Abstract
Atherosclerosis is a chronic disease of the large arteries and the underlying cause of myocardial infarction and stroke. Atherosclerosis is driven by cholesterol accumulation and subsequent inflammation in the vessel wall. Despite the clinical successes of lipid-lowering treatments, atherosclerosis remains one of the major threats to human health worldwide. Over the past 20 years, insights into cardiovascular immunopathology have provided a plethora of new potential therapeutic targets to reduce the risk of atherosclerosis and have shifted the therapeutic focus from lipids to inflammation. In 2017, the CANTOS trial demonstrated for the first time the beneficial effects of targeting inflammation to treat cardiovascular disease by showing that IL-1β inhibition can reduce the recurrence rate of cardiovascular events in a large cohort of patients. At the same time, preclinical studies have highlighted nanotechnology approaches that facilitate the specific targeting of innate immune cells, which could potentially generate more effective immunomodulatory treatments to induce disease regression and prevent the recurrence of cardiovascular events. The clinical translation of such nanoimmunotherapies and their application to treat patients with ischaemic heart disease are challenges that lie ahead.
Atherosclerotic plaques with a large lipid-rich necrotic core and a thin fibrous cap are classically classified as being at high risk of causing atherothrombotic events. In addition, superficial plaque erosion is increasingly recognized as an alternative mechanism underlying atherothrombosis. The two distinct plaque phenotypes differ in their inflammatory profile, with eroded plaques often lacking inflammation, whereas plaques with lipid-rich necrotic cores and a thin fibrous cap have abundant inflammation1.
Pathologists recognized the involvement of immune cells in cardiovascular diseases as early as the 19th century. The German pathologist Rudolf Virchow was the first investigator to postulate that immune cells have a principal role in atherosclerosis2. Since then, our understanding of the mechanisms by which immune cells drive atherogenesis has evolved substantially, leading us to realize that Virchow’s perspective was valid3.
In this Review, we discuss how the scientific discoveries in cardiovascular immunopathology from the past decade, together with advances in bioengineering techniques, have guided the development of innovative nanoimmunotherapeutic strategies to modulate immune responses in cardiovascular disease.
Cardiovascular immunopathology
From monocyte to macrophage
Atherosclerosis encompasses a complex immunological response in which neutrophils, dendritic cells, mast cells, T cells, and B cells have distinct roles. Macrophages are considered to be a particularly important cell type driving disease progression, and extensive investigations in the past 30 years have focused on the pathological process of macrophage accumulation in atherosclerotic plaque3. According to the classical view, plaque macrophages are derived from circulating monocytes that infiltrate the vessel wall through a process regulated by the endothelium3. Consequently, endothelial dysfunction is an important mechanism in the development of atherosclerosis. Endothelial dysfunction predominantly occurs at arterial branch points with low or oscillatory shear stress, which explains the focal nature of atherosclerosis4-6. Pro-inflammatory activation of endothelial cells can be triggered by stimuli such as dyslipidaemia or pro-inflammatory mediators3. Activated endothelial cells secrete chemoattractants and express adhesion molecules, which enable monocyte recruitment to atherosclerotic lesions3. Plaque neovascularization accompanies inflammation and facilitates monocyte invasion into the vessel wall7.
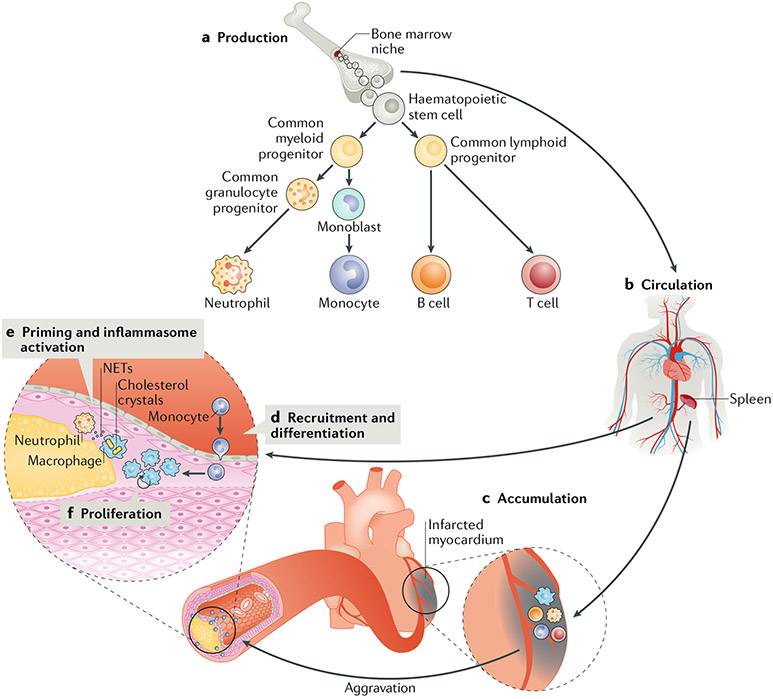
Circulating monocytes originate from haematopoietic stem cells in the bone marrow and from a splenic monocyte reservoir8. Hypercholesterolaemia stimulates leukocyte production, which results in increased infiltration of inflammatory monocytes in atherosclerotic lesions9,10. Upon acute injury or stress, such as myocardial infarction (MI), myelopoiesis increases, and the splenic monocyte reservoir is rapidly mobilized8,11. Physical stress, via sympathetic nervous signalling, causes the release of haematopoietic stem and progenitor cells from bone marrow niches to the circulation and their subsequent seeding in the spleen to amplify extramedullary myelopoiesis8. This process activates the recruitment of inflammatory monocytes to the atherosclerotic plaque and aggravates plaque inflammation11. Interestingly, mental stress, a major risk factor for MI12, also stimulates the proliferation of haematopoietic progenitors in the bone marrow via sympathetic nervous signalling, which increases the output of inflammatory monocytes and worsens plaque inflammation13. In 2017, a study unravelled how somatic mutations in haematopoietic cells can promote the expansion of mutant cells and accelerate the development of atherosclerosis through clonal haematopoiesis of indeterminate potential (CHIP), a phenomenon associated with ageing14. Fuster and colleagues showed that partial bone marrow reconstitution with cells deficient in methyl-cytosine dioxygenase TET2, an important enzyme for DNA demethylation, markedly enhanced the development of atherosclerosis in Ldlr−/− mice14. This finding was corroborated by a subsequent study that showed that individuals who were identified as CHIP carriers with mutations in ASXL1, DNMT3A, JAK2, or TET2 had increased coronary artery calcification, as assessed by cardiac CT, and were at increased risk of coronary heart disease15. These findings illustrate the tight involvement of medullary and extramedullary haematopoiesis in the process of atherosclerotic disease (FIG. 1).
Fig. 1 |. Cardiovascular immunopathology.
a | Production of myeloid cells and lymphoid cells in the bone marrow. b | Myeloid cells are released into the circulation; the spleen acts as a reservoir for monocytes. c | Myeloid cells accumulate at sites of inflammation such as atherosclerotic plaques and the infarcted myocardium. Acute events such as myocardial infarction aggravate local plaque inflammation. d | After recruitment to the plaque, monocytes differentiate into LesionaL macrophages. e | Plaque macrophages can be primed by various stimuli, including neutrophil extracellular traps (NETs) and oxidized LDL, to produce cytokines and become chronically activated by cholesterol crystals. f | Plaque macrophages have the capacity for Local proliferation.
The paradigm that tissue macrophages are solely derived from circulating monocytes has been revisited in the past 5 years. Studies using experimental models of inflammation have shown that tissue macrophages can be self-maintained by local proliferation16. The self-renewing capacity of macrophages was also observed during atherosclerosis: Robbins and colleagues showed that in atherosclerotic Apoe−/− mice, the accumulation of plaque macrophages depends in large part on local proliferation, not only on monocyte influx, even though the proliferating lesional macrophages ultimately derive from non-proliferating circulating monocytes17. Local macrophage proliferation has also been observed in human atherosclerotic plaques18 (FIG. 1).
Macrophage activation
Macrophages are part of the innate immune system. Macrophages form the first line of defence against pathogens but also have a central role in restoring damage and maintaining tissue homeostasis19. Macrophages have pattern recognition receptors (PRRs) that can recognize pathogens and damage-associated molecular patterns. Upon identification of these molecular patterns, the macrophages become activated, which stimulates phagocytosis and induces the release of cytokines and chemokines that activates and recruits other immune cells19.
The molecular stimuli that activate PRRs and chronically activate plaque macrophages have been intensively investigated. In a landmark study using the Apoe−/− mouse model of atherosclerosis, Duewell and colleagues showed, through a combination of laser reflection and fluorescence confocal microscopy, that small cholesterol crystals appear at the earliest stages of atherosclerosis development20. In addition, the study showed that cholesterol crystals cause phagolysosomal damage in macrophages and induce NLRP3 inflammasome activation as well as IL-1β and IL-18 release20. The activation of the NLRP3 inflammasome involves two independent steps: priming (signal 1) and activation (signal 2). The inflammasome must be primed before activation, and this process is mediated by PRRs or cytokine receptors that activate nuclear factor-κB (NF-κB)19. Priming can be induced by various stimuli via Toll-like receptors (TLRs). For example, oxidized LDL (oxLDL) cholesterol primes the inflammasome via activation of a signalling complex formed by a heterodimer of TLR4 and TLR6 together with the scavenger receptor class B member 1 (SRB1; also known as CD36)21. SRB1 has a very important role given that this receptor is involved in both signal 1 (priming) and signal 2 (oxLDL uptake and crystal formation) of NLRP3 inflammasome activation22. Neutrophils are also involved in this process. Cholesterol crystals induce the release of neutrophil extracellular traps (NETs) — extracellular webs consisting of DNA and proteins that form a physical barrier and can kill pathogens23. In atherosclerosis, these NETs can prime macrophages for cytokine production24 (FIG. 1).
Macrophages store the ingested lipoprotein cholesterol in their cytoplasm as cholesteryl ester droplets, a process that gradually transforms the cells into foam cells25. In 2016, a study revealed that these foam cells can become senescent26. Cellular senescence is a state of permanent growth arrest triggered by various processes, including DNA damage, telomere shortening, and elevated oxidative stress26. Foam cells can acquire a senescence-associated secretory phenotype with deleterious effects during atherogenesis by producing substances involved in proteolysis (such as matrix metalloproteinases (MMPs)), monocyte chemotaxis (such as C-C motif chemokine 2 (CCL2; also known as MCP1) and vascular cell adhesion protein 1 (VCAM1)), and inflammation (such as tumour necrosis factor (TNF) and IL-1α)26.
Altogether, decades of research have established an important mechanistic framework in which lipids, recognized as danger signals by PRRs, are ingested by macrophages, leading to inflammasome activation and macrophage transformation into senescent foam cells. These immune responses initiate and perpetuate chronic low-grade inflammation in the vessel wall. The interaction of macrophages with neutrophils and T cells in the plaque further sustains this inflammatory process3,24,27. Experimental studies have indicated that reducing inflammation can modulate the development of atherosclerosis3. However, whether reducing vascular inflammation translates into cardiovascular benefit in humans remained unproven until 2017. The paradigm of focusing on lipid-lowering to treat atherosclerosis was challenged by the CANTOS trial28. This trial included >10,000 patients with previous MI and with increased plasma levels of C-reactive protein (CRP) measured by high-sensitivity assay. Patients were treated with placebo or canakinumab, a monoclonal antibody targeting IL-1β, a cytokine that is produced by monocytes and macrophages and that is central to the systemic inflammatory response. After a median follow-up of 3.7 years, the composite end point of nonfatal MI, nonfatal stroke, or cardiovascular death was reduced by 15% in the group receiving canakinumab compared with the placebo group28. This study is an important step forward that supports the rationale for developing anti-inflammatory therapies to treat atherosclerotic cardiovascular disease.
A shift in nanotherapeutic approaches
The chemical properties of drugs or other therapeutic modalities dictate their route of administration as well as their pharmacokinetic and pharmacodynamic characteristics; however, these profiles can be drastically altered by nanotechnology. Nanoparticle formulations can facilitate the delivery of drugs with poor water solubility, the targeted delivery of drugs to specific tissues or cell populations to enhance efficacy, the delivery of drugs to intracellular targets otherwise difficult to reach, and the transcytosis of therapeutic compounds across an endothelial barrier. Nanoparticle formulations can also enable the co-delivery of multiple therapeutic modalities at once, prevent drugs from interacting with certain blood constituents and tissues to avoid toxicity and enhance circulatory half-life, and protect the drugs from inactivation and degradation. An additional advantage is that nanoparticles can be labelled to track their delivery to the site of action, evaluate their in vivo stability, and assess their pharmacokinetics and biodistribution by imaging29-31. Non-invasive imaging readouts help to improve nanoparticle design and can be simultaneously employed to quantify treatment efficacy. The application of nanotechnology for imaging purposes has been extensively reviewed elsewhere32. Nanotechnological approaches for the delivery of therapeutic modalities have emerged in various fields of medicine over the past 25 years. Doxil, a liposomal formulation of doxorubicin, was the first nanomedicine to gain FDA approval in 1995 (REF.33). Currently, >24 nanotherapeutic compositions are approved for clinical use, ranging from applications in oncology (such as protein-bound paclitaxel (Abraxane)) to infectious diseases (such as amphotericin B (AmBisome))34,35. The use of nanomedicines in the cardiovascular field is still scarce, although clinical trials to investigate novel potential applications are ongoing. As an example, Fitzgerald and colleagues studied the effect of a second-generation lipid nanoparticle carrying a small interfering RNA (siRNA) targeted to PCSK9 in healthy individuals with elevated plasma LDL-cholesterol levels36. Another example of cardiovascular nanomedicine is the use of prednisone-containing liposomes, which was investigated in patients with atherosclerotic disease37,38.
Traditionally, nanomedicine has been used as a targeting strategy, primarily against tumours. A multitude of nanoparticle platforms exist that are either approved for clinical use or investigated in clinical trials, including liposomal, polymeric, albumin-based, nanocrys-talline, polymeric micelles, dendrimer, virus-based, lipoprotein-based, metallic-based, ceramic-based, and polysaccharide-based platforms29,30. To achieve efficient targeting, nanoparticle systems are designed to evade uptake by innate immune cells, for instance, through polymeric coatings. However, although surface modification by polyethylene glycol (PEG) results in drastically prolonged circulatory half-lives39, nanoparticles are cleared by the mononuclear phagocytic system, which results in their accumulation in the liver and the spleen. In this respect, the development of coatings from leukocyte membranes, which prevents nanoparticles from being cleared by the immune system while enabling the transport and release of their cargo across the endothelium, is interesting40. Another promising development is the incorporation of self-peptides (for instance, leukocyte surface antigen CD47), which delays macrophage-mediated clearance of nanoparticles and increases their half-life in the circulation41. The accumulation of long-circulating nanoparticles in the atherosclerotic plaque is governed by the enhanced permeability of microvessels in the vessel wall and a retention-effect mechanism42. Alternatively, active targeting can be achieved by using nanoparticles with their surface functionalized with ligands that are specific to epitopes expressed on the angiogenic vasculature43,44, on collagen45,46, or on macrophages47.
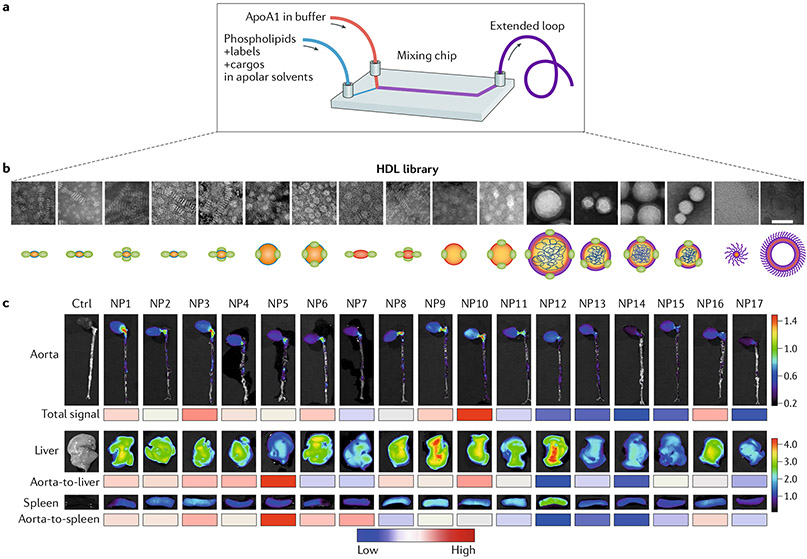
While bioengineering techniques are rapidly progressing and yielding methods to produce and characterize new nanomaterials (FiG. 2a), our knowledge of cardiovascular disease biology, in particular immunology, is also advancing dramatically. The cardiovascular pathology field has made great advances over the past decade and has moved from a focus on lipids to a focus on inflammation. Nanomedicine for cardiovascular applications should be aimed at targeting immune cells not only in atherosclerotic plaques or in the ischaemic myocardium but also systemically, particularly in haematopoietic organs. In addition to cell location, several other variables, including the cell type, the function of the cell in the context of its environment, and its role in the pathological process, should be considered to design rational and creative approaches for the selection of specific nanomaterials. Size, shape, electrokinetic potential, surface hydrophilicity, drug encapsulation efficiency, and in vivo stability are important aspects to consider in the engineering of nanomedicines. The targeting of monocytes and macrophages provides a unique opportunity to modify inflammatory responses and should be a primary goal in therapies against cardiovascular diseases. However, the toxicity of immunomodulatory drugs has to be taken into consideration, particularly in relation to liver uptake. In 2017, our group reported on the use of microfluidics technology (FiG. 2a) to generate a nanobiologic library containing HDL mimetics in the size range of 10–30 nm (REF48). The size of the HDL nanobiologics could also be increased from 40 nm to 150 nm and up to 400 nm by the inclusion of a polymeric core, which also resulted in a change in nanoparticle shape from discoidal to spherical49 (FiG. 2b). Fluorescent dyes were also easily integrated to allow the screening of HDL nanobiologics in vitro and in vivo in atherosclerotic Apoe−/− mice with the use of optical methods, including near-infrared fluorescence imaging of intact organs, which revealed the highest accumulation of nanoparticles in the liver, spleen, and kidney49 (FIG. 2c). Flow cytometry of digested tissues showed that the nanobiologics efficiently targeted monocytes and macrophages in atherosclerotic lesions and in the reticuloendothelial organs49. From the nanobiologic library, we selected a nanobiologic with low liver avidity to improve the toxicity profile of a liver X receptor agonist (GW3965) while preserving its therapeutic function in atherosclerotic plaques.
Fig. 2 |. HDL nanobiologic library.
a | Microfluidic technology allows the generation of an HDL nanobiologic library. b ü Transmission electron micrographs and schematic representations of an HDL nanobiologic library. c | Apoe−/− mice with advanced atherosclerotic plaques were intravenously injected with the different nanobiologics. Representative near-infrared fluorescence images of the accumulation of nanobiologics in the aorta, liver, and spleen. The heat map below the images of the aorta ranks the mean total fluorescent signal of the tissue; the heat map below the liver images ranks the total aorta-to-liver signal; the heat map below the spleen images ranks the mean aorta-to-spleen accumulation ratio, with the red and blue indicating a high and a low ratio, respectively. ApoA1, apolipoprotein A1; Ctrl, control; NP, nanoparticle. Part a adapted with permission from REF48, John Wiley & Sons. Parts b and c adapted with permission from REF49, Proceedings of the National Academy of Sciences, USA.
Targeting the innate immune system
Given its central role in atherosclerosis, the innate immune system is an attractive therapeutic target. However, interfering with this intricate system might debilitate its prime function, which is to fight off infection. In the past decade, insights into how we can modulate immune responses while minimizing systemic effects have evolved substantially, and nanotechnology approaches could be of specific relevance for such strategies.
Regulating monocyte recruitment
Monocytes are short-lived cells (1–7 days) that are derived from haematopoietic stem cells through a common macrophage–dendritic cell progenitor in the bone marrow50. The interaction of C-C chemokine receptor type 2 (CCR2) with its ligand CCL2 is required for monocytes to exit to the circulation51. Different monocyte subsets with distinct phenotypes and functions have been identified: classical, intermediate, and nonclassical monocytes can be distinguished on the basis of the expression of monocyte differentiation antigen CD14 and low-affinity immunoglobulin-γ Fc region receptor III-A (FcRIII; also known as CD16) in humans and lymphocyte antigen 6 C (LY6C) in mice. Nonclassical monocytes (CD14+CD16++ in humans and LY6Clow in mice) patrol the inner lining of the vessel to defend endothelial integrity. The role of nonclassical monocytes in atherosclerosis is unclear, and this subset does not contribute to the plaque macrophage population directly52,53. Classical monocytes (CD14++CD16− in humans and LY6Chigh in mice) compose the majority of the circulating monocyte pool (approximately 85%) and are rapidly recruited to inflammation sites. Monocyte infiltration into the atherosclerotic lesion involves a close interplay between monocytes and the endothelium, which can be divided into a capture and rolling phase and a subsequent transmigration phase54. This process requires the interaction of adhesion molecules expressed by activated endothelial cells, for example, P-selectin, intercellular adhesion molecule 1 (ICAM1), VCAM1, and platelet endothelial cell adhesion molecule (PECAM1), with glycosylated ligands and integrins expressed by the monocytes, such as P-selectin glycoprotein ligand 1 (PSGL1), integrin α4, and integrin αL. Different chemokine–receptor interactions, including CCL2–CCR2, CCL5–CCR5, and C-X3-C motif chemokine 1 (CX3CL1; also known as fractalkine)–CX3C chemokine receptor 1 (CX3CR1), facilitate the infiltration of monocytes across the endothelium54. In atherosclerotic plaque, monocytes directly contribute to inflammation and differentiate into lesional macrophages55. The critical participation of monocytes in the progression of atherosclerosis renders these cells an attractive therapeutic target. However, considering their primary role in homeostasis and host defence, high precision is paramount to modulating monocyte dynamics.
Targeting monocytes
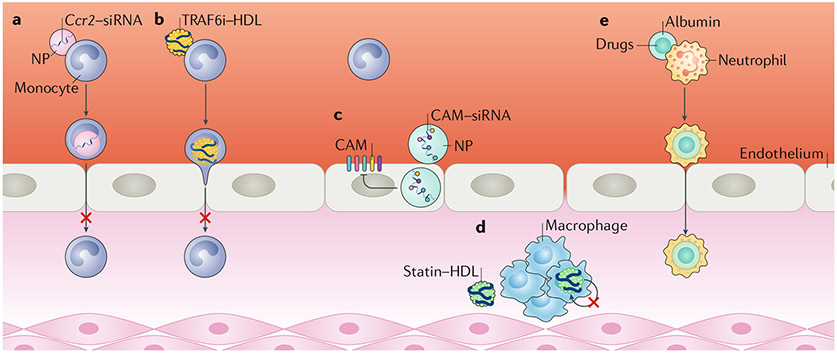
Leuschner and colleagues presented an innovative nanotechnology approach in which siRNA accumulated in cellular subsets in the spleen and reduced the expression of a surface receptor involved in leukocyte migration56,57 (Figs 3,4a). In this study, an siRNA against Ccr2 was incorporated in a lipid-based nanoparticle (size 70–80 nm) composed of designed amphiphiles — C12-200 lipid and PEG–dimyristolglycerol—as well as naturally occurring distearoyl phosphatidylcholine and cholesterol56. The targeting efficiency of the particles was investigated in leukocytes from the blood, spleen, and bone marrow. Low nanoparticle uptake was observed in lymphocytes, whereas innate immune cells (monocytes, macrophages, dendritic cells, and neutrophils) showed a marked internalization of nanoparticles. The therapy effectively decreased Ccr2 expression in monocytes and impaired their in vitro migratory capacity in response to CCL2. The effect of the nanoparticles was investigated in Apoe−/− mice aged 6 months with advanced atherosclerosis (fed a Western diet for 4 months)56. Mice treated twice per week for a period of 3 weeks with nanoparticles containing the siRNA against Ccr2 showed an 82% reduction in monocyte and macrophage numbers and a 53% reduction in LY6Chigh monocyte counts in atherosclerotic plaques compared with mice receiving nanoparticles containing siRNA control56. Next, the investigators studied the effects of the therapy on monocyte recruitment after MI. MI causes a rapid inflammatory response; the resulting infiltration of LY6Chigh monocytes into the infarct area increases the infarct size and is associated with the development of heart failure after MI58. In the study, the mice were treated with the nanoparticles 3 days before and on the day of the MI. Treatment with Ccr2–siRNA decreased LY6Chigh monocyte infiltration in the infarct area by 71% compared with siRNA control and markedly reduced the infarct:area-at-risk ratio. The beneficial effect of this therapy was reproduced in mice treated only 1 h after induction of the MI56.
Fig. 3 |. Nanotherapeutic approaches to reduce atherosclerotic plaque inflammation.
a | Small, lipid-based nanoparticLes (NPs) loaded with a short interfering RNA (siRNA) targeted against Ccr2 (Ccr2–siRNA) can impair monocyte migratory capacity. b | Small nanobioLogics derived from natural HDL loaded with a TNF receptor-associated factor 6 (TRAF6) inhibitor (TRAF6i) inhibited the CD40–TRAF6 interaction, resulting in diminished monocyte recruitment. c | PoLyamine-based nanoparticLes with high affinity for endothelial cells are used to deliver siRNA targeting five different ceLL adhesion molecules (CAMs) expressed on endothelial cells. d | Reconstituted HDL nanoparticLes with high cellular specificity for plaque macrophages can be Loaded with statins. Statin–HDL particles reduce Local macrophage proliferation. e | Neutrophils attached to the endothelium, but not circulating neutrophils, internalize aLbumin-based nanoparticLes Loaded with drugs and are exploited to facilitate transport of the nanoparticLes across the endothelium.
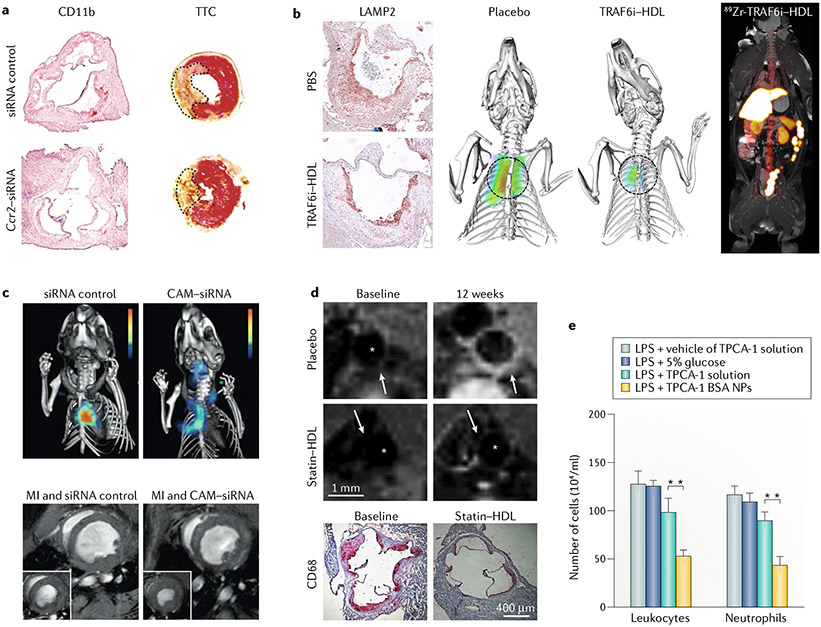
Fig. 4 |. Highlighted studies using nanotechnology to resolve inflammation in cardiovascular disease.
a | Treatment with nanoparticles loaded with short interfering RNA (siRNA) targeted against Ccr2 (Ccr2–siRNA) reduced the number of lymphocyte antigen 6C (LY6C)high monocytes in atherosclerotic mice, as shown by integrin αM (also known as CD11b) staining. In atherosclerotic mice that underwent myocardial infarction, 2,3,5-triphenyltetrazolium chloride (TTC) staining revealed that the infarct area was significantly reduced with Ccr2–siRNA compared with the siRNA control. b | Treatment with HDL nanoparticles loaded with an inhibitor of TNF receptor-associated factor 6 (TRAF6i–HDL) in atherosclerotic mice reduced the area positive for lysosome-associated membrane glycoprotein 2 (LAMP2; also known as MAC3) compared with treatment with the vehicle, as a result of diminished transendothelial migration of monocytes. Fluorescence molecular tomography–CT (FMT–CT) showed diminished protease activity, while PET–MRI showed the biodistribution in a non-human primate after injection with radiolabelled HDL-TRAF6i. c | Treatment with nanoparticles loaded with siRNA against five cell adhesion molecules (CAMs) expressed by endothelial cells led to decreased protease activity measured by FMT–CT in atherosclerotic mice compared with treatment with nanoparticles containing siRNA control. In mice with myocardial infarction (MI) induced by coronary artery ligation, treatment with siRNA targeted against CAM (CAM–siRNA) led to a significantly less impaired left ventricular ejection fraction compared with treatment with siRNA control. d | Treatment of atherosclerosis in mice with statin–HDL nanoparticles resulted in reduced plaque area compared with placebo, as assessed by T1-weighted MRI. A short-term, high-dose regimen resulted in an impressive 80% reduction in macrophage plaque content compared with placebo, as determined by histological staining for microsialin (also known as CD68) . Asterisks indicate the vessel lumen. White arrows indicate locations of signal enhancement in the vessel wall 24 h after infusion of gadolinium-labelled statin-HDL. e | In a mouse model of acute lung inflammation, neutrophil-mediated delivery of albumin nanoparticles loaded with 2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide (TPCA-1), a nuciear factor-κB (NF-κB) inhibitor, resulted in a lower number of leukocytes and neutrophils than with treatment with the inhibitor in solution. **, P < 0.01. BSA, bovine serum albumin; LPS, lipopolysaccharide; NPs, nanoparticles. Part a adapted from REF.56, Springer Nature Limited. Part b adapted from REF.60, Springer Nature Limited. Part c adapted with permission from REF.63, American Association for the Advancement of Science. Part d adapted from REF.71, Springer Nature Limited. Part e adapted with permission from REF83, American Chemical Society.
The electrokinetic potential of the particles can also have a role in their targeting and therapeutic efficacy. Getts and colleagues compared the effect of immune-modifying microparticles with ζ-potentials varying from −50 mV to +40 mV (REF.59). The investigators showed that highly negatively charged carboxylated poly(lactic-co-glycolic acid) (PLGA)-derived particles were effectively taken up by circulating LY6Chlgh monocytes via the macrophage receptor MARCO, a scavenger receptor, which induced cell uptake in the spleen and subsequent apoptosis. In a mouse model of MI, in which treatment with negatively charged PLGA particles was initiated 12 h after MI and continued for 3 days, monocyte and macrophage infiltration in the infarct site was reduced by 30% compared with non-treated mice and was accompanied by improved cardiac function, as evaluated by ultrasonography59.
Another strategy to regulate monocyte recruitment is the delivery of small-molecule drugs that impair intracellular signalling pathways. This concept was explored to inhibit the interaction between CD40 receptor and TNF receptor-associated factor 6 (TRAF6) (FiGS 3b,4b). CD40 signalling, mediated through TRAF6 in monocytes and macrophages, has a critical role in atherosclerosis60,61. A TRAF6 inhibitor (TRAF6i) was encapsulated in myeloid-cell-specific HDL nanobiologics (size 20 nm) prepared from apolipoprotein A1 and the phospholipids MHPC and DMPC. The specificity of TRAF6i–HDL nanoparticles for leukocytes was investigated in the blood, spleen, bone marrow, and atherosclerotic plaque. The uptake of the nanoparticle by macrophages and LY6Chlgh monocytes was approximately 80–90% in all the aforementioned tissues. Neutrophils and dendritic cells took up nanobiologics to a much lesser extent, and the uptake by lymphocytes was negligible. TRAF6i–HDL infusions in Apoe−/− mice aged 20 weeks reduced plaque macrophage and LY6Chigh monocyte content by 66% and 49%, respectively, compared with no treatment and empty HDL, after only 1 week of therapy (four infusions). Comparative, whole-transcriptome analysis and in vitro transendothelial migration assays confirmed that monocyte recruitment was impaired after treatment with TRAF6i–HDL, mainly through the inhibition of integrin activation. Importantly, the study extensively investigated the toxicity of the therapy and its effect on the systemic immune system in both mice and non-human primates, a crucial step in evaluating the translational potential of the nanoparticles. No signs of liver and other organ toxicity or systemic immunosuppressive effects were observed60,61.
Targeting transendothelial migration
The studies discussed above revolve around the concept of direct monocyte targeting. A 2014 study explored the potential of nanoparticles to reduce monocyte transendothelial migration through siRNA delivery62 (FiGS 3c,4c). The nanoparticles were formulated from 7C1 — a conjugate of polyethylenimine and a C15 polymer alkyl tail — and C14PEG2000 by a microfluidic manufacturing process, and siRNAs were encapsulated in the nanoparticle. The resulting nanoparticles measured 45 nm and had a strong affinity for endothelial cells and a fairly low uptake by hepatocytes and immune cells62. The endothelial-cell avidity of this nanoparticle formulation is not well understood, but seems to involve the interaction of 7C1 with serum proteins, which might promote delivery of the nanoparticle to certain cell types62. Sager and colleagues used these nanoparticles for the combined delivery of five siRNAs directed against Icam1, Icam2, Sele, Selp, and Vcam1 (REF.63). Fluorescent labelling of the nanoparticles revealed an absence of uptake by plaque macrophages, high uptake by endothelial cells, and low uptake by circulating monocytes and neutrophils. Treatment of Apoe−/− mice with three infusions of the therapy over the course of 2 weeks resulted in a significant reduction in the number of LY6Chigh monocytes in atherosclerotic plaques compared with control siRNA, which coincided with reduced inflammatory activity in the plaque. Similarly, in an experimental model of MI, LY6Chigh monocyte recruitment to the heart was markedly reduced in mice treated with nanoparticles, and cardiac MRI showed improved preservation of left ventricular ejection fraction63.
The studies discussed above illustrate that intelligent integration of materials science and bioengineering allows the design of nanotherapeutics with avidity for myeloid or endothelial cells. Advances in the field of monocyte biology have identified novel targets that will continue to inspire nanotherapy design. Conceptually, the differentiation of monocyte precursors in the bone marrow, the release of haematopoietic stem cells from the bone marrow, monocyte egress from the bone marrow and the splenic monocyte reservoir, and the monocyte–endothelial cell interaction can be explored for therapeutic targeting.
Modulating macrophage dynamics
Macrophages are long-lived cells that have an important role in host defence and tissue homeostasis, with distinct functions and phenotypes depending on their environment and localization in the body. As discussed previously, in atherosclerosis, these cells are activated by oxLDL and cholesterol crystals, which induce their polarization towards an inflammatory phenotype. In this disturbed state, macrophages locally stimulate the endothelium and produce chemokines to attract monocytes. The secretion of MMPs and cysteine proteases leads to extracellular matrix degradation, which contributes to plaque instability and the occurrence of atherothrombotic events3,64.
Targeting macrophage polarization
The therapeutic potential of myeloid-cell-specific nanomedicines depends not only on the core loading but also on the materials used in the shell. For example, sugar-based amphiphilic macromolecule constructs can be used to target and modulate macrophages. The similarity in charge and hydrophobicity of the constructs with oxidized lipoproteins facilitates their binding to macrophage scavenger receptors and competitively blocks oxLDL uptake by the macrophages65. On the basis of this technology, Lewis and colleagues designed a library of individual nanoparticles with different charges, hydrophobicity, and stereochemistry65. The investigators evaluated the library in vitro by measuring the effects of the particles on oxLDL uptake and scavenger receptor expression in a human macrophage cell line. The nanoparticle formulation (approximately 160 nm in size) with the most favourable characteristics was selected for further in vivo testing. Upon intravenous injection, the nanoparticles accumulated in atherosclerotic plaques in Apoe−/− mice. After four infusions of the therapy in a period of 4 weeks, histological analysis showed a marked decrease in plaque size, lipid content, cholesterol cleft formation, and neointimal formation in the treated mice compared with controls65.
In another study, Beldman and colleagues reported nanoparticles in which the primary building block was also the bioactive molecule66. Naturally occurring hyaluronan, a principal component of tissue extracellular matrix, was used to formulate nanoparticles with a mean size of approximately 90 nm. Upon intravenous infusion, the hyaluronan nanoparticles accumulated in the vessel wall and effectively targeted plaque macrophages. Subsequently, a 12-week treatment regimen with the nanoparticle in Apoe−/− mice resulted in a 30% decrease in plaque macrophage content, a decrease in plaque size, and an increase in plaque collagen content compared with vehicle-treated mice66. The above-discussed studies demonstrate the concept of achieving a therapeutic effect on the basis of the biochemical properties of the nanoparticle material itself and not on the delivery of a therapeutic cargo65,66.
Other studies utilized nanoparticles as carrier vehicles to deliver various therapeutic compounds. Nakashiro and colleagues exploited a PLGA nanoparticle system in which a potent agonist of peroxisome proliferator-activated receptor-γ (PPARγ) was incorporated67. PPARγ is a nuclear receptor that regulates lipid metabolism and affects inflammatory activity in macrophages68. In vitro, the therapy elicited macrophage polarization towards a less inflammatory phenotype and lowered IL-1β production. After establishing the affinity of the particles for plaque monocytes and macrophages, the investigators conducted a therapeutic study. Weekly intravenous infusions of the nanoparticles loaded with inhibitor decreased MMP production in the brachiocephalic arteries of Apoe−/− mice after a 4-week regimen compared with control nanoparticles, which was quantified in vivo with fluorescence reflectance imaging. Histological analysis revealed that the size and macrophage content of atherosclerotic plaques in the brachiocephalic arteries were not modified by the therapy; however, the number of buried fibrous caps decreased, whereas their thickness increased67.
Courties and colleagues proposed a macrophage polarization technology that consisted of 70 nm lipidoid nanoparticles loaded with an siRNA to silence Irf5 (REF.69). The transcription factor interferon regulatory factor 5 (IRF5) is an important regulator of macrophage polarization70. IRF5 is highly expressed by macrophages in MI, and its expression wanes as inflammation resolves70. In an Apoe−/− mouse model of MI, macrophages in the myocardium were adequately targeted by the Irf5–siRNA nanoparticles, resulting in significant suppression of IRF5 levels compared with the use of control siRNA nanoparticles. Histology and fluorescence molecular tomography (FMT)–CT, respectively, revealed fewer macrophages and considerably decreased protease activity in the infarcted myocardium of mice treated with Irf5–siRNA nanoparticles compared with control siRNA nanoparticles. The expression of genes associated with an inflammatory macrophage phenotype, including Tnf and Il1b, was also decreased in the treated mice, whereas cardiac MRI established that preservation of left ventricular function was improved69.
Targeting macrophage biology
In addition to macrophage polarization, other functional features of macrophages can be targeted. Our group has explored the effects of an injectable HDL nanoparticle for the delivery of simvastatin to plaque macrophages71 (FiGS 3d,4d). Statins suppress intracellular cholesterol synthesis by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase, a rate-limiting step in the mevalonate pathway72. We first showed in vitro that the statin–HDL nanoparticle (approximately 25 nm in size) suppressed the inflammatory response of macrophages through inhibition of the intracellular mevalonate pathway. Subsequently, we showed by in vivo MRI that statin–HDL (labelled with a contrast agent) targeted the plaque in Apoe−/− mice. The localization of the nanoparticles was confirmed with ex vivo near-infrared fluorescence imaging and confocal microscopy of dual fluorescently labelled statin–HDL particles, whereas their specificity for plaque macrophages was demonstrated by flow cytometry. To investigate the efficacy of statin–HDL particles, we performed two therapeutic studies in Apoe−/− mice. In vivo MRI showed that a 12-week, low-dose, biweekly infusion regimen inhibited plaque progression. The in vivo data were corroborated by histological analysis of the aortic root, which showed a 30% decrease in plaque size and a 40% decrease in plaque macrophage content with the statin–HDL nanoparticles compared with oral statin or bare HDL. A short-term, high-dose, 1-week regimen reduced macrophage content by approximately 80% in mice with established atherosclerotic plaques compared with any of the control treatments71.
Similarly, Katsuki and colleagues used PLGA nanoparticles to deliver pitavastatin in Apoe−/− mice fed a high-fat diet and infused with angiotensin II, a mouse model of plaque destabilization and rupture. Several assays demonstrated that PLGA nanoparticle therapy reduced plaque destabilization73. Our group decided to investigate further the therapeutic mechanism underlying the anti-atherosclerotic effect of statin–HDL particles74. Statin–HDL treatment did not affect LY6Chigh monocyte recruitment to the plaque. In addition, no differences in the number of LY6Chigh monocytes in the circulation or in the plaque were observed, and the expression of important genes related to monocyte recruitment was unchanged. However, in vivo 5-bromodeoxyuridine (BrdU) pulse–chase experiments and staining for the proliferation marker protein Ki67 indicated that inhibition of local proliferation of plaque macrophages was the principal mechanism underlying the observed decrease in plaque inflammation. This effect was confirmed in vitro in a murine macrophage cell line and involved the inhibition of the mevalonate pathway74.
A very different approach is to target macrophages in the spleen, as Dutta and colleagues demonstrated in a study published in 2015 (REF75). The objective of this study was to block splenic macrophage maturation by decreasing splenic macrophage VCAM1 expression. VCAM1 expression is essential to retain haematopoietic stem cells in the spleen and promotes extramedullary myelopoiesis. This process has a pivotal role in the pathogenic cardiovascular immune responses in atherosclerosis and MI, as described earlier. The investigators developed a nanoparticle containing an siRNA to silence the expression of Csf1 (which encodes the receptor for macrophage colony-stimulation factor; M-CSF). Treatment with the nanoparticles containing the targeted siRNA three times per week for 3 weeks decreased haematopoietic stem cell counts in the spleen, dramatically reduced the number of circulating LY6Chigh monocytes, and caused a marked reduction in plaque size as well as suppression of plaque monocyte and macrophage content in Apoe−/− mice compared with control nanoparticles. In mice with MI induced by coronary artery ligation, treatment with the therapeutic nanoparticles on day 1 and day 3 after MI resulted in decreased monocyte and macrophage content in the infarcted tissue compared with control nanoparticles. Similar findings were observed when using a nanoparticle loaded with an siRNA directed at Vcam1 (REF.75). The study highlights that macrophages can be targeted in the spleen to mitigate extramedullary haematopoiesis, thereby preventing a surplus of inflammatory cells from migrating to atherosclerotic plaques or infarcted myocardium, where they cause harm.
Collectively, the reviewed studies show that targeting macrophage dynamics yields potent therapeutic opportunities to resolve pathogenic cardiovascular immune responses. Monocyte to macrophage differentiation, macrophage polarization, inflammatory and metabolic activity, foam cell formation, cellular senescence, and local macrophage proliferation are among the potential cellular processes that can be targeted. Beneficial effects can be achieved by targeting macrophages directly in the atherosclerotic plaque or the infarcted myocardium and also by targeting them indirectly in the spleen. By combining in-depth knowledge of disease biology with advanced nanoengineering, this approach provides a wide array of therapeutic opportunities to explore and exploit.
Therapeutic targeting of neutrophils
Neutrophils are short-lived cells (5–90 h) that constitute an essential part of the innate immune system76. Neutrophils are the most abundant leukocyte subclass in the circulation (50–70%) and are produced in bulk in the bone marrow. The cytoplasm of neutrophils is packed with granules containing antimicrobial substances. Neutrophils are the first responders to acute inflammation and are guided by chemoattractants to inflammation sites77. Upon encountering a pathogen, neutrophils activate multiple defence responses that include degranulation, formation of NETs, and phagocytosis before being cleared by macrophages77. Neutrophils have been identified in atherosclerotic plaques in humans, and neutrophilia is an independent risk factor for cardiovascular disease77-81. Experimental studies support the role of neutrophils in the progression of atherosclerosis82. Neutrophils promote the recruitment of monocytes through the secretion of granule proteins with chemoattractant properties and the production of cytokines by macrophages through the release of NETs, as mentioned before24,77.
A potential therapeutic strategy involves targeting the engagement of neutrophils in atherosclerosis by impairing their transendothelial migration. Wang and colleagues published a noteworthy study in which they developed an albumin-based nanoparticle (size 100 nm) loaded with piceatannol, a tyrosine-protein kinase SYK-inhibiting compound that reduces the adhesion and migration of neutrophils across the endothelium by blocking integrin signalling82. The nanoparticle displayed a high affinity for activated neutrophils attached to the endothelium, whereas unattached neutrophils remained unaffected. In mice injected with TNF to stimulate vascular inflammation, real-time fluorescence intravital microscopy in the post-capillary venules of the cremaster muscle revealed that the injection of the nanotherapy markedly reduced neutrophil adhesion to the endothelium and inhibited vascular inflammation82. Theoretically, this nanotherapeutic approach could be applied to prevent neutrophil migration into the atherosclerotic plaque, although this situation was not investigated. Blocking NETosis or degranulation is another potential strategy to prevent neutrophils from attracting monocytes to the atherosclerotic plaque. However, given that neutrophils are highly involved in combating microbial pathogens, this therapeutic approach might disable the first response system of the body to infection, which warrants precaution for the application of the therapy in the treatment of atherosclerosis.
A more rational approach would involve not interfering with neutrophil functions but exploiting their qualities instead, as reported by Chu and colleagues, who used the above-mentioned albumin-based nanoparticle to specifically target activated neutrophils adherent to the vessel wall83 (FIGS 3e,4e). The nanoparticles were internalized by neutrophils, which subsequently promoted the transport of the particles across the endothelial barrier to the inflammation site. Nanoparticles loaded with different drugs — an NF-κB inhibitor and an antibiotic — were delivered to the inflamed site without affecting the mobility and function of the neutrophils83. If and how the drugs were released from the neutrophils were not investigated. However, given that neutrophils are short-lived cells and are eventually engulfed by macrophages, the drugs might have been released into the extracellular space or might have accumulated in macrophages. Neutrophil-mediated delivery of nanotherapeutics with undisturbed neutrophil function might be an interesting concept to explore in atherosclerosis.
Translational considerations and outlook
Research into nanoimmunotherapy for atherosclerosis and ischaemic heart disease has intensified in the past decade. Nanoengineering and advancing insights in cardiovascular immunobiology have fuelled these developments, which are anticipated to lead to new cures for patients. Various nanoparticle constructs, each with distinct targeting properties, as well as different types of cargo (for example, drugs or siRNA), have been tested in experimental models, mostly in mice. The studies described in this Review highlight the success of this therapeutic approach. Indeed, most studies report impressive decreases in plaque and infarct inflammation. Strikingly, these effects are, without exception, achieved in a matter of weeks or even within a single week. However, modulating immune cell responses and disabling the first line of defence against pathogens are two sides of the same coin. A large therapeutic effect might generate an equally large inhibition of the host defence mechanism. High precision is required to modulate the immune response selectively, which can be accomplished by careful selection of the therapeutic compound in combination with cell-specific targeting properties of the drug-delivery vehicle. Nanoimmunotherapy holds the promise of achieving the goal of targeting the pathogenic process with high specificity without damaging vital immune functions.
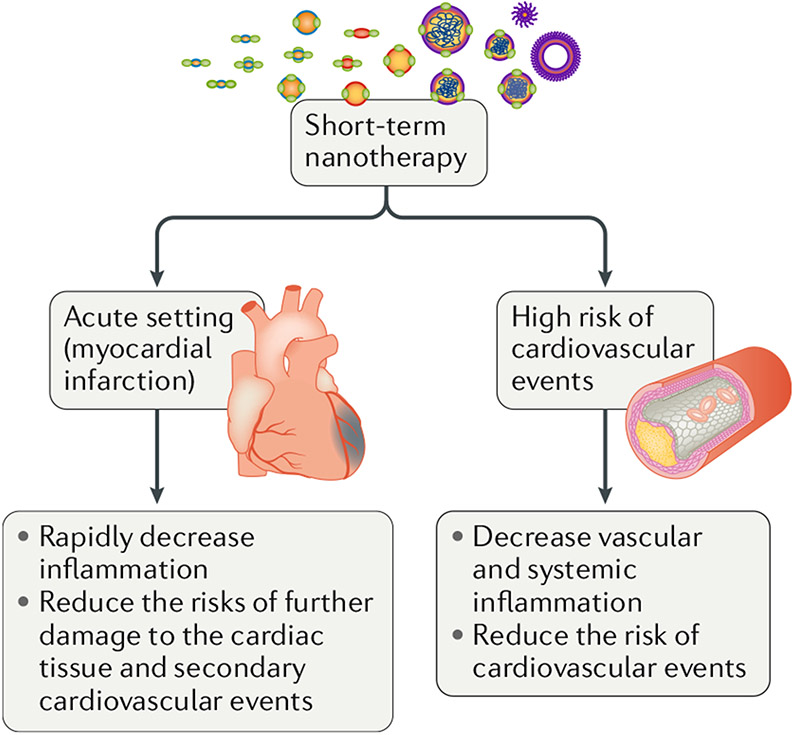
Until a few years ago, immunotherapies had not been considered for clinically targeting the inflammatory process that aggravates atherosclerosis and ischaemic heart disease. However, the CANTOS trial28, which showed that chronic therapy with an IL-1β-blocking antibody reduced the number of cardiovascular events in patients, has opened new therapeutic horizons. The concept of manipulating the immune response to prevent ischaemic heart disease seems valid, which encourages us to develop potential clinical scenarios in which nanoimmunotherapy could benefit patients (FIG. 5). One of these scenarios involves patients with acute coronary syndrome, in which innate immune cells are acutely mobilized from the bone marrow and spleen to invade atherosclerotic lesions and the ischaemic myocardium, where they have harmful effects. This pathogenic process might be prevented with short-term nanoimmunotherapy aimed at decreasing leukocyte migration into these tissues. A second conceivable scenario could be to select patients in whom inflammation is believed to be an important contributing factor to their risk of cardiovascular disease (including increased levels of serum biomarkers such as CRP or imaging biomarkers such as 18F-FDG PET–CT). Short-term induction nanoimmunotherapy could facilitate rapid suppression of plaque inflammation while minimizing the risks associated with chronic immunosuppression.
Fig. 5 |. Potential clinical applications of nanoimmunomodulating therapies.
In an acute setting in which a patient experiences a cardiovascular event such as myocardial infarction, treatment with nanoimmunomodulating drugs could rapidly decrease inflammation and prevent further damage. High-risk patients with elevated biomarkers that are indicative of increased systemic inflammation might be considered for a nanotherapeutic approach to suppress vascular inflammation.
Nanoparticle strategies are particularly suited to target phagocytic cells, which are primarily innate immune cells such as monocytes, macrophages, and dendritic cells; however, effects on adaptive immunity can also be achieved by blunting or promoting co-stimulation, for example. Indeed, one of the latest publications from our group explores this concept60 (FIGS 3b,4b). We are not aware of studies on atherosclerosis in which T cells and B cells are directly targeted by nanodrugs. Santamaria and colleagues reported an alternative approach84,85 in which they developed nanoparticles coated with autoimmune disease-relevant peptides bound to major histocompatibility complexes (pMHCs). These pMHC nanoparticles blunted autoimmune responses by triggering the differentiation and expansion of antigen-specific regulatory T cells in vivo. This approach might also be useful in the context of ischaemic heart disease.
Conclusions
Nanotherapeutic applications to modulate immune responses in cardiovascular disease are rapidly emerging. Studies exploiting innovative nanoimmunomodulation strategies demonstrate promising therapeutic benefits. Lessons learned from the oncology field highlight that benefits to patients rely on clinical translation, which should be prioritized. Major challenges include large-scale manufacturing, the costs inherently associated with nanoparticle drugs, and the design and financing of cardiovascular trials. Small-scale imaging trials might offer an attractive alternative to select therapeutic strategies that are worth evaluating in large patient cohorts37,86. At the present pace and facilitated by large programmes, such as the National Heart, Lung, and Blood Institute’s Program of Excellence in Nanotechnology and the 7th Framework Programme NanoAthero, the development of nanoimmunotherapy can be expected to modify the landscape of cardiovascular medicine in the years to come.
Key points.
The therapeutic focus in atherosclerosis has shifted from lipid lowering to treating inflammation.
In the past decade, novel therapeutic targets for atherosclerosis have been identified as our understanding of the complex immune processes involved in this pathology has increased.
Advances in bioengineering have yielded innovative techniques to produce libraries of nanomaterials that engage immune cells.
The combined advances in nanoengineering and immunobiology have fuelled the development of novel nanoimmunotherapies, mainly aimed at modulating innate immune responses in cardiovascular diseases.
Large studies in animal models focusing on efficacy as well as safety are required to pave the way for clinical translation of cardiovascular nanoimmunotherapy.
Acknowledgements
The authors are supported by grants from the Netherlands Organization for Scientific Research: ZonMW Veni 016156059 (R.D.), ZonMW Vidi 91713324 (W.J.M.M.), and ZonMW Vici 91818622 (W.J.M.M.); AHA grant 17PRE33660729 and the Foundation “De Drie Lichten” in the Netherlands (M.L.S.); AHA grant 16SDG31390007 (C.P.M.); NIH grants R01 HL118440, R01 HL125703, and P01 HL131478 (W.J.M.M.), R01 EB009638 (Z.A.F.), and R01 HL144072 (W.J.M.M. and Z.A.F.); NIH Program of Excellence in Nanotechnology (PEN) Award HHSN368201000045C (Z.A.F.); and the Massachusetts General Hospital Research Scholar Award (M.N.).
RELATED LINKS
7th Framework Programme NanoAthero: http://www.nanoathero.eu.
National Heart, Lung, and Blood institute’s Program of excellence in Nanotechnology: http://nhlbi-pen.net.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Quillard T, Franck G, Mawson T, Folco E & Libby P Mechanisms of erosion of atherosclerotic plaques. Curr. Opin. Lipidol 28, 434–441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melanie CM, Roland L, Harald S, Ruediger N & Wick SG Atherosclerosis research from past to present — on the track of two pathologists with opposing views, Carl von Rokitansky and Rudolf Virchow. Virchows Arch. 449, 96–103 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Moore KJ & Tabas I Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caro CG, Fitz-Gerald JM & S. R Arterial wall shear and distribution of early atheroma in man. Nature 223, 1159–1161 (1969). [DOI] [PubMed] [Google Scholar]
- 5.Malek AM & Alper SL Hemodynamic shear stress and its role in atherosclerosis. JAMA 282, 2035–2042 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Chatzizisis YS et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling. molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 49, 2379–2393 (2007). [DOI] [PubMed] [Google Scholar]
- 7.van Hinsbergh VWM, Eringa EC & Daemen MJAP Neovascularization of the atherosclerotic plaque: interplay between atherosclerotic lesion, adventitia-derived microvessels and perivascular fat. Curr. Opin. Lipidol 26, 405–411 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Swirski FK et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 235, 612–616 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swirski FK et al. Ly-6 Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest 117, 195–205 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yvan-Charvet L et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328, 1689–1693 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosengren A. et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 364, 953–962 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Heidt T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med 20, 754–758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuster JJ et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaiswal S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med 377, 111–121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez Perdiguero E et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors Most haematopoietic cells renew from adult haematopoietic stem cells (HSCs). Nature 518, 547–551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins CS et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med 19, 1166–1172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandl R. et al. Topographic analysis of proliferative activity in carotid endarterectomy specimens by immunocytochemical detection of the cell cycle-related antigen Ki-67. Circulation 96, 3360–3368 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki A & Medzhitov R Control of adaptive immunity by the innate immune system. Nat. Immunol 16, 343–353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duewell P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart CR et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol 11, 155–161 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheedy FJ et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol 14, 812–820 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinkmann V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Warnatsch A, Ioannou M, Wang Q & Papayannopoulos V Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349, 316–320 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Small DM & Shipley GG Physical-chemical basis of lipid deposition in atherosclerosis. Science 185, 222–229 (1974). [DOI] [PubMed] [Google Scholar]
- 26.Childs BG et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koltsova EK et al. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J. Clin. Invest 122, 3114–3126 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridker PM et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med 377, 1119–1131 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Medina C. et al. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat. Commun 7, 11838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y. et al. Augmenting drug-carrier compatibility improves tumour nanotherapy efficacy. Nat. Commun 7, 11221 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pérez-Medina C. et al. In vivo PET imaging of HDL in multiple atherosclerosis models. JACC Cardiovasc. Imag 9, 950–961 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissleder R, Nahrendorf M & Pittet MJ Imaging macrophages with nanoparticles. Nat. Mater 13, 125–138 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Barenholz Y. Doxil® — The first FDA-approved nano-drug: lessons learned. J. Control. Release 160, 117–134 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Wagner V, Dullaart A, Bock AK & Zweck A The emerging nanomedicine landscape. Nat. Biotechnol 24, 1211–1217 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Zhang L. et al. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther 83, 761–769 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald K. et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: A randomised, single-blind, placebo-controlled, phase 1 trial. Lancet 383, 60–68 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Valk FM et al. Prednisolone-containing liposomes accumulate in human atherosclerotic macrophages upon intravenous administration. Nanomedicine 11, 1039–1046 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Valk FM et al. Liposomal prednisolone promotes macrophage lipotoxicity in experimental atherosclerosis. Nanomedicine 12, 1463–1470 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Milla P, Dosio F & Cattel L PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab 13, 105–119 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Parodi A. et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol 8, 61–68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez PL et al. Minimal ‘Self’ peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 339, 971–975 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lobatto ME et al. Atherosclerosis targeting mechanism of long-circulating nanoparticles established by multimodal imaging. ACS Nano 9, 1837–1847 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winter PM et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with αvβ3-integrin-targeted nanoparticles. Circulation 108, 2270–2274 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Winter PM et al. Endothelial αvβ3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol 26, 2103–2109 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Kamaly N et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano 10, 5280–5292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fredman G et al. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med 7, 275ra20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulder WJM et al. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn. Reson. Med 58, 1164–1170 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Gaytan BL et al. Real-time monitoring of nanoparticle formation by FRET imaging. Angew. Chemie Int. Ed 56, (2923–2926 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang J. et al. Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc. Natl Acad. Sci. USA 113, E6731–E6740 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geissmann F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serbina NV & Pamer EG Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol 7, 311–317 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Hamers AAJ et al. Bone marrow–specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ. Res 110, 428–438 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Hanna RN et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ. Res 110, 416–427 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore KJ, Sheedy FJ & Fisher EA Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol 13, 709–721 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hilgendorf I, Swirski FK & Robbins CS Monocyte fate in atherosclerosis. Arterioscler. Thromb. Vasc. Biol 35, 272–279 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Leuschner F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol 29, 1005–1010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee H. et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat. Nanotechnol 7, 389–393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nahrendorf M, Pittet MJ & Swirski FK Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Getts DR et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med 6, 219ra7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lameijer M et al. Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nat. Biomed. Eng 2, 279–292 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seijkens TTP et al. Targeting CD40-induced TRAF6 signaling in macrophages reduces atherosclerosis. J. Am. Coll. Cardiol 71, 527–542 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dahlman JE et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol 9, 648–655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sager HB et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci. Transl. Med 8, 342ra80 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Honold L & Nahrendorf M Resident and monocyte-derived macrophages in cardiovascular disease. Circ. Res 122, 113–127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis DR et al. Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo. Proc. Natl Acad. Sci. USA 112, 2693–2698 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beldman TJ et al. Hyaluronan nanoparticles selectively target plaque-associated macrophages and improve plaque stability in atherosclerosis. ACS Nano 11, 5785–5799 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakashiro S et al. Pioglitazone-incorporated nanoparticles prevent plaque destabilization and rupture by regulating monocyte/macrophage differentiation in ApoE−/− mice. Arterioscler. Thromb. Vasc. Biol 36, 491–500 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Brown JD & Plutzky J Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation 115, 518–533 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Courties G. et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J. Am. Coll. Cardiol 63, 1556–1566 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krausgruber T et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol 12, 231–238 (2011). [DOI] [PubMed] [Google Scholar]
- 71.Duivenvoorden R. et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat. Commun 5, 3065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tobert JA Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov 2, 517–526 (2003). [DOI] [PubMed] [Google Scholar]
- 73.Katsuki S. et al. Nanoparticle-mediated delivery of pitavastatin inhibits atherosclerotic plaque destabilization/rupture in mice by regulating the recruitment of inflammatory monocytes. Circulation 129, 896–906 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Tang J. et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci. Adv 1, e1400223 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dutta P. et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J. Exp. Med 212, 497–512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tak T, Tesselaar K, Pillay J, Borghans JAM & Koenderman L What’s your age again? Determination of human neutrophil half-lives revisited. J. Leukoc. Biol 94, 595–601 (2013). [DOI] [PubMed] [Google Scholar]
- 77.Soehnlein O, Steffens S, Hidalgo A & Weber C Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol 17, 248–261 (2017). [DOI] [PubMed] [Google Scholar]
- 78.Drechsler M, Megens RTA, van Zandvoort M, Weber C & Soehnlein O Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122, 1837–1845 (2010). [DOI] [PubMed] [Google Scholar]
- 79.Ionita MG et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler. Thromb. Vasc. Biol 30, 1842–1848 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Hellings WE et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation 121, 1941–1950 (2010). [DOI] [PubMed] [Google Scholar]
- 81.Guasti L. et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularization: a systematic review on more than 34,000 subjects. Thromb. Haemost 106, 591–599 (2011). [DOI] [PubMed] [Google Scholar]
- 82.Wang Z, Li J, Cho J & Malik AB Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat. Nanotechnol 9, 204–210 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chu D, Gao J & Wang Z Neutrophil-mediated delivery of therapeutic nanoparticles across blood vessel barrier for treatment of inflammation and infection. ACS Nano 9, 11800–11811 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singha S. et al. Peptide-MHC-based nanomedicines for autoimmunity function as T cell receptor microclustering devices. Nat. Nanotechnol 12, 701–710 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Clemente-casares X. et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530, 434–440 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Fayad ZA et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 378, 1547–1559 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]