL. Total synthesis of dan shen diterpenoid quinones. Tetrahedron Lett. 1992;33:1149–52. [Google Scholar]
- 10.Xue M, Cui Y, Wang HQ, Hu ZY, Zhang B. Reversed phase liquid chromatographic determination of cryptotanshinone and its active metabolite in pig plasma and urine. J Pharmaceut Biomed Anal. 1999;21:207–13. doi: 10.1016/s0731-7085(99)00098-9. [DOI] [PubMed] [Google Scholar]
- 11.Yokozawa T, Chen CP. Role of Salvia miltiorrhiza radix extract and its compounds in enhancing nitric oxide expression. Phytomedicine. 200;7:55–61. doi: 10.1016/S0944-7113(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhou G, Jiang W, Zhao Y, et al. Sodium tanshinone IIA sulfonate mediates electron transfer reaction in rat heart mitochondria. Biochem Pharmacol. 2003;65:51–7. doi: 10.1016/s0006-2952(02)01447-8. [DOI] [PubMed] [Google Scholar]