Abstract
While health care policies are frequently signed into law well before they are implemented, such lags are ignored in most empirical work. This paper demonstrates the importance of implementation lags in the context of Medicare Part D, the prescription drug benefit that took effect two years after it was signed into law. Exploiting the differential responses of chronic and acute drugs to anticipated future prices, I show that individuals reduced drug utilization for chronic but not acute drugs in anticipation of Part D’s implementation. Accounting for this anticipatory response substantially reduces the estimated total treatment effect of Part D.
Keywords: Medicare Part D, Prescription drugs, Anticipation effects, Intertemporal substitution, Forward-looking behavior, H51, I13, I18
1. Introduction
Many health care policies are implemented with a significant lag from their enactment date, including major recent reforms such as the expansion of prospective payment for Medicare under the Balanced Budget Act, the introduction of Medicare Part D, and the Affordable Care Act (ACA), which was signed into law in March 2010, but did not have its major provisions implemented until 2014.1 Understanding the consequences of these “implementation lags” is important from both a policy and program evaluation perspective. The ACA and other policies that are announced in advance may begin to affect individual and firm behaviors ahead of implementation. Yet many program evaluations estimate only contemporaneous program effects – comparing outcomes pre- and post-policy implementation – often ignoring the anticipatory effects of policy announcements and resulting in potentially biased estimated policy impacts.2 Moreover, implementation lags themselves are policy decisions, but there is little economic evidence on the consequences of these lags.
In this paper, I examine anticipation effects in the case of Medicare Part D, which went into effect two years after it was signed into law. Part D, which added outpatient prescription drug coverage to Medicare, was the largest single expansion of the Medicare program since its inception. At a cost of $32 billion in the first year, Part D substantially reduced the out-of-pocket price of drugs for Medicare beneficiaries. While Part D was implemented in January 2006, it was signed into law in December 2003 as part of the widely publicized Medicare Prescription Drug, Improvement, and Modernization Act (MMA). Given this two-year lag between when the program was announced and when it was implemented, it is possible that forward-looking individuals changed their drug consumption behavior before Part D took effect in anticipation of future subsidized drug coverage. The direction of this pre-implementation utilization response is theoretically ambiguous due to opposing intertemporal substitution and income effects. On the one hand, individuals may have strategically deferred initiating new therapies or reduced the use of ongoing medications until after Part D was implemented, when drugs would be cheaper. On the other hand, since Part D lowered the total cost of long-term therapies and increased lifetime income, individuals may have begun drug therapies prior to implementation that they would not have otherwise started. As a result, an anticipatory effect could manifest as a dip or a spike in drug utilization in the period immediately before Part D took effect.
Prior studies of Part D have implicitly assumed a myopic response to the policy by comparing outcomes before and after the 2006 implementation date, largely ignoring the possibility of behavioral responses in the intervening years of 2004 and 2005.3 I find evidence that previous estimates of the demand response to Part D have been overstated by not accounting for anticipatory effects during these years. More generally, a large literature has estimated the price elasticity of demand for prescription drugs by focusing solely on contemporaneous responses to current prices (summarized in Goldman et al., 2007). Yet individuals’ demand for prescription drugs might also respond to future prices. This paper contributes to the broader literature on the price-responsiveness of medical care by exploiting the advanced announcement of Part D as a new test of forward-looking behavior for drug demand.
To quantify the anticipation effects of Part D, I estimate the causal demand response to the announcement of Part D in 2003.4 Using detailed drug utilization data from the Medicare Current Beneficiary Survey (MCBS) and the Medical Expenditure Panel Survey (MEPS) that spans the pre-announcement to post-implementation periods, my empirical strategy consists of several tests which exploit heterogeneity in the predicted responses of drugs and individuals to anticipated future price changes driven by Part D.
First, in my main approach, I test for an anticipatory response by exploiting the predicted differential responses of chronic and acute drugs to anticipated future prices. The intuition for this approach is that demand for acute drugs (e.g. antibiotics), which treat illnesses that require immediate treatment, should be relatively insensitive to future prices since there is little scope for postponing treatment into future periods. On the other hand, chronic drugs (e.g. statins), treat long duration illnesses and produce health benefits in many periods. Consequently, the use of chronic drugs can be more readily deferred to later time periods, making demand for chronic drugs likely to be more responsive to future prices than acute drugs.5 Both acute and chronic drugs have been shown to be highly responsive to contemporaneous prices in other settings (e.g. see Landsman et al., 2005 and Skipper, 2013 for acute drugs and Goldman et al., 2004 for chronic drugs), thus finding relatively larger and sharp changes in the utilization of chronic drugs compared to acute drugs after the policy announcement would provide evidence that individuals are responding to the anticipated future price changes brought about by Part D.
Second, I add complementary evidence using a difference-in-difference strategy comparing changes in utilization before and after the announcement of Part D for the elderly aged 65 and over who are currently eligible for Medicare relative to those under age 65, who are not yet eligible. The Medicare-ineligible group is less affected by the impending implementation of Part D and would be expected to be less responsive to the future price changes. Third, I compare the utilization response to the announcement of Part D for those with and without employer-sponsored drug insurance. Individuals with employer-sponsored drug insurance were the least likely group to enroll in Part D (only 19% enrolled in 2006), thus their drug use should be relatively unaffected by the announcement of the program in 2003. Finally, I also compare the effects of the announcement on utilization for Medicare beneficiaries across age and income groups. If Part D’s announcement led to changes in drug utilization, one would predict the largest responses for the youngest Medicare beneficiaries, for whom the health costs of delaying treatment are lowest relative to older beneficiaries, and individuals with low income, who are the most liquidity constrained.
I find a substantial decline in overall drug use by the elderly following the announcement of Part D of approximately 6%. This comes after several years of consistent upward growth in drug utilization. Then, in the implementation year, drug utilization reverts upward to the long-run utilization trend (see Fig. 1). These findings are consistent with a dominating intertemporal substitution effect – that is, consumers responding to expected future price reductions by shifting the timing of drug use to future periods. As predicted, this reduction in utilization after Part D’s announcement is driven entirely by a reduction in chronic drug use. Acute drug use does not respond to the announcement. However, the use of both acute and chronic drugs increases after the implementation of Part D. This is consistent with the main theoretical prediction that chronic drugs respond to both current and future prices, whereas acute drugs are only responsive to current price. The observed pattern is supportive of the interpretation of the pre-implementation reduction in drug use as an anticipatory response. Moreover, the results from the age group comparisons show that the decline in drug utilization following the announcement only occurs for Medicare beneficiaries over 65 and does not occur for those aged 50–58 I also find that the anticipatory effects are concentrated among the groups of individuals that would be predicted to be most responsive to the change in future drug prices: those without employer-sponsored drug insurance, the youngest Medicare beneficiaries, and those with below-median incomes.
Fig. 1.
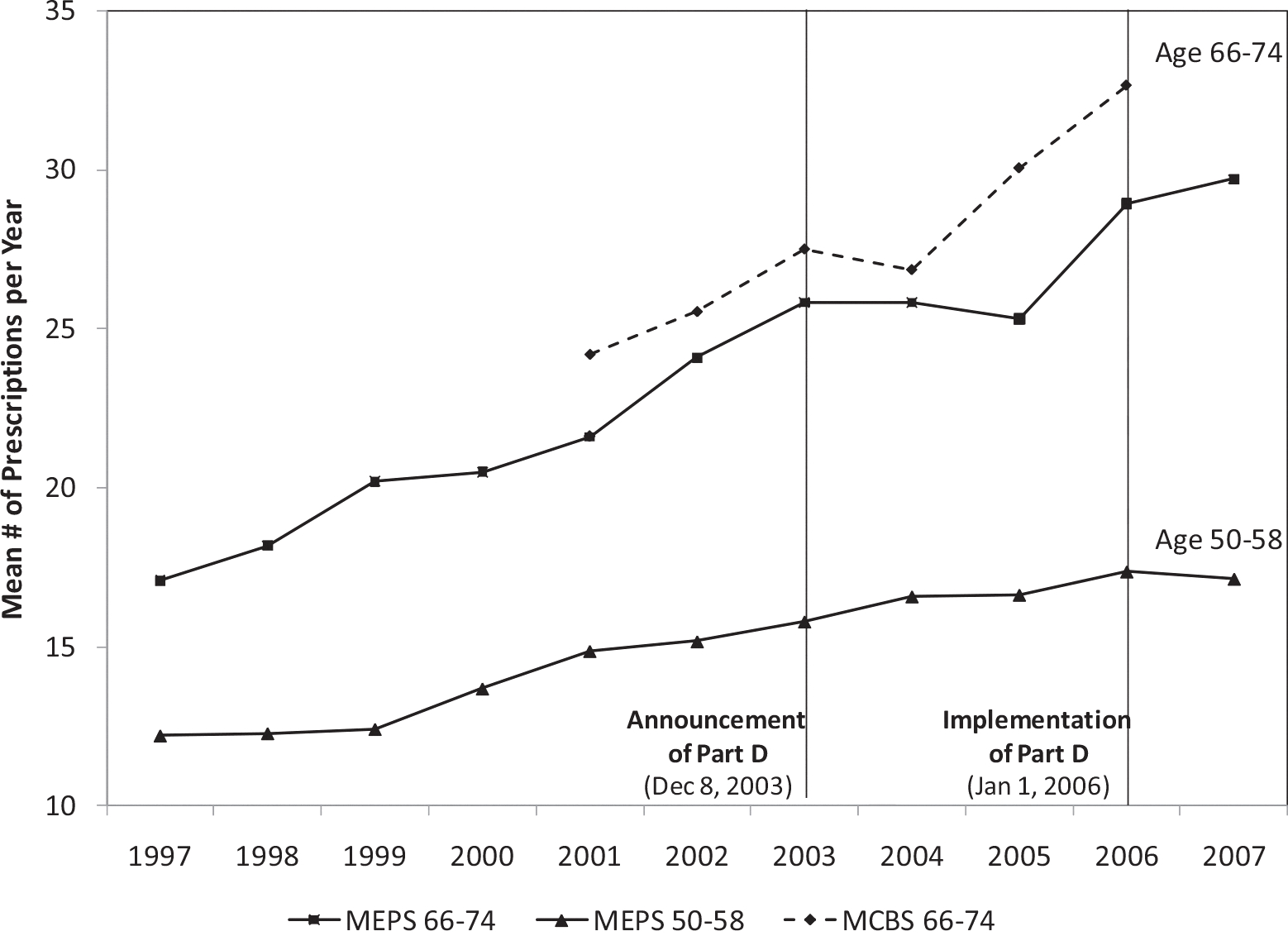
Mean annual drug utilization in MEPS and MCBS.
Notes: Author’s calculation using MEPS 1997–2007 and MCBS 2001–2006, non-institutionalized population aged 66–74, weighted. Includes individuals who appear in the sample for 2 or more consecutive years.
Finally, I evaluate two alternative supply-side explanations for the observed reduction in utilization after the announcement. First, pharmaceutical firms may have begun to increase drug prices as soon as the law was passed in anticipation of the reduced price-sensitivity of consumers under Part D, thus generating a contemporaneous negative demand effect. However, I do not find empirical support for this explanation, given that price growth changes after 2003 were negative and statistically insignificant for drugs differentially used by Medicare beneficiaries. I also consider the possibility that insurers discontinued drug coverage or reduced benefit generosity before the implementation of Part D, thus increasing out-of-pocket costs. While I find a small decline in certain types of drug insurance coverage after the announcement, I show that this change is likely driven by individuals’ take-up decisions and can thus be considered part of the demand-side anticipation effect. Moreover, neither of these supply-side responses can explain the differential effect for chronic and acute drugs. Taken together, the evidence on drug utilization responses to Part D’s announcement across drug types, age groups, insurance status, and income levels demonstrate that drug utilization responds to predictable changes in future drug prices in an economically meaningful way. My analysis implies that the total estimated treatment effect on utilization in the first year of the Part D program is reduced by about one-half when anticipatory effects are taken into account.
This study has important parallels for the ACA, which implemented its key provisions with a lag from when they were announced. As one example, the “Cadillac tax” on high cost health insurance plans, which takes effect in 2020, may have already led insurers to lower insurance premiums and firms to switch to lower cost health insurance plans (Abelson, 2013; Piotrowski, 2013) in anticipation of the future tax. Future studies of the effects of this tax on premiums, wages and other outcomes will need to account for such pre-implementation responses, since comparisons of outcomes immediately before and after 2020 will miss the full impact of this policy. Similarly, other anticipatory responses could have occurred in the run-up to ACA implementation in 2014 with respect to individuals’ decisions about whether to purchase insurance and healthcare services, premium and pricing decisions by insurers and providers, decisions by employers about whether to offer insurance coverage, and decisions by individuals and firms about employment, among others. The results of this study illustrate the importance of accounting for anticipation effects when policies are announced in advance of implementation, which has specific implications for the evaluation of Part D and broader implications for the ACA and many other policies. By extension, this paper also relates to public programs in which future eligibility can be anticipated, such as Medicare coverage which can be perfectly anticipated as one approaches age 65. In that case, individuals may defer some medical care until they receive Medicare coverage at age 65 (Card et al., 2008).
This paper also contributes to a burgeoning literature that examines whether individuals are forward-looking in responding to future prices of medical care that are anticipated when individuals change their insurance status or plan (Aron-Dine et al., 2015; Cabral, 2015; Gross, 2009; Long et al., 1998) or face changing prices throughout the calendar year due to non-linear insurance contracts (Einav et al., 2015; Kowalski, 2016).6 For example, Aron-Dine et al. (2015) studies within-year price changes using variation in the timing of when employees join firms within a calendar year, which generates different expected year-end prices for medical care due to non-linear insurance contracts. They use a similar strategy to estimate drug utilization responses to future prices exploiting variation in the timing of when individuals first enroll in Medicare based on their birth month. The authors find that new employees and new Medicare beneficiaries are highly responsive to expected year-end prices for medical care and prescription drugs, estimating demand elasticities with respect to future price of −0.16 and −0.25, respectively. These elasticities are not directly comparable to this paper’s results since within contract-year price changes are mechanically dependent on consumption decisions (i.e., consuming more at the beginning of the contract-year makes it more likely that the individual will meet the deductible and end the year in the coinsurance phase of the insurance contract), whereas the across-year changes driven by the introduction of Part D are not. However, these estimates are supportive of forward-looking behavior in drug demand. Other recent papers test for forward-looking behavior by estimating structural models of prescription drug consumption over the non-linear insurance contract of Medicare Part D – finding mixed results. Two studies (Dalton, et al., 2015; Abaluck, et al., 2015) find that Medicare beneficiaries are largely myopic with respect to expected year-end prices, as evidenced, for example, by the discontinuous drop in drug utilization when beneficiaries enter the Part D “donut hole”. These papers, similar to Aron-Dine et al. (2015), focus on within contract-year variation in price. On the other hand, Einav et al. (2015) show striking evidence of forward-looking behavior when considering across-year price changes. They find that individuals defer some drug treatments at the end of the calendar year (around the time when they would enter the donut hole) until January, when the coinsurance schedule resets and drugs are cheaper. This intertemporal substitution response is analogous to deferring drug use after the announcement of Part D until the program was implemented in 2006. They argue that this type of anticipatory response is important to account for when evaluating alternative coinsurance schedules, such as the ACA’s provision to fill in the donut hole in 2020. When they account for across-year substitution in a counterfactual policy simulation, the effect of filling in the donut hole on utilization is reduced by two-thirds. I find that accounting for an analogous type of intertemporal substitution is also critical for estimating the effect of the introduction of Part D on utilization – it reduces the effect by about one-half. This smaller effect may be due to the slightly longer time horizon over which individuals may be deferring treatments.
These recent studies contrast with a long standing literature in economics that estimates the price elasticity of medical care assuming that individuals respond to a single static price. Relative to these few previous studies, the advantages of the Part D setting in estimating forward-looking behavior are that the price change resulting from the introduction of Part D is exogenous to individual decisions and health status; the announcement of the policy was widely publicized, reducing the need for strong informational assumptions about the ability of individuals to forecast future price changes; and drug use is a highly prevalent and highly frequent outcome for the elderly, so there is broad scope for a response. In addition, this paper provides one of the first tests of forward-looking behavior using policy-variation for prescription drug demand.
2. Background
2.1. Program coverage and participation
Medicare is an over $500 billion federal program that provides health insurance to the elderly, aged 65 and over, and qualifying non-elderly disabled individuals. The traditional program consists of Part A and Part B, which together cover most medical services including physician-administered drugs such as chemotherapy. Outpatient prescription drugs were not covered by traditional Medicare until the introduction of Part D in 2006.7 After the implementation of Part D, Medicare’s share of total national spending on prescription drugs increased from 2% in 2005 to 22% in 2006 (KFF, 2007).
Enrollment in Part D is voluntary. By January 2007, 54% of Medicare beneficiaries had enrolled in Part D (KFF, 2007), over one-third of whom did not have any source of drug insurance two years earlier (Levy and Weir, 2010). Individuals who were dually eligible for Medicaid and Medicare were automatically enrolled in Part D and most Medicare Advantage (Part C) plans began to offer Part D benefits (Levy and Weir, 2010). Medicare beneficiaries who had received drug benefits from employer-sponsored insurance were least likely to take-up Part D, with only 19 percent enrolling in 2006 (Levy and Weir, 2010). This low participation rate can likely be attributed to the employer Retiree Drug Subsidy. Levy and Weir (2010) estimate that the fraction of the elderly who were drug-uninsured declined from 24% to 7% in the first year of Part D.
2.2. How did Part D lower drug costs?
Part D is administered by stand-alone private drug plans (PDPs) and Medicare Advantage plans (MA-PDs) that compete for Medicare enrollees within defined regions of the U.S. The program lowered the out-of-pocket cost of drugs for enrollees primarily through two mechanisms. The first was through the coinsurance design. All plans must offer a benefit that is at least actuarially equivalent to a standard benefit defined by Medicare. The standard benefit provides a drug subsidy that is non-linear in annual expenditures. Plans typically require an annual premium, which was on average $384 in 2006 (KFF, 2006). The first $250 of drug expenditures are borne fully out-of-pocket, while the next $2000 are subsidized by 75 percent. After reaching a spending threshold of $2250, the beneficiary enters what is known as the “donut-hole” in which he again bears 100 percent of the costs. After $5100 in total drug spending, catastrophic coverage begins and a 95 percent subsidy takes effect for all remaining expenditures for the year. Low-income beneficiaries receive additional subsidies, such as reduced premiums and deductibles, smaller coinsurance, and subsidized coverage in the “donut hole” region.8 In addition to lowering enrollees’ out-of-pocket payments mechanically through the coinsurance design, PDPs and MA-PDs could also lower spending by using their bargaining power to negotiate lower prices from manufacturers and pharmacies. Duggan and Scott Morton (2010) show evidence that this type of strategic behavior has led to a reduction in prices of brand name drugs by approximately 20% for enrollees who moved from not having drug insurance to Part D. Together, the coinsurance design and strategic behavior of plan providers have contributed to a 13 to 22% decline in the share of drug spending paid out-of-pocket by Medicare beneficiaries following the implementation of Part D (Ketcham and Simon, 2008; Yin et al., 2008).
2.3. Part D utilization effect estimates
Given the large decline in the out-of-pocket price of drugs after Part D went into effect, we would expect to see an increase in the demand for prescription drugs. High rates of drug non-compliance and sub-optimal take-up of medically beneficial therapies among the elderly prior to Part D (Adams et al., 2001; Mojtabai and Olfson, 2003), combined with moral hazard effects, suggest that this utilization effect potentially could be large. In other contexts, a large body of literature has estimated insurance price elasticities of drug demand ranging from −0.2 to −0.6 (Goldman et al., 2007).
Several studies have evaluated the impact of the implementation of Part D on drug utilization. The three most widely cited studies employ a difference-in-difference strategy comparing drug use for the elderly aged 65+ and the near-elderly (who are not yet eligible for Medicare) right before and after the implementation of Part D (Ketcham and Simon, 2008; Lichtenberg and Sun, 2007; Yin et al., 2008). Using large samples of pharmacy claims, these studies have estimated that drug utilization increased by 4–10% in the first or second year of the program9 with implied elasticities ranging from −0.2 to −0.7.10
One critical limitation of these previous difference-in-difference studies is that they do not possess a long enough time series of data to account for possible anticipation effects. In each of these studies, the “pre-period” begins in 2004 – nearly one year after the announcement of Part D. If there were anticipation effects, the baseline period is effectively “treated.” The DID estimator will overstate the program effect if the announcement of Part D caused Medicare beneficiaries to shift the timing of drug purchases until after implementation, leading to a transitory pre-implementation decline in utilization. The near-elderly group is not an adequate control for anticipatory responses by the elderly because they would not be expected to respond to the announcement with the same intensity as those who are already Medicare-eligible. By not accounting for anticipatory effects, the DID estimate will falsely attribute the increase in drug use following the transitory dip to the Part D program effect. This identification problem is structurally similar to the “Ashenfelter dip” that has been widely discussed in the job training literature.11 Conversely, if the announcement caused beneficiaries to increase drug use in the pre-implementation period, the DID estimator will understate the program effect, since part of the real impact of Part D occurs before the program is implemented. Thus, using only a small window of data around the implementation date generates biased treatment effect estimates if there are anticipation effects.
3. Accounting for anticipatory responses
3.1. Conceptual framework
In contrast to the previous studies of Part D, I take a more dynamic view of drug demand. Given that the lag in program implementation allowed individuals to forecast price changes two years in advance, individuals’ demand for prescription drugs may respond not only to current prices at the time of implementation, but also to expectations of future prices at the time of the policy announcement. Thus, estimates of the total treatment effect of Part D should combine the effects of both the announcement and implementation.
The notion that future prices can affect present behavior is well-established. This idea is central to models of dynamic commodity demand and intertemporal labor supply.12 Similarly, the demand for healthcare is part of a life-cycle decision-making process (Grossman, 1972). For forward-looking individuals, current demand should be a function of everything that is known about the lifetime path of prices. All else equal, individuals should allocate greater drug use to periods when drugs are cheaper. The announcement of Part D in 2003 changed individuals’ expectations about the future path of prices for drugs. Since this reform represented a permanent change, it lowered the entire stream of out-of-pocket prices in all future periods beginning on the implementation date. The life-cycle model predicts that individuals should have immediately used this new information to re-optimize their consumption path.13
While the life-cycle model suggests that we should observe a change in drug utilization following the announcement of Part D, from an empirical standpoint it is difficult to disentangle aggregate changes in drug utilization caused by anticipatory behavior from other consumption fluctuations. I propose a test for an anticipatory response (or equivalently, a test for forward-looking life-cycle behavior) that exploits the fact that different types of drugs – namely, chronic and acute drugs – should respond differentially to anticipated future price changes. This will form the basis of a difference-in-difference strategy.
For this analysis, the key difference between acute and chronic drugs is their average duration of use. Acute drugs (e.g. antibiotics) treat illnesses that are largely unpredictable, short in duration, and require immediate treatment; meanwhile, chronic drugs treat long-term illnesses. Put differently, acute drugs typically produce a health benefit in the current period, while chronic drugs can produce health benefits in many periods. Since there is not much scope for shifting acute drug use to future periods, anticipated future prices should have little impact on current use. Thus, the announcement of Part D is likely to have a much larger effect (in absolute value) for chronic drugs than for acute drugs. Moreover, this utilization effect could be either negative or positive due to opposing intertemporal substitution and income effects.
First, the announcement could produce a negative utilization response if intertemporal substitution effects dominate: individuals delay the use of some drugs until after the program is implemented, when the out-of-pocket price is lower. For example, individuals may have asked their physicians to delay the initiation of chronic treatments for which they were newly eligible or reduced their adherence to “less-essential” medications that they believed could be suspended temporarily without posing an immediate health risk. Postponement of acute drug use is less likely given the reasoning noted above. It should be emphasized that in order for the intertemporal substitution effect to generate a pre-reform decline in utilization relative to the counterfactual trend, it must be the case that elderly who would have otherwise taken a drug or initiated a new treatment in the absence of Part D decided to postpone treatment after learning of the announcement. One concern is that, for drugs that are taken for an entire lifetime, this response would not fit a standard model of rational behavior.14 Nevertheless, most drugs are used for a finite period of time, at least in expectation, because they fail to be effective with some probability, better drugs enter the market, or their usefulness is eventually outlived. Thus, given the uncertainty of treatment duration, it may be optimal to defer use or experiment with new treatments of unknown effectiveness in periods when the price of drugs is lower.15
Second, there will be a positive anticipatory response to Part D if income effects dominate. Part D increased lifetime income by lowering the cost of drugs in each period. Since this income effect is distributed across the life-cycle, it could increase drug use (and other consumption) in any period after the announcement. Thus, individuals could begin drug therapies that they would not have otherwise started or initiate them earlier. Importantly, the magnitude of the income effect varies with the size of the expected benefit of Part D. Chronic drug users should anticipate a large subsidy from Part D given the expected persistence in their drug use; whereas purely acute drug users, facing uncertain future health shocks, may anticipate a much smaller subsidy in expectation. Again, chronic drugs are predicted to be more responsive to the future price change than acute drugs since the income effect will be larger. There is also an intra-temporal substitution effect. To the extent that individuals take into account the entire cost of a therapy before deciding whether to initiate a treatment, substitution between drug therapies and other consumption may lead to an increase in chronic drug use. These positive effects would be reinforced for drugs that exhibit strong complementarities in marginal health benefits across time periods. The intuition for this response is analogous to the model for “rational addiction” (Becker and Murphy, 1988). These drugs have the feature that a larger stock of past consumption raises the marginal health benefit from current consumption. Thus individuals who anticipate increasing drug use in the future (because of an anticipated future reduction in price) should increase use in the current period in order to increase the benefit in the next period.
It should be noted that a reduction in the use of chronic drugs relative to acute drugs prior to Part D implementation, which I will observe in the data, can be generated only by a dominating intertemporal substitution effect combined with the existence of anticipation effects. I will find that chronic drug utilization did react to Part D’s announcement while acute drug utilization did not. Furthermore, the evidence for anticipation effects is reinforced by findings that this utilization change occurred only for those without employer prescription drug coverage and for those eligible or nearly-eligible for Medicare.
3.2. Salience and timing of the Part D announcement
My test of anticipatory behavior relies on two informational assumptions: first, that the announcement of Part D was salient; second, that the timing of the announcement was a surprise. Part D was signed into law as part of the MMA on December 8, 2003, but the program did not actually begin until January 1, 2006. This implementation date was stipulated by the MMA and thus was known in advance. Given the wide media coverage of the passage of the legislation, it is reasonable to assume that many elderly anticipated a reduction in their future drug expenditures. In a monthly Kaiser Family Foundation Poll, nearly 75% of the elderly said they followed the Medicare prescription drug benefit “very closely” or “somewhat closely” after February 2003 (see Appendix Figure A.1). Moreover, the elderly followed the debate most closely in the month that the law was signed and least carefully in the months after it was passed, suggesting awareness that the debate had ended. Another KFF poll quizzed individuals about whether the bill had passed 2 months after it was signed into law. 32% of the elderly aged 65+ responded correctly, while 41% were uncertain (among the non-elderly, 21% responded correctly). Even if elderly individuals were not fully aware of the passage of the MMA, physicians and family-members may have been better informed. Furthermore, the size of the benefit was immediately known as major news sources such as the New York Times (Pear, 2003) reported the precise coinsurance schedule on the day the bill was signed into law.
Finally, the timing of the announcement was unanticipated, which is necessary to pin down the time period in which to estimate the anticipatory response. Adding prescription drug coverage to Medicare had been the subject of nearly two decades of debate and failed legislative proposals (Oliver et al., 2004). The prescription drug bill was highly controversial throughout the debate and press accounts suggest that it was far from certain that a bill would pass at any point in time. The final conference bill that passed in the House and Senate did so with very thin margins, 220 to 215 and 54 to 44 respectively. Thus anticipatory responses are unlikely to have occurred prior to the passage of the law.
4. Data and descriptive statistics
4.1. Data description
The primary data source for this paper is the Medicare Current Beneficiary Survey (MCBS) Cost and Use module for 2001–2006 and a secondary source is the Medical Expenditure Panel Survey (MEPS) for 1997–2007. Both surveys collect nationally representative data on non-institutionalized individuals’ healthcare utilization and expenditures. The MCBS sample consists of only Medicare beneficiaries, while the MEPS surveys households of all ages. Importantly, both datasets provide detailed records for each prescription drug purchased (including refills) during the calendar year including the drug name and therapeutic drug class. The MCBS will serve as the primary data source for the analysis since the sample size for the population of interest is more than twice as large as in the MEPS. One key advantage of the MEPS is that it samples non-disabled individuals under age 65, which serves as an informative comparison group for the Medicare-eligible elderly. However, since the sample size in the MEPS is small, it does not allow for obtaining precise estimates; therefore these data will be used in only limited analyses where the near-elderly are used as a comparison group.
From the initial MCBS sample of 74,139 observations, I exclude individuals with incomplete drug utilization records for the calendar year. This involves dropping individuals who were not interviewed in every round, had partial year Medicare eligibility, or became institutionalized (20.6% of the sample). I also exclude individuals with missing demographic characteristics. The final MCBS sample of Medicare beneficiaries aged 66–85 includes 41,475 observations.16 In many specifications, I use a sample of the youngest Medicare beneficiaries aged 66–74 which includes 20,072 observations.
One caveat is that, unlike other studies of Part D that use pharmacy claims records, the drug utilization data used in this paper are self-reported and thus subject to reporting error. I can estimate the severity of misreporting using the 2006 MCBS. In 2006, survey records were matched to Medicare administrative data for the first time for those enrolled in Part D. The MCBS identifies which drug records are extracted from the survey only, the claims only, or both the survey and claims. Among all prescription claims, 18.9% of prescription records are reported only in administrative claims and thus would have been absent from the survey data in previous years. Nevertheless, since the emphasis of my analysis is on changes in utilization and not on levels, the misreporting error will not confound my estimates if the magnitude of misreporting does not vary across years and is orthogonal to my explanatory variables of interest. It should be noted that in my analysis I exclude claims-only drug records in 2006 for comparability with previous years.
Despite this limitation, there are a number of advantages to using survey data over pharmacy claims. The survey data provide a nationally representative sample, richer demographic and health insurance status characteristics, and importantly, a long enough time frame to examine utilization patterns before the announcement of Part D. Also, as noted in Ketcham and Simon (2008), Part D may have changed the extent to which people use multiple pharmacies or it may have induced people to use different pharmacies than their usual store. Thus utilization changes may be better captured in nationally representative survey data than using data from a single pharmacy.
4.2. Descriptive statistics
In Table 1, descriptive statistics are reported for sub-groups from the MCBS and MEPS. Column 4 presents characteristics of the elderly aged 66–74 from the MCBS, which is the sample used in most of the analyses. Prescription drug use is highly prevalent among this group. 92 percent of the elderly purchase at least one prescription each year – filling on average 28 prescriptions at a total cost of $1789. In addition to receiving Medicare coverage, 11 percent of the sample are dually enrolled in Medicaid and 67 percent are covered by supplementary private insurance plans such as Medigap or retiree employer benefits. I estimate that 16 percent of elderly did not have any drug insurance coverage prior to 2006.17 This is a slightly lower estimate than other sources. For example, Levy and Weir (2010) find that 24% are drug-uninsured in the Health and Retirement Survey in 2004. Comparing the MCBS to the MEPS for the same age group (Columns 3 and 4) demonstrates that mean utilization and expenditures are slightly higher in the MCBS. This may be partially explained by differences in demographic characteristics across the samples. The MCBS sample is slightly older and more educated.
Table 1.
Descriptive statistics, 2001–2006.
| MEPS |
MCBS |
|||||
|---|---|---|---|---|---|---|
| Age 50–58 |
Age 59–64 |
Age 66–74 |
Age 66–74 |
Acute |
Chronic |
|
| (1) | (2) | (3) | (4) | (5) | (6) | |
|
| ||||||
| Outcomes | ||||||
| Any prescriptions filled | 0.76 | 0.83 | 0.90 | 0.92 | 0.55 | 0.86 |
| Avg. # of prescriptions | 16.18 | 20.83 | 25.63 | 28.21 | 3.26 | 22.14 |
| Annual drug expenditures | 1,106.60 | 1,354.87 | 1,600.07 | 1,789.24 | 161.57 | 1,379.97 |
| Avg. tot. paid per script (excl. 2006) | 60.94 | 63.27 | 62.19 | 67.94 | 48.01 | 69.62 |
| Fract. paid out-of-pocket (excl. 2006) | 0.47 | 0.47 | 0.56 | 0.45 | 0.54 | 0.43 |
| Demographics | ||||||
| Age | 53.80 | 61.33 | 69.83 | 70.29 | 70.35 | 70.31 |
| Male | 0.48 | 0.48 | 0.45 | 0.45 | 0.41 | 0.43 |
| Black | 0.10 | 0.10 | 0.09 | 0.09 | 0.08 | 0.09 |
| Hispanic | 0.08 | 0.07 | 0.07 | 0.07 | 0.06 | 0.06 |
| No college | 0.46 | 0.53 | 0.62 | 0.56 | 0.55 | 0.56 |
| Some college | 0.23 | 0.19 | 0.17 | 0.26 | 0.26 | 0.26 |
| College | 0.31 | 0.28 | 0.21 | 0.19 | 0.19 | 0.19 |
| Employed | 0.81 | 0.61 | 0.25 | 0.19 | 0.17 | 0.18 |
| Insurance Coverage | ||||||
| Medicare | 0.05 | 0.08 | 0.99 | 1.00 | 1.00 | 1.00 |
| Medicare HMO | - | - | - | 0.17 | 0.17 | 0.17 |
| Medicaid | 0.07 | 0.06 | 0.10 | 0.11 | 0.12 | 0.11 |
| Private insurance | 0.79 | 0.78 | 0.59 | 0.67 | 0.69 | 0.68 |
| Drug insurance (excl. 2006) | 0.81 | 0.78 | 0.52 | 0.79 | 0.81 | 0.81 |
| Observations | 20,719 | 9,617 | 10,358 | 20,072 | 11,229 | 17,362 |
| # of Unique persons | 12,934 | 6,262 | 6,544 | 10,079 | 6,840 | 9,036 |
| # of Prescriptions | 352,889 | 211,718 | 278,753 | 573,720 | 66,874 | 450,909 |
Notes: Means are weighted and pooled for 2001–2006 unless otherwise noted. In Columns 5 and 6, unconditional means are shown for outcome variables and the remaining variables show means conditional on purchasing an acute or chronic drug. Drug classes that could not be classified as either acute or chronic are excluded in Columns 5 and 6.
Drug utilization is lower for the primary comparison group of adults aged 50–58 (Column 1). This group purchases nearly two-thirds as many prescriptions as the elderly and has a rate of drug use of 76%. Naturally, the largest differences in demographic characteristics across the two age groups are in employment status and insurance coverage.
In Columns 5 and 6, means are reported for individuals who filled at least one acute prescription or at least one chronic prescription. Many individuals purchased both types of drugs and are included in both samples. The elderly fill on average 22 prescriptions of chronic drugs and 3 prescriptions of acute drugs per year.
5. Empirical framework
5.1. Baseline model
I estimate the announcement effect of Part D on drug utilization by using a difference-in-difference estimator with group-specific linear trends. The basic strategy compares deviations from drug utilization trends for a treatment group that is more affected by the announcement of Part D with the deviation from trend for a comparison group that is less affected. As motivated by the conceptual framework, my primary test compares deviations from utilization trends for chronic drugs relative to acute drugs. I extend this test further by comparing drug utilization trends for individuals who are age-eligible for Medicare with those who are age-ineligible in Section 7.3 and also examine heterogeneity in the effects across age, income, education, and health insurance status. Taken together these complementary tests – with their corresponding advantages and limitations – provide more comprehensive evidence on the existence of anticipation effects than a single test on its own.
First, I test for differential responses of chronic and acute drugs to the announcement. I find that chronic and acute drugs do not exhibit parallel utilization trends in the pre-announcement period. The empirical strategy accounts for these differential trends by allowing each drug type (chronic and acute) to have its own linear trend. This is implemented by including an interaction term between a chronic indicator and a linear time trend in Equation 1 below . With group-specific linear trends, we interpret the difference-in-difference estimate as the deviation from the pre-announcement trend for chronic drugs relative to the deviation for the acute trend.18
The key identifying assumption is that in the absence of the announcement, any utilization differences between treatment and comparison groups would continue along the same differential linear trends.19 Any relative deviation from the pre-trends after 2003 is attributed to Part D. While it is not possible to test this assumption directly, I provide indirect evidence that key potential alternative explanations for utilization changes do not appear to be occurring concurrently with the announcement (e.g., changes in drug prices and insurance coverage or generosity). I also verify that there were no breaks in the chronic and acute trends prior to the announcement.20
Specifically, I estimate variants of the following difference-in-difference equation which includes the announcement and implementation as separate policies:
| (1) |
For the chronic and acute drug comparison, the outcome is the number of prescriptions (new and refill) purchased by individual in year in drug category (where is chronic or acute). That is, each individual receives two observations in the regression for each year – one for the number of chronic drugs that they purchase and one for the number of acute drugs they purchase, including zeros. I also consider the log of the number of prescriptions in some specifications. To account for zeros in the data, the log transformation is log (number of prescriptions +1) 21 is an indicator which equals one if the observation is for chronic drugs, and zero if the observation is for acute drugs. ANNOUNCEt is an indicator variable which turns on in 2004 and 2005, the time period between the announcement and implementation of Part D, and IMPLEMENTt is an indicator which turns on in 2006 after the program has been implemented. The omitted time period is 2001 to 2003 is a vector of individual level control variables including male, age, age-squared, married, three education dummies, three race dummies, three region dummies, metro-area, employment status, Medicaid enrollment, and Medicare HMO enrollment. Standard errors are clustered at the person level to allow for an arbitrary variance–covariance matrix across the two drug groups and over time. I account for the observed differential trends for chronic and acute drug groups by interacting a linear time trend (which takes on a value of 1 in 2001) with the chronic indicator. It should be noted that Equation 1 allows only for an intercept shift in trends for the announcement and implementation effects. While I cannot estimate a slope shift for the implementation effect given that I use only one year of post-implementation data, I do estimate slope shifts for the announcement effect in some specifications. The key variable of interest is the interaction between the announcement and chronic indicators. With drug group specific linear trends, this difference-in-difference estimate is interpreted as the deviation from the pre-announcement trend for chronic drugs relative to the deviation for the acute trend. The test of interest is whether is positive or negative (indicating the direction of the anticipatory effect) and whether it is statistically significantly different from zero (indicating whether the deviation from trend was larger for chronic drugs relative to acute drugs). A non-zero coefficient is evidence of a causal announcement effect.
While the main analysis exploits variation in the predicted impact of the announcement on chronic and acute drug utilization within the Medicare-eligible sample, in Section 7.3, I also compare overall drug utilization for adults who are currently eligible for Medicare (aged 66–74) with two groups of adults who are not yet eligible (aged 50–58 and 59–64). Taking seriously the idea of forward-looking behavior, even those who are not yet eligible for Medicare may anticipate future subsidized coverage and respond to the announcement. Those who are further from age 65 should be less responsive than those who are closer to eligibility. For the age-eligible and age-ineligible comparison, I include two treatment indicators: and 22 is an indicator for Medicare-eligible adults aged 66–74 and is an indicator for Medicare-ineligible adults aged 59–64 who are close to the eligibility threshold. The omitted comparison group are adults aged 50–58 who are furthest from Medicare eligibility. In this specification, the outcome is the total number of prescriptions filled. Thus, each individual receives only one observation per year.
5.2. Defining chronic and acute drugs
I use an empirical approach for categorizing drugs as chronic and acute based on observed treatment duration. This method exploits average treatment patterns in the population as opposed to clinical recommendations which may or may not be adopted. The classification method (which is illustrated in Appendix Figure A.2) proceeds as follows. First, I pool MCBS drug records for the elderly aged 65+ from 2002 to 2003. I use data from before the announcement so as not to confound underlying utilization patterns with the treatment effect of Part D. For each person, I count the number of purchases of each drug in each year. This generates person–year–drug level observations, where the number of purchases is the variable of interest. There are no zeros, since I condition on purchasing the drug. Second, I combine these person–year–drug observations across individuals to construct empirical distributions of the number of prescriptions purchased in each therapeutic drug class. Each drug is assigned one of 38 possible First Data Bank drug class categories.23 For example, if a person fills 1 prescription of Amoxicillin and 2 prescriptions of Cefaclor (both Antiinfectives), and 5 prescriptions of Zocor (a Cardiovascular drug) in a given year, then she contributes a 1 and 2 to the Antiinfectives distribution of prescriptions filled and a 5 to the Cardiovascular distribution. As is apparent in Appendix Figure A.2, Antiinfectives are clearly an acute drug class since their distribution has a large mass point at 1, meaning that the vast majority of drugs in this class are filled only once a year. Cardiovascular drugs, on the other hand, are more chronic in nature since they are typically filled many times per year.
Finally, I compute the median number of purchases across person–year–drug observations within each drug class. I take the median of the number of prescriptions purchased across individuals, instead of across drugs, so that the classification puts more weight on drugs in the class that are used more. I define a drug class as chronic if the median person purchases drugs in this class more than 2 times per year and acute if the median person purchases drugs 2 or fewer times per year. In other words, the drug class is chronic if more than 50% of individuals purchase drugs in the class for chronic use (more than 2 times per year) and acute if more than 50% purchase drugs for acute use (2 times or fewer per year). This is my most conservative classification.
I assign this classification to all drugs within the therapeutic class. 11 out of 32 classes used by the elderly are classified as acute, including Analgesics, Eye, Ear, Nose, and Throat (EENT) Preparations, and Antiinfectives (see full list in Appendix Table A.1). Cardiovascular drugs, Diuretics, and Hypoglycemics are among the most frequently purchased chronic treatments. With this approach, there is some measurement error since some drugs in chronic classes actually acute and vice versa and some drugs can be used for both indications. The extent of the measurement error varies across drug classes depending on how heterogeneous treatment duration is within the class. Consequently, this test for anticipation effects is conservative given that misclassification should bias against observing any effect. In sensitivity analyses, I exclude the most heterogeneous drug classes from the sample by repeating the basic classification algorithm with more stringent cutoffs. Appendix Figure A.2 describes the classification rules in order of increasing stringency. For example, in the “65% rule,” a drug class is classified as acute if more than 65% of individuals fill drugs in this class 2 times or less per year and chronic if more than 65% of individuals fill drugs in this class more than 2 times. Heterogeneous drug classes for which fewer than 65% of drugs can be classified as either acute or chronic (e.g. 45% acute, 55% chronic) are dropped from the sample.
I validated this method by comparing the empirical classifications with classifications made independently by three family medicine physicians. Both classification methods corresponded very closely. Each physician was asked to report whether drugs in each class were “somewhat more likely to be acute than chronic,” “much more likely to be acute than chronic,” “somewhat more likely to be chronic than acute,” or “much more likely to be chronic than acute.” In the most conservative classification (median rule), the empirical algorithm matches the physicians’ classifications of chronic versus acute drug classes 85% of the time. The match rate improves as the classification rule for the empirical algorithm becomes more stringent. For example, using the “65%” rule, the empirical classifications match the physician classifications 96% of the time. Appendix Figure A.3 illustrates the match for three examples.
6. Results
I begin the analysis by comparing drug utilization changes following the announcement and implementation of Part D for elderly who are eligible for Medicare relative to the near-elderly who are not yet eligible. Medicare-eligibility status is a natural first cut for identifying the announcement effect. This strategy has been used in most previous studies of Part D. I select adults aged 50–58 as the initial comparison group because they are far enough away from eligibility that they are unlikely to respond to the announcement, and Medicare beneficiaries aged 66–74 who are closest in age to the comparison group. Fig. 1 plots aggregate trends in drug utilization for these age groups in the MCBS and MEPS from 1997 to 2007. The two datasets provide largely comparable measures of drug utilization. For the elderly, the average number of prescriptions filled per year had been rising since 1997. Then immediately following the 2003 Part D announcement there was a distinct leveling off and eventual decline in drug utilization. In contrast, no trend break after the announcement is observed for the near-elderly. After 2006, when Part D took effect, drug use for the elderly reverted upward toward its pre-2003 trend. The pre-program “dip” in utilization for the elderly is consistent with a dominating intertemporal substitution effect, in which beneficiaries delay some drug use until after Part D is implemented. Consequently, the increase between 2005 and 2006 may constitute both the treatment effect of Part D and mean reversion. Thus, studies that use small windows of data around the implementation date could overstate the implementation effect.
While the striking graphical evidence is strongly suggestive of a negative announcement effect, we might be concerned that the 50–58 age group does not provide a sufficient measure of counterfactual drug utilization for the Medicare-eligible elderly. For example, there might be time-varying factors related to Social Security benefits, Medicare benefits, the markets of certain drugs, and so forth that could explain part of the differential pre-program decline in utilization for the elderly. Due to this concern, the next part of my analysis uses identifying variation for the announcement effect within the elderly age group, which should alleviate concerns that age-specific secular trends are driving the results.
The outline of the remainder of the analysis proceeds as follows. I first estimate the aggregate announcement effect for Medicare beneficiaries and heterogeneity in the intensity of this effect across age and demographic groups. Since aggregate effects are estimated from a basic time series model – estimating changes in utilization relative to the pre-existing trend – they may be biased if other aggregate shocks to utilization occur during this time period. To address this concern, I compare the differential effects of the announcement on chronic and acute drug utilization among beneficiaries. I revisit the age-ineligible comparison group in the final section to test whether those who are nearing eligibility are also responsive to the announcement relative to younger adults who are further from eligibility. I also test whether the anticipation effects occur primarily among groups that would be predicted to be more responsive to future price changes from Part D, such as those without employer-provided prescription drug coverage. Taken together, these tests aim to identify whether the decline in drug utilization observed in Fig. 1 represents a causal response to the announcement of Part D.
6.1. Aggregate drug utilization effects for the elderly
Before presenting difference-in-difference results comparing chronic and acute drug use, I examine aggregate changes in drug utilization, which has been the focus of previous evaluations of Part D. I estimate the announcement and implementation effects as deviations from the prior utilization trend in a simple interrupted time series model for the Medicare sample as follows:
| (2) |
Table 2 reports the OLS results for variants of this equation. The dependent variable is total prescriptions. In Column 1, only the implementation indicator is included along with the time trend and controls, under the assumption that (i.e. no announcement effect). This specification is analogous to previous studies that identify the treatment effect by comparing drug utilization right before and after the implementation date, ignoring possible anticipatory effects. Using this specification, the implementation effect is large, positive, and statistically significant at the 1% level, representing an average annual increase of 3 prescriptions or a 10.6% increase relative to the sample mean. From this estimate – which is comparable to the effect size found in other studies of 4 to 10% – it would appear that Part D had a large positive effect on utilization in the first year of the program.
Table 2.
Aggregate announcement and implementation effects.
| Dependent variable: | Total prescriptions |
Log (total prescriptions) |
||||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | |
|
| ||||||
| Announce | −2.1140*** | −1.6064*** | −0.0475*** | −0.0342 | ||
| (0.430) | (0.621) | (0.018) | (0.030) | |||
| Implement | 2.9943*** | 0.9064 | 0.0682** | 0.0237 | ||
| (0.724) | (1.115) | (0.030) | (0.050) | |||
| t | 1.2970*** | 1.9667*** | 1.7788*** | 0.0514*** | 0.0666*** | 0.0616*** |
| (0.177) | (0.157) | (0.240) | (0.008) | (0.007) | (0.011) | |
| Observations | 20,072 | 20,072 | 20,072 | 20,072 | 20,072 | 20,072 |
Notes:
p < 0.01
p < 0.05
p < 0.1.
Clustered standard errors at the person level. Regressions are weighted and include a full set of control variables. MCBS 2001–2006; Ages 66–74.
If the assumption of no anticipatory effects is correct, controlling for the announcement indicator should not change the estimate of On the contrary, I find that after adding the announcement indicator in Column 3, the implementation effect shrinks from 3.0 to 0.9 and becomes statistically insignificant although, since it has large standard errors, it is imprecisely estimated. The announcement effect itself is statistically significant and negative, representing a decline of 1.61 prescriptions (a 6% decline relative to the sample mean). This announcement response is also economically important given that it is nearly equivalent to the average annual growth rate of utilization during this time period, suggesting that utilization growth nearly halted for two years. Excluding Medicaid beneficiaries in Appendix Table A.2 produces similar estimates.24 This set of analyses provides the first piece of evidence that there may be a large upward bias in the implementation effect if anticipatory responses are not taken into account.
When I repeat the above exercise with log prescriptions as the dependent variable in Columns 4–6, I find a smaller percent decline in utilization after the announcement which is statistically insignificant. Since the log transformation places more weight on smaller prescription counts, this smaller effect relative to the level specification suggests possible treatment effect heterogeneity, with the announcement having a larger effect for elderly with high levels of drug utilization. I investigate this claim further by estimating quantile regressions of the same interrupted time series model. Confidence intervals are block bootstrapped at the person level to preserve the serial correlation structure of the error term. The estimated conditional quantile treatment effects and 95% confidence intervals are presented graphically in Appendix Figure A.4 for each quantile of the conditional distribution of total prescriptions. The negative announcement effect increases monotonically across quantiles and only becomes statistically significant beginning with the 75th quantile and above. In contrast, the implementation effect conditional on the announcement indicator is close to zero and statistically insignificant for every quantile of the distribution. This provides evidence that announcement effects are concentrated among elderly with high (conditional) drug use.
In Appendix Section A.1, I estimate the announcement and implementation effects for alternative outcomes and specifications. I find that the announcement and implementation of Part D have no effect on the probability of any drug use – which is not surprising given the nearly universal use of drugs among the elderly. Further, log expenditures and out-of-pocket expenditures decline following the announcement, although the effect is not statistically significant.25 Finally, I estimate alternative specifications that control more flexibly for time trends. I include a quadratic trend, allow for slope shifts in addition to the level shift, and estimate a non-parametric trend. I also estimate Equation 2 with a negative binomial model to better account for the count nature of the data and address zero counts. The estimates are largely robust across specifications.
The results in this section confirm the visual impressions from Fig. 1 and suggest that the rise in utilization during the first year of Part D may largely represent a recovery from the anticipatory decline with little net increase in utilization generated by Part D. This causal interpretation will be investigated in detail in the following sections.
6.2. Treatment heterogeneity by demographic characteristics and insurance status
Next, I examine how aggregate anticipatory responses vary across age, income, education, and insurance groups. In the above sections, I have focused on the 66–74 age group which has been shown to experience a sharp decline in drug use after the announcement of Part D. I now expand the sample to include the elderly aged 75–85. Columns 1 and 2 of Table 3 (Panel A) compare age groups 66–74 (repeated from Table 2) and 75–85. Only the younger age group exhibits an anticipatory utilization response. While there is no announcement effect for the older age group 75–85, there is a large positive contemporaneous implementation effect. This is as predicted, given that the costs of deferring drug use is greater for those who are older and in poorer health. Further, there may have been less cognitive awareness of the announcement of Part D among older Medicare beneficiaries.
Table 3.
Aggregate announcement and implementation effects – heterogeneous effects.
| Panel A: Demographic characteristics | ||||||
|---|---|---|---|---|---|---|
| Dependent variable: |
Total prescriptions |
|||||
| Sub-sample: | Age 66–74 |
Age 75–85 |
< = HS Educ |
>HS Educ |
< = Median income |
>Median income |
| (1) | (2) | (3) | (4) | (5) | (6) | |
|
| ||||||
| Announce | −1.606*** | 0.157 | −1.920 ** | −1.177 | −2.204** | −0.859 |
| (0.621) | (0.592) | (0.866) | (0.876) | (0.995) | (0.879) | |
| Implement | 0.906 | 3.650*** | 1.210 | 0.548 | 0.366 | 1.317 |
| (1.115) | (1.005) | (1.584) | (1.543) | (1.721) | (1.557) | |
| t | 1.779*** | 1.368*** | 2.038*** | 1.376*** | 2.437*** | 1.058*** |
| (0.240) | (0.218) | (0.333) | (0.341) | (0.363) | (0.340) | |
| Observations | 20,072 | 21,403 | 11,352 | 8,720 | 10,384 | 9,688 |
| Panel B: Insurance status | ||||
|---|---|---|---|---|
| Dependent variable: |
Total prescriptions |
|||
| Sub-sample: | Excluding individuals with employer drug ins. |
Individuals with employer drug ins. |
||
| (1) | (2) | (3) | (4) | |
|
| ||||
| Announce | −2.159*** | −0.498 | ||
| (0.827) | (1.030) | |||
| Implement | 3.796*** | 0.998 | 1.927* | 1.277 |
| (0.986) | (1.478) | (1.080) | (1.794) | |
| t | 1.619*** | 2.262*** | 0.685** | 0.836** |
| (0.223) | (0.306) | (0.286) | (0.412) | |
| Observations | 12,760 | 12,760 | 7,312 | 7,312 |
Notes:
p < 0.01
p < 0.05
p < 0.1.
Clustered standard errors at the person level. Regressions are weighted and include a full set of control variables. Medicaid beneficiaries are included. Columns 3–6 in Panel A and Columns 1–4 in Panel B are estimated for the elderly aged 66–74. Median income is computed separately for each year. MCBS 2001–2006.
I also consider heterogeneous effects for other demographic groups. Since the announcement effect is only apparent for the 66–74 age group, I look within this age group for variation in the effect size by income and education levels. Comparing Columns 5 and 6, we can see that the negative announcement effect for the 66–74 age group is concentrated among elderly with income below the median. This also conforms to predictions, since these individuals are most liquidity constrained and also anticipate larger benefits from Part D, given the additional subsidies provided to low income beneficiaries. Finally, after controlling for income, individuals with low education levels also have a larger negative announcement effect (Column 3). A priori, the direction of the education effect is ambiguous. On the one hand, the less educated may be less informed of the announcement of Part D. On the other hand, less educated individuals may be more likely to engage in risky health behaviors – such as postponing beneficial drug treatments – or they may be less well-informed about the health risks of postponing treatments.26 The results suggest that the latter effect dominates, since we observe that less educated individuals are more likely to postpone drug treatments after the announcement, trading-off health for additional consumption.
Finally, I examine heterogeneity in the announcement effect by insurance status for the 66–74 age group. In Table 3 (Panel B), I compare the announcement and implementation effects for Medicare beneficiaries who receive drug benefits from employer-sponsored insurance (e.g. retiree benefits) with all other Medicare beneficiaries. The anticipatory response is driven entirely by beneficiaries without employer-sponsored drug insurance, who are more likely to enroll in Part D (Kaestner and Khan, 2012; Levy and Weir, 2010). The announcement effect for beneficiaries without employer-sponsored drug insurance is −2.16 and is statistically significant at the 1% level compared to a statistically insignificant −0.50 for those with employer-sponsored drug insurance. Consequently, the estimated announcement effect is primarily driven by the subgroups that we would predict should be most responsive to changes in future prices from Part D.
6.3. Chronic and acute difference-in-difference estimates
6.3.1. Basic results
If the observed decline in drug utilization is the result of anticipatory behavior, we would expect to find differential utilization responses for chronic and acute drugs. In this section, I test this hypothesis for the 66–74 age group. Fig. 2 plots sample means of total prescriptions for chronic and acute drugs in each year and predicted counterfactual trends in the post-announcement period. I find differential trends in drug utilization that follow the pattern predicted by the life-cycle model. While both drug types exhibit smooth linear trends before the announcement of Part D, there is a substantial negative trend break after the announcement in 2003 for chronic drugs, whereas acute drug utilization continues along its pre-existing trend.27 After Part D is implemented in 2006, utilization increases relative to the counterfactual trend for both acute and chronic drugs. This pattern is consistent with the prediction that chronic drugs respond to both current and future prices, whereas acute drugs are only responsive to current price.
Fig. 2.
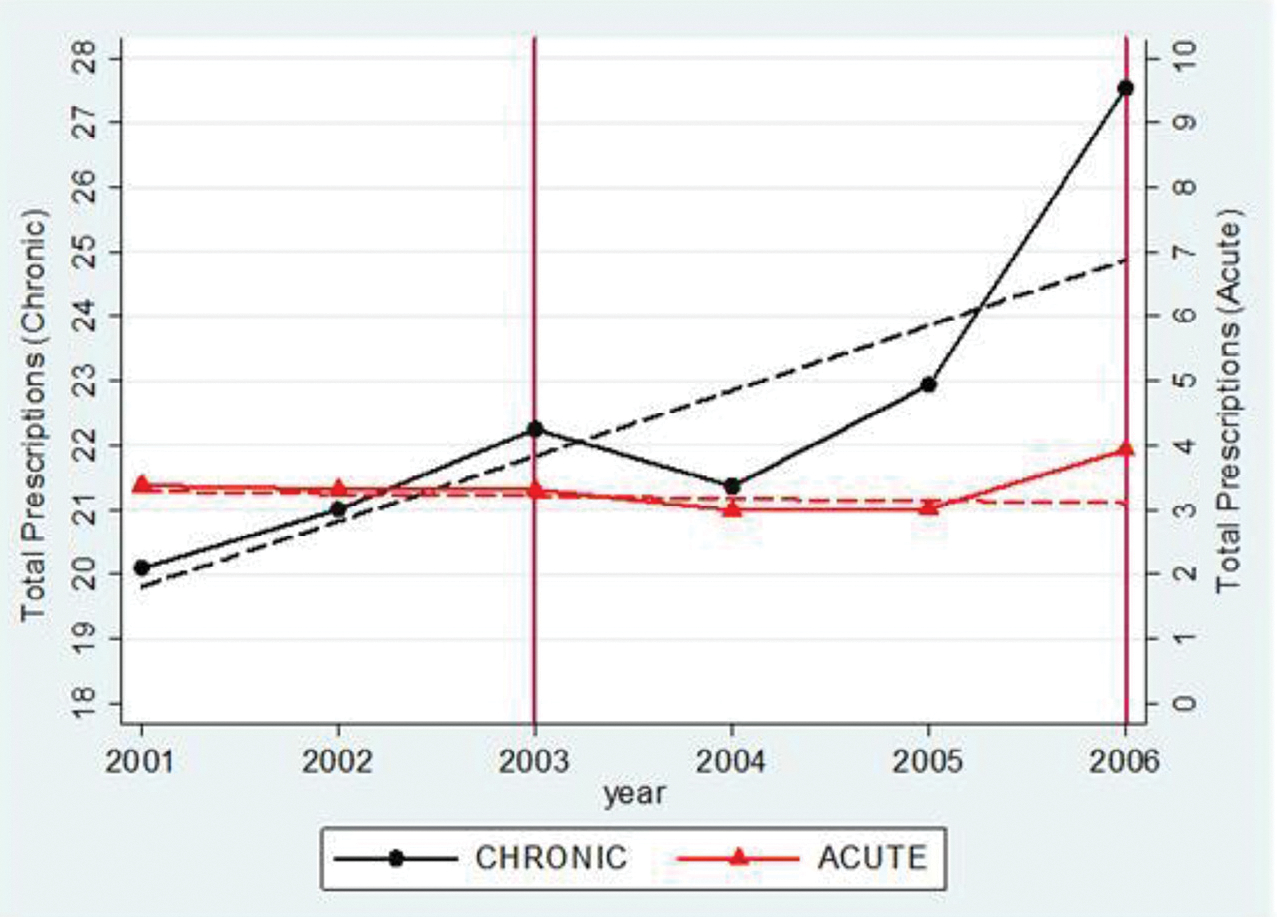
Chronic and acute announcement and implementation effects.
Notes: MCBS 2001–2006, weighted. The points represent weighted sample means. Chronic and acute categories are defined by the median assignment rule and correspond to the results in Table 4. The dashed lines show preannouncement trends projected forward. These are obtained by estimating the basic model, Equation 1, without controls. To implement this, I first estimate the coefficients in the basic model from Equation 1. Then I set the announcement and implementation indicators to zero for all observations and compute the predicted values for total prescriptions in each year. Chronic and acute trends have separate y-axes.
Table 4 formalizes this graphical evidence by reporting the difference-in-difference regression results from estimating Equation 1 – that is, comparing the change in utilization for chronic drugs relative to acute drugs before and after the announcement and implementation of Part D. Columns 1 and 2 present results that use the most conservative method for classifying drugs as chronic or acute (the median classification rule), while Columns 3 and 4 use the more stringent 65% classification rule. In the latter classification method, drug classes that are nearly equally likely to be either chronic or acute are dropped from the sample and the total count of acute and chronic prescriptions purchased are recalculated and reduced for each person. Consequently, the mean number of chronic and acute prescriptions filled under the median rule is 22.14 and 3.26, respectively, while the mean number under the 65% rule is 17.28 and 1.37. I report the results from the 65% classification rule since it balances stringency with representativeness by including 73% of the prescriptions in the sample.
Table 4.
Chronic and acute announcement and implementation effects.
| Dependent variable: |
Total prescriptions |
Log(total prescriptions) |
||||||
|---|---|---|---|---|---|---|---|---|
| Classification method: | >50% In drug group |
>65% In drug group |
>50% In drug group |
>65% In drug group |
||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
|
| ||||||||
| Chronic*Announce | −1.5667*** | −1.4160*** | −0.0543 | −0.1127*** | ||||
| (0.509) | (0.444) | (0.035) | (0.036) | |||||
| Chronic*Implement | 3.5449*** | 1.5100 | 3.2464*** | 1.4074* | 0.0088 | −0.0618 | 0.0404 | −0.1060* |
| (0.602) | (0.924) | (0.534) | (0.807) | (0.035) | (0.058) | (0.036) | (0.060) | |
| Announce | −0.1567 | −0.0322 | −0.0179 | 0.0191 | ||||
| (0.159) | (0.090) | (0.026) | (0.020) | |||||
| Implement | 0.9651*** | 0.7608*** | 0.3655*** | 0.3230** | 0.1547*** | 0.1314*** | 0.1031*** | 0.1278*** |
| (0.163) | (0.264) | (0.091) | (0.151) | (0.025) | (0.042) | (0.019) | (0.033) | |
| Chronic | 16.2625*** | 15.4795*** | 12.9526*** | 12.2450*** | 1.4718*** | 1.4447*** | 1.5046*** | 1.4483*** |
| (0.474) | (0.495) | (0.404) | (0.418) | (0.031) | (0.033) | (0.033) | (0.034) | |
| t | −0.0494 | −0.0023 | −0.0048 | 0.0049 | −0.0187*** | −0.0133 | −0.0128*** | −0.0185** |
| (0.041) | (0.058) | (0.023) | (0.033) | (0.006) | (0.009) | (0.005) | (0.007) | |
| Chronic*t | 0.5777*** | 1.0473*** | 0.6883*** | 1.1127*** | 0.0419*** | 0.0582*** | 0.0551*** | 0.0889*** |
| (0.144) | (0.196) | (0.124) | (0.168) | (0.009) | (0.013) | (0.010) | (0.013) | |
| Announce + Chronic*Announce | −1.7234*** | −1.4481*** | −0.0722** | −0.0936*** | ||||
| (0.526) | (0.441) | (0.031) | (0.032) | |||||
| Implement + Chronic*Implement | 4.5100*** | 2.2707** | 3.6119*** | 1.7304** | 0.1635*** | 0.0697 | 0.1435*** | 0.0218 |
| (0.622) | (0.957) | (0.531) | (0.802) | (0.032) | (0.053) | (0.033) | (0.054) | |
| Observations | 40,144 | 40,144 | 40,144 | 40,144 | 40,144 | 40,144 | 40,144 | 40,144 |
Notes:
p < 0.01
p < 0.05
p < 0.1.
Clustered standard errors at the person level. Regressions are weighted and include a full set of control variables. Medicaid beneficiaries are included. The bottom panel presents linear combinations of the coefficients and their standard errors to show absolute announcement and implementation effects for chronic drugs. The classification method presented is either the median assignment rule (more than 50% of drugs in the therapeutic class are either chronic or acute) or the assignment rule in which more than 65% of drugs in the class are either chronic or acute (classes with fewer than 65% of drugs in both groups are excluded). MCBS 2001–2006; Ages 66–74.
Consistent with the graphical results, I find a large decline in chronic drug use relative to acute drug use after the announcement of Part D (Table 4, Column 2). This is robust across both classification methods. For the median classification method, the use of chronic drugs declined by 1.72 prescriptions in absolute terms (bottom panel of Column 2) compared to a decline of 0.16 prescriptions for acute drugs relative to pre-announcement trends. This decline is only statistically significant for chronic drugs. The difference-in-difference estimate of the relative change in utilization for chronic drugs is −1.57 (a 7% decline relative to the mean for chronic drugs) and is also statistically significant at the 1% level. To the extent that changes in acute drug use control for underlying aggregate shocks to health, insurance coverage, pharmaceutical prices (I consider these directly in Section 7.2), and other possible confounding factors, the DID estimate represents the causal announcement effect. The fact that the announcement effect for acute drugs is both qualitatively small and not significantly different from zero alleviates major concerns of a potential bias from coincident aggregate shocks. As before, the negative announcement effect for chronic drugs suggests that Medicare beneficiaries delayed drug use in anticipation of subsidized Part D coverage. Meanwhile, acute drug use does not respond to the announcement of the future price change as predicted. An alternative test of chronic versus acute responses to the announcement would be to compare overall drug utilization among individuals with chronic conditions relative to individuals without chronic conditions. The results of this test are in Appendix Table A.6 and are similar.28
I next turn to the implementation effects to estimate the treatment effect bias from ignoring anticipation effects. First, I estimate the model in Equation 1 assuming that there are no anticipatory effects by excluding the announcement indicator and the announcement × chronic interaction term. In other words, I assume that and (recall that the second assumption was rejected in the section above). In this (misspecified) model, the implementation effect is positive, large, and statistically significant for chronic drugs, representing an absolute increase of 4.51 and 3.61 prescriptions for the median and 65% classification rules, respectively. However, when announcement controls are added in Columns 2 and 4, the implementation effect drops to 2.27 and 1.73 for the two classification rules. Thus, while we observe a positive implementation effect of Part D on drug utilization in 2006, accounting for the negative announcement effect reduces the estimate for chronic drugs by about one-half. This suggests a potentially large upward bias in previous studies that evaluate the first year impact of the program. Meanwhile, there is a large increase in acute drug use relative to trend after the implementation of 23.6% (.323/1.37). This effect is stable across the specifications with and without announcement controls, as expected, given that we could not reject that there was a zero announcement effect for acute drugs, or .29
Given that the Part D announcement and implementation occurred at the national level and identification results from comparing differences in outcomes across two groups – chronic and acute drugs – the standard errors from the main specification have the potential to be misleading (Bertrand etal., 2004). In Appendix Table A.7, I present results using an alternative inference procedure motivated by Donald and Lang (2007) when the number of clusters is small. This procedure involves estimating the announcement and implementation effects using adjusted group means.30 Wooldridge (2003) notes (see p. 136) that t-statistics from this procedure converge to the standard normal distributions as the number of observations in each cluster becomes large, allowing us to use the critical values from a standard normal distribution. It is reassuring that this highly conservative approach generates broadly similar results as the main specification in Table 4 in terms of both the coefficients’ size and significance. For example, the chronic × announcement interaction term has a coefficient of −1.5667 (standard error of 0.509) in the main specification compared to −1.7098 (standard error of 0.686) using the Donald and Lang (2007) procedure.
6.3.2. Robustness tests
I now consider several alternative specifications as robustness tests. One concern is that there is limited scope for downward adjustment for acute drugs (relative to chronic drugs) due to their low mean utilization. Furthermore, given the large difference in baseline means, it is not clear that differences in level effects alone can be interpreted as different treatment impacts.31 To address these concerns, I compare proportional changes for chronic and acute drugs using log prescriptions as the dependent variable in Columns 5–8 of Table 4. Similar negative anticipation effects for chronic drugs are observed for proportional changes in utilization and the difference between chronic and acute drugs is large (e.g., in the most conservative classification in Column 6, utilization of acute drugs fell by 1.8% while utilization of chronic drugs fell by 7.2%). While this estimate is statistically insignificant for the most conservative classification method (50% rule), it becomes larger and statistically significant at the 1% level for the more stringent classification (65% rule), as would be expected as the measurement error in the classifications is reduced. Importantly, I find that the announcement effect for acute drugs is still close to zero and statistically insignificant for both classification methods, suggesting that the low mean for acute drugs is not driving the smaller announcement effect size. Furthermore, previous studies have found that acute drug use is highly responsive to contemporaneous prices (e.g. Landsman et al., 2005; Skipper, 2013). Thus, it is not expected that the zero effect is driven by an inherent price inelasticity for acute drugs. Instead our results suggest that acute drug use is relatively insensitive to future prices and responsive to contemporaneous price, as evidenced by the zero announcement effect and large implementation effect for acute drugs.
I conduct additional sensitivity tests of the drug classification method. As previously noted, measurement error in the empirical classification method will lead some chronic drugs to be misclassified as acute drugs and vice versa. This could bias the chronic announcement effect toward zero and the acute effect away from zero. In Table 5, I repeat the analysis in the above section for the 50%, 55%, 60%, 65%, 70%, and 75% classification rules for both level and log prescriptions. Moving from 50% to 75% reduces classification measurement error, but also lowers the total number of prescriptions included in the sample by construction. Since an 80% classification rule would exclude 93% of the original sample, I do not go beyond the 75% threshold. The results for the announcement and implementation effects are extremely stable across classification methods. Importantly, the acute announcement effect is close to zero and statistically insignificant in every single level and log specification. The announcement effect for chronic drugs relative to acute drugs is negative and statistically significant for the level specification across all definitions of chronic and acute drugs. The log specifications are also largely robust as they are statistically significant at the 5% or 1% level for the most stringent classifications (60%, 65%, 70%) and significant at the 10% level for the 75% classification. Out of the twelve specifications, the two log-specification coefficients that are not statistically significant are also the most conservative classifications (50%, 55%). When considering the differences across classification methods, there are tradeoffs. Using lower thresholds (e.g., 50%) induces more classification error and should bias the estimate toward zero; these estimates are mechanically conservative. However, we lose data as we exclude more drug classes in the more stringent classifications so the variance increases. The patterns observed are consistent with this – I find that the effects generally become quantitatively larger and more significant as I increase the classification thresholds.
Table 5.
Robustness across classification methods.
| Panel A: Dependent variable is total prescriptions | ||||||
|---|---|---|---|---|---|---|
| Dependent variable: |
Total prescriptions |
|||||
| Classification method: | >50% |
>55% |
>60% |
>65% |
>70% |
>75% |
| (1) | (2) | (3) | (4) | (5) | (6) | |
|
| ||||||
| Chronic*Announce | −1.5667*** | −1.3048*** | −1.3587*** | −1.4160*** | −0.9821** | −0.3056* |
| (0.509) | (0.480) | (0.441) | (0.444) | (0.387) | (0.180) | |
| Chronic*Implement | 1.5100 | 1.4968* | 0.8539 | 1.4074* | 1.4556** | 0.5967* |
| (0.924) | (0.875) | (0.798) | (0.807) | (0.705) | (0.321) | |
| Announce | −0.1567 | −0.1628 | −0.1315 | −0.0322 | −0.0282 | −0.0229 |
| (0.159) | (0.157) | (0.141) | (0.090) | (0.082) | (0.074) | |
| Implement | 0.7608*** | 0.7448*** | 0.7365*** | 0.3230** | 0.2732** | 0.2794** |
| (0.264) | (0.261) | (0.235) | (0.151) | (0.137) | (0.120) | |
| Chronic | 15.4795*** | 14.2163*** | 10.9330*** | 12.2450*** | 10.1698*** | 1.7792*** |
| (0.495) | (0.470) | (0.417) | (0.418) | (0.365) | (0.159) | |
| Announce + Chronic*Announce | −1.7234*** | −1.4676*** | −1.4902*** | −1.4481*** | −1.0102*** | −0.3285** |
| (0.526) | (0.494) | (0.447) | (0.441) | (0.383) | (0.167) | |
| Implement + Chronic*Implement | 2.2707** | 2.2416** | 1.5903** | 1.7304** | 1.7288** | 0.8761*** |
| (0.957) | (0.902) | (0.810) | (0.802) | (0.698) | (0.304) | |
| Observations | 40,144 | 40,144 | 40,144 | 40,144 | 40,144 | 40,144 |
| Chronic: Mean prescriptions | 22.14 | 20.62 | 17.46 | 17.28 | 14.63 | 4.22 |
| Acute: Mean prescriptions | 3.26 | 3.26 | 2.85 | 1.37 | 1.24 | 1.24 |
| Total # of prescriptions | 517,783 | 486,815 | 413,313 | 379,278 | 322,131 | 110,391 |
| Panel B: Dependent variable is log(total prescriptions) | ||||||
|---|---|---|---|---|---|---|
| Dependent variable: |
Log(total prescriptions) |
|||||
| Classification method: | >50% |
>55% |
>60% |
>65% |
>70% |
>75% |
| (1) | (2) | (3) | (4) | (5) | (6) | |
|
| ||||||
| Chronic*Announce | −0.0543 | −0.0526 | −0.0777** | −0.1127*** | −0.0993*** | −0.0643* |
| (0.035) | (0.035) | (0.037) | (0.036) | (0.036) | (0.033) | |
| Chronic*Implement | −0.0618 | −0.0638 | −0.1112* | −0.1060* | −0.0908 | −0.0481 |
| (0.058) | (0.059) | (0.061) | (0.060) | (0.060) | (0.055) | |
| Announce | −0.0179 | −0.0180 | −0.0159 | 0.0191 | 0.0192 | 0.0187 |
| (0.026) | (0.026) | (0.025) | (0.020) | (0.020) | (0.020) | |
| Implement | 0.1314*** | 0.1315*** | 0.1324*** | 0.1278*** | 0.1260*** | 0.1239*** |
| (0.042) | (0.042) | (0.041) | (0.033) | (0.032) | (0.032) | |
| Chronic | 1.4447*** | 1.3715*** | 1.1692*** | 1.4483*** | 1.3089*** | 0.2467*** |
| (0.033) | (0.034) | (0.035) | (0.034) | (0.034) | (0.030) | |
| Announce + Chronic*Announce | −0.0722** | −0.0706** | −0.0936*** | −0.0936*** | −0.0802** | −0.0456* |
| (0.031) | (0.031) | (0.032) | (0.032) | (0.032) | (0.028) | |
| Implement + Chronic*Implement | 0.0697 | 0.0677 | 0.0212 | 0.0218 | 0.0352 | 0.0758 |
| (0.053) | (0.053) | (0.054) | (0.054) | (0.054) | (0.047) | |
| Observations | 40,144 | 40,144 | 40,144 | 40,144 | 40,144 | 40,144 |
| Chronic: Mean prescriptions | 22.14 | 20.62 | 17.46 | 17.28 | 14.63 | 4.22 |
| Acute: Mean prescriptions | 3.26 | 3.26 | 2.85 | 1.37 | 1.24 | 1.24 |
| Total # of prescriptions | 517,783 | 486,815 | 413,313 | 379,278 | 322,131 | 110,391 |
Notes:
p < 0.01
p < 0.05
p < 0.1.
Clustered standard errors at the person level. Regressions are weighted and include a linear time trend t, Chronic*t, and a full set of control variables, which are not reported. Medicaid beneficiaries are included. The bottom panel presents linear combinations of the coefficients and their standard errors to show absolute announcement and implementation effects for chronic drugs. Each column represents a classification assignment rule of increasing stringency as described in Section 5.2. MCBS 2001–2006; Ages 66–74.
I consider two additional specification tests. First, in Appendix Table A.8, I relax the linear time trend assumption for the most conservative 50% classification method. As might be expected from inspection of Fig. 2, the results are highly robust across specifications, lending support to the suitability of the linear time trend. Columns 1 and 5 repeat the baseline specification. Columns 2 and 6 allow for slope shifts in addition to the level shift and the results are almost identical to the baseline specification. Columns 3 and 7 include a quadratic trend. The results are similar (slightly larger in the level specification and slightly smaller in logs); however given only three years of pre-announcement data, the trend and trend-squared terms are likely difficult to separately identify and these estimates should be interpreted with caution. Columns 4 and 8 estimate the trend non-parametrically by including a full set of interactions between chronic and year indicators (excluding the 2001 interaction term). To interpret the coefficients, it is useful to consider the linear combinations in the bottom panel. The first estimate compares the one-year change in utilization for chronic drugs relative to acute drugs in the post-announcement period (2003–2004) versus the one-year change in the pre-announcement period (2002–2003). This is analogous to the main difference-in-difference results presented in the other columns. Here the results are larger (−1.94 for levels and −0.09 for logs) and statistically significant for both levels and logs. In the second estimate, I compare 2004–2005 to 2002–2003. The results are similar for logs and again statistically significant. For levels, the effect is not significant and this shows that the effect is concentrated in the first year after the announcement. The third estimate represents the relative implementation effect for chronic drugs.
Second, I estimate a negative binomial model. The marginal effects are reported in Appendix Table A.9. As with other non-linear models, the marginal effects are conditional on the independent variables and vary across observations. Thus, computing marginal effects for the interaction terms in non-linear models requires explicit calculation of the cross-partial or (in this case) “double-difference” of the conditional expectation function (Ai and Norton, 2003). The marginal effects are derived in Appendix Section A.2. The average announcement and implementation marginal effects for chronic and acute drugs in the negative binomial are very similar to the OLS results. The change in chronic drug utilization relative to acute use after the announcement is −1.34 compared to −1.42 in the OLS model and is statistically significant at the 1% level. As before, acute drug use does not respond significantly to the announcement.
Finally, another possible concern is that the negative announcement effect could be driven by an idiosyncratic shock that affected a single drug class (e.g. a major product discontinuation). I decompose the main announcement effect reported in Table 2 by running the basic model separately for each of the 32 therapeutic drug classes.32 In Table 6, I report the coefficients for the announcement and implementation indicators for the 8 classes of chronic and acute drugs with the highest utilization in the MCBS. I find that the negative announcement effect is not driven by a single drug class, but is a widespread phenomenon. For example, among the top 8 chronic drug classes, there are significant negative anticipatory responses for Diuretics, Hypoglycemics, Psychotherapeutic drugs, and Gastrointestinal preparations. Some chronic drugs such as Cardiac drugs and Autonomic drugs are not responsive to the announcement of Part D. Some of this heterogeneity in utilization patterns within the chronic drug class may reflect price-inelasticity to current and future prices (i.e. non-deferability), rather than failure of the intertemporal substitution hypothesis. To explore this, I compare these estimates with a physician’s coding of each drug class as “non-deferrable” versus “deferrable” in Appendix Table A.10.33 Since a physician’s interpretation of which drugs are deferrable may differ from a patient’s interpretation, this coding is likely to be conservative. The Cardiac and Autonomic drugs are classified as most likely to be non-deferrable, whereas the other chronic drug classes with significant negative anticipatory responses are classified as most likely to be deferrable or borderline deferrable/non-deferrable. The one exception is psychotherapeutic drugs which is classified as non-deferrable, but has a large negative announcement effect. In sharp contrast, the announcement effect is close to zero and statistically insignificant for nearly all acute drug classes. Consistent with these results, all of the acute drug classes were classified as non-deferrable. Taken together, the results presented in this section provide strong evidence of the prediction that chronic drugs are more responsive to the announcement than acute drugs, and that beneficiaries are postponing precisely the types of drugs that are less essential to their immediate health, suggesting an anticipatory effect of Part D.
Table 6.
Announcement and implementation effects for top chronic and acute therapeutic classes.
| Dependent variable: | Total prescriptions |
Mean # of prescriptions | Total # of prescriptions | |
|---|---|---|---|---|
| Announce | Implement | |||
|
|
|
|
|
|
| Drug class: | (1) | (2) | (3) | (4) |
|
| ||||
| Panel A: Top 8 chronic drug classes | ||||
| Cardiovascular | −0.3079* | 0.9655*** | 5.8901 | 120,965 |
| (0.173) | (0.310) | |||
| Cardiac drugs | −0.0665 | 0.1012 | 1.9975 | 41,022 |
| (0.108) | (0.185) | |||
| Diuretics | −0.2064** | −0.1903 | 1.7612 | 36,169 |
| (0.091) | (0.154) | |||
| Hypoglycemics | −0.2360** | 0.5286** | 1.7488 | 35,916 |
| (0.113) | (0.209) | |||
| Autonomic drugs | −0.0941 | 0.1252 | 1.6904 | 34,716 |
| (0.086) | (0.149) | |||
| Psychotherapeutic drugs | −0.2197** | 0.1103 | 1.5812 | 32,474 |
| (0.104) | (0.180) | |||
| Gastrointestinal preparations | −0.2556*** | 0.0419 | 1.5326 | 31,474 |
| (0.092) | (0.156) | |||
| Antiarthritics | −0.1277* | −0.1048 | 1.0987 | 22,563 |
| (0.068) | (0.118) | |||
| Panel B: Top 8 acute drug classes | ||||
| Analgesics | −0.1340** | 0.1826 | 0.7640 | 15,690 |
| (0.063) | (0.112) | |||
| EENT preparations | 0.0312 | 0.2302* | 0.7469 | 15,339 |
| (0.075) | (0.119) | |||
| Antiinfectives | −0.0143 | 0.048 | 0.5549 | 11,395 |
| (0.038) | (0.062) | |||
| Antihistamines | −0.0359 | −0.0187 | 0.4190 | 8,604 |
| (0.046) | (0.075) | |||
| Antiinfectives, miscellaneous | −0.0402 | 0.0826 | 0.2980 | 6,120 |
| (0.034) | (0.055) | |||
| Skin preparations | 0.0127 | 0.0554 | 0.2546 | 5,228 |
| (0.034) | (0.052) | |||
| Muscle relaxants | −0.0091 | 0.0275 | 0.1408 | 2,891 |
| (0.020) | (0.034) | |||
| Cough and cold preparations | 0.0377* | 0.1265*** | 0.1352 | 2,776 |
| (0.021) | (0.033) | |||
Notes:
p < 0.01
p < 0.05
p < 0.1.
Clustered standard errors at the person level. Columns 1 and 2 are coefficients from 16 regressions of total prescriptions for the drug class on the announcement and implementation indicators, a linear time trend, and a full set of control variables. Regressions are weighted and Medicaid beneficiaries are included. The mean number of prescriptions include zeros. MCBS 2001–2006; Ages 66–74.
7. Additional tests of the anticipatory effect
7.1. Mechanisms
Two possible mechanisms for a negative utilization response are reductions in the probability of treatment initiation and increases in the probability of treatment discontinuation. Using two-year panels, I estimate the probability of initiating treatment with an indicator which equals 1 if a person uses at least one drug in class in period conditional on not having used any drugs in that class in period . The discontinuation probability is defined in the opposite way. Trends for the transition probabilities are plotted in Appendix Figures A.6 and A.7. I find a pre-reform decline in the initiation probability followed by a steep increase in 2006. While this pattern is consistent with elderly delaying the initiation of new treatments, without access to more years of data it is difficult to determine whether this represents a break from the pre-existing trend. Discontinuation probabilities are noisier, making it difficult to draw conclusions. If there is a reduction in initiation, we should also observe a decline in doctor visits since they are a necessary condition for starting new treatments. There is a close correspondence between the trend in utilization and doctors’ visits as shown graphically in Appendix Figure A.8.
7.2. Alternative hypotheses
The results presented in the above sections provide strong evidence that the decline in utilization following the announcement of Part D can be explained by an anticipatory delay in drug use. Below I examine the potential significance of three possible alternative supply-side explanations for the decline in drug use after 2003.
First, one alternative explanation is that pharmaceutical firms, responding to the reduced price-sensitivity of the elderly under Part D, may have found it optimal to increase drug prices. In order to avoid the potential political backlash from increasing prices after implementation, firms may have started to raise prices as soon as the announcement was made. Thus the observed decrease in drug utilization could reflect a contemporaneous response to current price rather than anticipatory behavior by the elderly. I test for this directly by estimating whether prices increased after the announcement for drugs that are most likely to be used by Medicare beneficiaries (which is similar in spirit to Duggan and Scott Morton, 2010). I estimate the following model:
| (3) |
The outcome is the price of drug in year and is computed by dividing total expenditures over total prescriptions in the MEPS. I include a full set of year fixed effects, drug fixed effects, and interactions of year fixed effects with the Medicare market share (MMS). The MMS is the fraction of prescriptions that are purchased by Medicare beneficiaries for drug in the 2002–2003 MEPS. This regression is estimated at the drug level for the top 200 brand-name drugs in terms of 2003 sales as reported in Drug Topics magazine. If suppliers respond as hypothesized, drugs that are differentially used by Medicare beneficiaries should see the greatest price growth. In results reported in Table 7, I find evidence of negative and statistically insignificant relative price growth for drugs with higher Medicare market share immediately after the announcement. The absence of a significant price hike among top drugs suggests that the decline in utilization did not result from contemporaneous price changes.
Table 7.
Announcement and implementation effects for pharmaceutical prices by Medicare market share.
| Dependent variable: | Price |
Log(price) |
|---|---|---|
| (1) | (2) | |
|
| ||
| MMS*Year2001 | −1.2987 | −0.1006 |
| (10.673) | (0.134) | |
| MMS*Year2002 | 5.5946 | −0.0473 |
| (10.021) | (0.118) | |
| MMS*Year2003 | −4.5142 | −0.1458 |
| (10.656) | (0.115) | |
| MMS*Year2004 | −4.4471 | −0.1957* |
| (10.376) | (0.119) | |
| MMS*Year2005 | −1.2343 | −0.1451 |
| (10.988) | (0.117) | |
| MMS*Year2006 | −16.3658 | −0.2405* |
| (11.754) | (0.123) | |
| (MMS*2004-MMS*2003) – (MMS*2002-MMS*2001) | −6.8262 | −0.1033 |
| (10.349) | (0.142) | |
| (MMS*2005-MMS*2003) – (MMS*2003-MMS*2001) | 6.4955 | 0.0458 |
| (14.005) | (0.161) | |
| (MMS*2006-MMS*2005) – (MMS*2002-MMS*2001) | −22.0248* | −0.1488 |
| (12.032) | (0.147) | |
| Weighted by #Rx 02–03 | Y | Y |
| Year fixed effects | Y | Y |
| Drug fixed effects | Y | Y |
| Observations | 924 | 924 |
Notes:
p < 0.01
p < 0.05
p < 0.1.
Robust standard errors in parentheses. This regression is estimated at the drug-year level from Equation 3. The MMS is the fraction of prescriptions that are purchased by Medicare beneficiaries for each drug in the 2002–2003 pooled MEPS. The bottom panel presents linear combinations of the coefficients and their standard errors. MEPS 2000–2006.
Second, I also consider the possibility that insurers or employers discontinued drug coverage following the announcement of Part D. Increasing out-of-pocket costs could then explain a contemporaneous decline in drug use. Using a simple interrupted time series, I find a statistically significant 3.7 percentage point decline in drug insurance coverage after the announcement (Table 8). Decomposing this effect by insurance type, I find that this effect is driven by declining drug coverage from Medigap and Medicare HMO plans. Given the limitations of the data, I cannot distinguish whether this decline was due to changes in offer rates by insurers or changes in take-up. Reductions in take-up would be consistent with a demand-side anticipatory response as hypothesized above. In other words, an individual deciding to postpone drug utilization would also be more willing to defer drug insurance coverage, since it is less valuable when one intends to use fewer drugs. On the supply side, it does not seem plausible that Medigap and Medicare HMO plans would have the ability to discontinue coverage prior to Part D since they are guaranteed issue plans. Moreover, if reduction in coverage is due to lower offer rates, it should have affected both chronic and acute drug use alike which is inconsistent with the empirical findings. Thus, it is likely that changes in coverage can be explained by changes in take-up, which is mechanism through which the demand-side anticipation effect is operating.
Table 8.
Announcement effects for drug insurance coverage.
| Dependent variable: | I(Drug-insured = 1) |
||||||
|---|---|---|---|---|---|---|---|
| All drug insured |
Employer sponsored insurance |
Self-purchased private (excl. Medigap) |
Medigap |
Private HMO |
Medicare HMO |
||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |
|
| |||||||
| Announce | −0.0317*** | −0.0365*** | −0.0035 | 0.0235** | −0.0203*** | 0.0144 | −0.0158*** |
| (0.010) | (0.011) | (0.013) | (0.009) | (0.007) | (0.010) | (0.005) | |
| t | 0.0179*** | 0.0190*** | 0.0073 | −0.0079** | 0.0079*** | −0.0118*** | 0.0055*** |
| (0.004) | (0.004) | (0.005) | (0.003) | (0.002) | (0.004) | (0.002) | |
| Excluding Medicaid beneficiaries | N | Y | Y | Y | Y | Y | Y |
| Observations | 16,876 | 14,981 | 14,981 | 14,981 | 14,981 | 14,981 | 14,981 |
Notes:
p < 0.01
p < 0.05
p < 0.1.
Clustered standard errors at the person level. The regressions are weighted and include a full set of control variables. The coefficients are from an OLS regression of an indicator for drug insurance (for each type of drug insurance) on the announcement indicator and a linear time trend. MCBS 2001–2005.
Finally, I explore whether there were underlying shocks to physician office visits and medical care provision, more generally, which coincided with the announcement of Part D (which may have independently impacted drug utilization). To test for this, I examine the announcement and implementation effects for several medical procedures that are not covered by Part D. In particular, I examine screenings (mammograms, pap tests, and prostate exams) and flu shots (covered by Part B). This serves as a useful “placebo” test since a physician office visit is also often required to prescribe or administer these procedures (as with prescription drugs), though these procedures should be unaffected by PartD.AppendixTableA.11 shows interrupted time series estimates for the probability of obtaining each procedure. Indeed, the announcement and implementation effects for these procedures are all close to zero and statistically insignificant. The point estimates are slightly positive, which is consistent with possible income effects from Part D, though the estimates are not significant. These results are reassuring that the reduction in drug utilization after the announcement is not driven by other secular trends in medical care utilization.
7.3. Anticipatory effects before Medicare eligibility?
Finally, I return to the Medicare age-eligible and age-ineligible split to test whether the announcement of Part D affected consumption patterns for adults who are not yet eligible for Medicare. I use the MEPS to compare utilization patterns for two groups of age-ineligible adults aged 50–58 and 59–64 with the elderly aged 66–74 who are eligible for Medicare. Graphically, utilization declines relative to trend for the age-eligible and oldest age-ineligible groups after the announcement with no change for the youngest age group (Appendix Figure A.9). The regression results in Appendix Table A.12 confirm this observation; however due to the small sample size of the MEPS there is not enough power to estimate the effects precisely. Individuals who were aged 62 to 64 at the end of 2003 could expect to become eligible for Medicare in time for Part D, while those aged 59 to 61 would become eligible shortly thereafter. It is possible that the anticipatory effects could be even stronger for the Medicare-ineligible, since many individuals would anticipate gaining not only drug coverage, but also coverage of doctor visits which are complementary to drug use. The results provide suggestive evidence that individuals nearing Medicare eligibility also change their drug utilization in anticipation of future coverage.
8. Conclusion
The advance announcement of Part D in late 2003 provides an opportunity to evaluate the effects of program announcements in addition to providing a test of life-cycle behavior in the context of drug demand. Economic theory makes ambiguous predictions about the effect of a forecastable future price change on the direction of the utilization effect. For chronic drugs that treat long term illnesses, the effect could be either positive or negative. Meanwhile, acute drugs that treat short duration medical events are unlikely to be affected by future price changes.
The results of this study demonstrate a marked decline in drug use following the announcement of Part D of approximately 6% (or a decline of nearly 2 prescriptions per year), suggesting that the elderly delayed drug use in anticipation of lower future prices. The anticipatory effects are strongest for the youngest Medicare beneficiaries, those with below-median incomes, and those without employer prescription drug coverage. I also find suggestive evidence that individuals not yet eligible for Medicare may also respond to the anticipation of future coverage. Together, these results present strong evidence of important anticipation effects in the context of a major health care policy.
Since the negative anticipatory response can be observed across many drug classes, we can rule out the possibility that the anticipatory effect is driven by idiosyncratic shocks to a single class. Moreover, I find strong evidence that this anticipatory response is concentrated among chronic drugs which is consistent with the main theoretical predictions. The comparison of chronic and acute drugs is advantageous because any plausible alternative explanation must also explain this differential effect. I find little evidence that the decline in utilization is driven by anticipatory responses on the supply side.
Finally, the observed anticipatory decline in drug use has consequences for evaluating the program effect of Part D. When I take into account the negative announcement effect, my estimates of the implementation impact of Part D are reduced by about one-half. Thus, failing to account for anticipatory responses may overstate the impact of Part D on drug utilization.34 In a similar way, anticipation effects may also confound future evaluations of the 2010 Affordable Care Act and should be explicitly estimated.
Supplementary Material
Acknowledgements
I am especially grateful to Mark Duggan and Judith Hellerstein for their invaluable advice and support. I also thank Ana Abras, Paul Bailey, Juan Bonilla-Angel, Tami Calvez, Carolina Gonzalez-Velosa, Raymond Guiteras, Tamara Hayford, Mireille Jacobson, Ginger Jin, Darius Lakdawalla, Gabriel Lara-Ibarra, Kenneth Leonard, Willard Manning, David Powell, Jeff Prince, Kosali Simon, and seminar and conference participants at the University of California-Irvine, University of Chicago, University of Colorado-Denver, University of Georgia, University of Maryland, Vanderbilt University, University of Pittsburgh-School of Public Health, Conference of the American Society of Health Economists, Midwest Health Economics Conference, Southern California Conference in Applied Microeconomics, RAND, Mathematica, U.S. Treasury, and CBO for helpful discussions and suggestions. This work was supported by grant number R36HS019681 from the Agency for Healthcare Research and Quality. The content does not represent the views of the Agency for Healthcare Research and Quality. All errors are my own.
Footnotes
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.jhealeco.2016.06.004.
In other settings, implementation lag is also common, including changes to the minimum wage, taxes, Social Security, and welfare benefits. For example, the Fair Minimum Wage Act of 2007 phased-in three minimum wage hikes to take effect in 2007, 2008, and 2009; the Tax Reform Act of 1986 phased in over two years; the Social Security Amendments of 1983 increased the normal retirement age by two-months each year starting in 2000.
There are a few examples in which anticipatory behavior has been shown to lead to biased estimates of program treatment effects, including the Social Security Amendments of 1983 (Mastrobuoni, 2009), the Tax Reform Act of 1986 (e.g. Slemrod, 1995; Scholes et al., 1992), tort reform (Malani and Reif, 2015), and welfare reform in the UK (Blundell et al., 2010).
For example, see Lichtenberg and Sun, 2007; Yin et al, 2008; Ketcham and Simon, 2008.
The lag between the announcement and implementation of policies has also been used in tests of the “rational addiction” model (Gruber and Koszegi, 2001) and the life-cycle hypothesis (Wilcox, 1989).
This test is similar in spirit to Sorensen (2000), which exploits across-drug variation in the frequency of prescription fills to estimate the effect of search on price dispersion.
Long et al. (1998) use data from the SIPP to examine individuals’ medical care prior to gaining or losing insurance coverage, finding no evidence of forward-looking behavior. Gross (2009) exploits the forecastable change in health insurance status that occurs when teenagers lose their family’s coverage and become uninsured at age 19 (pre-ACA). The study finds no evidence that teenagers “stock up” on health care before losing insurance. Related to these studies, analysis of the RAND Health Insurance experiment has been criticized for its assumption of myopia in not accounting for within-year price variation (Kowalski, 2016; Manning et al., 1987).
Some outpatient prescription drug coverage has been provided through Part C, also known as Medicare Advantage (MA). Drug coverage under Part C was not very generous. In 2003, 69 percent of Part C enrollees in basic plans received drug coverage with 60 percent of plans covering only generic drugs (Achman and Gold, 2003).
Before Part D was implemented – between June 2004 and January 2006 – Medicare beneficiaries could enroll for a small fee in a drug discount card that provided discounts at the point-of-sale, with estimated savings of approximately 17% (Cubanski et al., 2004). Only 5.8 million Medicare beneficiaries had enrolled in the program six months after it was introduced, with the vast majority automatically enrolled due to their low income status. Moreover, most of the automatic enrollees did not activate their discount cards (Thomas et al., 2005).
These estimates are from the age-standardization that Ketcham and Simon (2008) perform to compare the results of Yin et al. (2008) and Lichtenberg and Sun (2007).
Another recent paper uses the MCBS to compare the previously drug-uninsured elderly with those with drug insurance (Kaestner and Khan, 2012). Finally, using IMS sales data, Duggan and Scott Morton (2010) examine whether drug use increased between 2003 and 2006 differentially for drugs that had a higher Medicare market share. They find a large utilization effect that is insignificant and statistically imprecise.
See for example Ashenfelter (1978); Heckman and Smith (1999).
These models begin with Lucas and Rapping (1970) and Friedman’s “permanent income hypothesis” (Friedman, 1957). The large literature that has followed for labor supply is surveyed in Card (1991).
Since Part D changed the lifetime price path of drugs for individuals of all ages, it is possible that the announcement affected consumption for even those not yet eligible for Medicare. However, the short-run effects are likely strongest for Medicare beneficiaries and individuals closest to eligibility.
In other words, individuals would find it optimal to purchase the drug when the expected lifetime path of prices for that treatment was higher (in the absence of the announcement), but not purchase the drug when the expected lifetime path of prices was lower (after learning of the announcement).
Moreover, even in the case of a lifetime therapy, we can appeal to behavioral models of contextual price effects (Thaler, 1985) to explain a delay in the timing of purchases. If we consider the announcement of Part D as introducing a new lower “reference price” for drugs, then purchasing drugs before implementation at a price that is higher than the reference price may be perceived as a “loss”, which generates transaction disutility. This disutility may consequently reduce drug use.
I exclude individuals over age 85 due to the non-comparable measurement of drug utilization for the institutionalized population.
A person is defined as drug-insured if they report that at least one of their insurance plans covers prescription drugs or if they receive drug coverage from a public program.
This strategy differs from a more typical difference-in-difference strategy where pre-trends are assumed to be parallel. When treatment and comparison groups are allowed to have different trends, this strategy is sometimes called a Comparative Interrupted Time Series (CITS) – i.e., an interrupted time series with a comparison group. CITS is commonly used in the education literature and program evaluation (e.g., see discussion in Dee and Jacob, 2011; Shadish et al., 2002), as well as in economics (e.g., Jayachandran et al., 2010) and is well suited for this application given the differential trends in chronic and acute drugs and the visibly linear pre-trends. The typical difference-in-difference estimator is a special case of CITS, since it imposes the stronger assumption of parallel trends.
More formally, in the standard difference-in-difference approach the parallel trends assumption is that the difference between the treatment and comparison group means remain constant in the absence of treatment. In my approach, the analogous assumption is that the difference between the growth rate (in levels) of means is constant (Mora and Reggio, 2012). I assume that any deviation from this difference in growth rates is due to Part D.
Results from a non-parametric event study (not shown) confirms that the only structural breaks in the chronic trend relative to the acute trend occur in 2004 (after the announcement) and again in 2006 (after the implementation). This is consistent with the analogous graphical evidence in Fig. 2.
I also estimate Equation 1 using a negative binomial model in the Appendix to better account for the count nature of the data.
32 classes have a positive number of prescriptions for the elderly. I exclude drugs with no therapeutic classification.
Medicaid Dual-Eligible beneficiaries were switched from Medicaid drug coverage to Medicare coverage in 2006 and would be unlikely to experience large changes in cost-sharing. As expected, we do not find statistically significant announcement effects for Medicaid Dual-Eligibles in Appendix Table A.2, though these estimates are imprecise since the sample size is very small.
Some of the loss in precision may reflect measurement error for expenditures, which may be less accurately reported than the number of prescription purchases.
Consistent with this, studies have shown that an education-gradient exists for compliance to drug treatments (e.g., Goldman and Smith, 2002).
While there is a slight reduction in acute drug utilization relative to trend after the announcement, this reduction is not statistically different from zero (as will be shown in Table 4). Moreover, it should be noted that the classification of acute and chronic drugs used in Fig. 2 is the most conservative (i.e., uses the 50% classification rule), thus some drugs in the acute category may actually be chronic and vice versa, which may explain the very slight reduction for acute drugs after the announcement. As the more stringent classification rules are imposed (which removes measurement error in the classification), the announcement effect for acute drugs becomes even closer to zero.
I proxy for chronic conditions by splitting the sample by self-reported health status and by the number of diagnosed conditions. The announcement effect is largest for the unhealthiest beneficiaries aged 66–74, who are likely to be using the most chronic drugs. An alternative approach to this test would be to compare the announcement effect across specific chronic conditions (e.g., diabetes, cancer, etc.). In results not reported, I conduct this test for a variety of conditions finding largely negative anticipation effects which is consistent with Appendix Table A.6. However, given the small sample size for each condition, this effect is statistically insignificant and imprecise for some conditions.
In this section I have focused on the absolute change in utilization relative to trend after the implementation, rather than the DID estimate, because acute drug use may also be responsive to the implementation of Part D.
The procedure requires two steps. First, I estimate a non-parametric version of Equation 1, including separate chronic × year interaction terms, chronic indicator, year fixed effects, and control variables. In the second step, I use the estimated coefficients on the chronic × year interaction terms, which represent the adjusted mean difference in prescriptions across chronic and acute drug groups in each year. I regress these coefficients on indicators for the announcement, implementation and a linear time trend. Following Wooldridge’s 2003 minimum distance approach – an extension of the Donald and Lang method – I estimate this regression using weighted least squares.
Delaying one acute treatment would lead to a decline in utilization of one or two prescriptions, whereas delaying one chronic treatment would lead to a decline of five or more prescriptions.
Each regression uses as an outcome the total number of prescriptions purchased in each drug class, including zeros.
I define non-deferrable drugs as those which need to be taken immediately to prevent severe health consequences or undesirable symptoms, whereas deferrable drugs could be postponed. Specifically, the physician was given four options for each drug class (“much more likely to be non-deferrable than deferrable”; “somewhat more likely to be non-deferrable than deferrable”; “somewhat more likely to be deferrable than non-deferrable”; “much more likely to be deferrable than nondeferrable”). I collapse the middle two categories to create three groups.
This smaller program effect size (compared to the large effects found in other studies) is also consistent with the partial-year enrollment of many beneficiaries and known administrative difficulties which may have hindered enrollment in the first year.
Uncited references
Abaluck, Gruber, 2011, Brown et al, 2014, Engelhardt, Gruber, 2011, Hall, 1978, Kling et al, 2012, MaCurdy, 1981
References
- Abaluck J, Gruber J, 2011. Choice inconsistencies among the elderly: evidence from plan choice in the Medicare Part D program. The American Economic Review 101 (4), 1180–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelson R High-end health plans scale back to avoid Cadillac tax. New York Times, May 27, 2013. [Google Scholar]
- Achman L, Gold M, 2003. Medicare+Choice Plans Continue to Shift More Costs to Enrollees. Mathematica Policy Institute, Inc. [Google Scholar]
- Adams A, Soumerai S, Ross-Degnan D, 2001. The case for a Medicare drug coverage benefit: a critical review of the empirical evidence. Annual Review of Public Health 22, 49–61. [DOI] [PubMed] [Google Scholar]
- Ai C, Norton E, 2003. Interaction terms in logit and probit models. Economics Letters 80 (1), 123–129. [Google Scholar]
- Aron-Dine A, Einav L, Finkelstein A, Cullen M, 2015. Moral hazard in health insurance: do dynamic incentives matter? Review of Economics and Statistics 97 (4), 725–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashenfelter O, 1978. Estimating the effect of training programs on earnings. Review of Economics and Statistics 60, 47–57. [Google Scholar]
- Becker G, Murphy K, 1988. A theory of rational addiction. The Journal of Political Economy 96 (4), 675–699. [Google Scholar]
- Bertrand M, Duflo E, Mullainathan S, 2004. How much should we trust differences-in-differences estimates? The Quarterly Journal of Economics 114, 249–275. [Google Scholar]
- Blundell R, Francesconi M, van der Klaauw W, 2010. Anatomy of policy reform evaluation: announcement and implementation effects. Mimeo. [Google Scholar]
- Brown J, Duggan M, Kuziemko I, Woolston W, 2014. How does risk selection respond to risk adjustment? Evidence from the Medicare advantage program. The American Economic Review 104 (10), 3335–3364. [DOI] [PubMed] [Google Scholar]
- Cabral M, 2015. Claim timing and ex post insurance selection. Mimeo. [Google Scholar]
- Card D, 1991. Intertemporal labor supply: an assessment. NBER Working Paper No. 3602. [Google Scholar]
- Card D, Dobkin C, Maestas N, 2008. The impact of nearly universal insurance coverage on health care utilization and health: evidence from Medicare. The American Economic Review 98 (5), 2242–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubanski J, Frank R, Epstein A, 2004. Savings from drug discount cards: relief for Medicare beneficiaries? Health Affairs W4, 198–209. [DOI] [PubMed] [Google Scholar]
- Dee T, Jacob B, 2011. The impact of No Child Left Behind on student achievement. Journal of Policy Analysis and Management 30 (3), 418–446. [Google Scholar]
- Donald S, Lang K, 2007. Inference with difference-in-differences and other panel data. The Review of Economics and Statistics 89 (2), 221–233. [Google Scholar]
- Duggan M, Scott Morton F, 2010. The impact of Medicare Part D on pharmaceutical prices and utilization. The American Economic Review 100 (1), 590–607. [DOI] [PubMed] [Google Scholar]
- Einav L, Finkelstein A, Schrimpf P, 2015. The response of drug expenditure to nonlinear contract design: evidence from Medicare Part D. The Quarterly Journal of Economics 130 (2), 841–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt G, Gruber J, 2011. Medicare Part D and the financial protection of the elderly. American Economic Journal: Economic Policy 3 (4), 77–102. [Google Scholar]
- Friedman M, 1957. A Theory of the Consumption Function. Princeton University Press, Princeton, N.J. [Google Scholar]
- Goldman D, Smith J, 2002. Can patient self-management help explain the SES health Gradient? Proceedings of the National Academy of Sciences of the United States of America 99 (16), 10929–10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Joyce GF, Escarce JJ, Pace JE, Solomon MD, Laouri M, Landsman PB, Teutsch SM, 2004. Pharmacy benefits and the use of drugs by the chronically ill. Journal of the American Medical Association 291 (19), 2344–2350. [DOI] [PubMed] [Google Scholar]
- Goldman D, Joyce G, Zheng Y, 2007. Prescription drug cost sharing. Associations with medication and medical utilization and spending and health. Journal of the American Medical Association 298 (1), 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross T, 2009. Using insurance before you lose it: health care consumption at the end of coverage. Mimeo. [Google Scholar]
- Grossman M, 1972. On the concept of health capital and the demand for health. The Journal of Political Economy 80, 223–255. [Google Scholar]
- Gruber J, Koszegi B, 2001. Is addiction “rational”? Theory and evidence. The Quarterly Journal of Economics 116 (4), 1261–1303. [Google Scholar]
- Hall R, 1978. Implications of the life cycle-permanent income hypothesis: theory and evidence. The Journal of Political Economy 86 (6), 971–987. [Google Scholar]
- Heckman J, Smith J, 1999. The pre-programme earnings dip and the determinants of participation in a social programme: implications for simple programme evaluation strategies. The Economic Journal 109 (457), 313–348. [Google Scholar]
- Jayachandran S, Lleras-Muney A, Smith K, 2010. Modern medicine and the twentieth century decline in mortality: evidence on the impact of sulfa drugs. American Economic Journal: Applied Economics 2, 118–146. [Google Scholar]
- Kaestner R, Khan N, 2012. Medicare Part D and its effect on the use of prescription drugs, use of other health care services and health of the elderly. Journal of Policy Analysis and Management 31 (2), 253–279. [Google Scholar]
- Ketcham JD, Simon KI, 2008. Medicare Part D’s effects on elderly drug costs and utilization. American Journal of Managed Care 11, SP14–SP22. [PubMed] [Google Scholar]
- Kaiser Family Foundation, 2006. The Medicare prescription drug benefit – fact sheet. <http://www.kff.org/medicare/7044.cfm>.
- Kaiser Family Foundation, 2007. The Medicare prescription drug benefit – fact sheet. <http://www.kff.org/medicare/7044.cfm>.
- Kling J, Mullainathan S, Shafir E, Vermeulen LC, Wrobel MV, 2012. Comparison friction: experimental evidence from Medicare drug plans. The Quarterly Journal of Economics 127 (1), 199–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski A, 2016. Censored quantile instrumental variable estimates of the price elasticity of expenditure on medical care. Journal of Business and Economic Statistics 34 (1), 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman P, Yu W, Liu X, Teutsch SM, Berger ML, 2005. Impact of 3-tier pharmacy benefit design and increased consumer cost-sharing on drug utilization. American Journal of Managed Care 11 (10), 621–628. [PubMed] [Google Scholar]
- Levy H, Weir D, 2010. Take-up of Medicare Part D: results from the health and retirement study. Journals of Gerontology: Series B, Gerontological Society of America, 65 (4), 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg F, Sun S, 2007. The impact of Medicare Part D on prescription drug use by the elderly. Health Affairs 26 (6), 1735–1744. [DOI] [PubMed] [Google Scholar]
- Long S, Marquis S, Rogers J, 1998. Do people shift their use of health services over time to take advantage of insurance? Journal of Health Economics 17, 105–115. [DOI] [PubMed] [Google Scholar]
- Lucas R, Rapping L, 1970. Real wages, employment and inflation. In: Phelps E. (Ed.), Microeconomic Foundations of Employment and Inflation Theory. Norton, New York. [Google Scholar]
- MaCurdy T, 1981. An empirical model of labor supply in a life-cycle setting. The Journal of Political Economy 89 (6), 1059–1085. [Google Scholar]
- Malani A, Reif J, 2015. Interpreting pre-trends as anticipation: impact on estimated treatment effects from tort reform. Journal of Public Economics 124, 1–17. [Google Scholar]
- Manning W, Newhouse JP, Duan N, Keeler EB, Leibowitz A, Marquis MS, 1987. Health insurance and the demand for medical care: evidence from a randomized experiment. The American Economic Review 77 (3), 251–277. [PubMed] [Google Scholar]
- Mastrobuoni G, 2009. Labor supply effects of the recent social security benefit cuts: empirical estimates using cohort discontinuities. Journal of Public Economics 93 (11–12), 1224–1233. [Google Scholar]
- Mojtabai R, Olfson M, 2003. Medication costs, adherence, and health outcomes among Medicare beneficiaries. Health Affairs 22 (4), 220–229. [DOI] [PubMed] [Google Scholar]
- Mora R, Reggio I, 2012. Treatment effect identification using alternative parallel assumptions. Mimeo. [Google Scholar]
- Oliver T, Lee P, Lipton H, 2004. A political history of Medicare and prescription drug coverage. The Milbank Quarterly 82 (2), 283–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear R Sweeping Medicare change wins approval in congress; president claims a victory. New York times. November 26, 2003. [Google Scholar]
- Piotrowski J Health policy brief: excise tax on ‘Cadillac’ plans, Health Affairs. September 12, 2013. [Google Scholar]
- Scholes M, Wilson G, Wolfson M, 1992. Firms’ responses to anticipated reductions in tax rates: the Tax Reform Act of 1986. Journal of Accounting Research 30, 161–185. [Google Scholar]
- Shadish W, Cook T, Campbell D, 2002. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. Houghton-Mifflin, Boston. [Google Scholar]
- Skipper N, 2013. On the demand for prescription drugs: heterogeneity in price responses. Health Economics 22, 857–869. [DOI] [PubMed] [Google Scholar]
- Slemrod J, 1995. Income creation or income shifting? Behavioral responses to the Tax Reform Act of 1986. The American Economic Review 85 (2), 175–180. [Google Scholar]
- Sorensen A, 2000. Equilibrium price dispersion in retail markets for prescription drugs. The Journal of Political Economy 108 (4), 833–850. [Google Scholar]
- Thaler R, 1985. Mental accounting and consumer choice. Marketing Science 4 (3), 199–214. [Google Scholar]
- Thomas C, Wallack S, Martin T, 2005. How do seniors use their prescription drug discount cards? Health Affairs W5, 180–190. [DOI] [PubMed] [Google Scholar]
- Wilcox D, 1989. Social security benefits, consumption expenditure, and the life cycle hypothesis. The Journal of Political Economy 97 (2), 288–304. [Google Scholar]
- Wooldridge J, 2003. Cluster-sample methods in applied econometrics. American Economic Review, Papers and Proceedings 93 (2), 133–138. [Google Scholar]
- Yin W, Basu A, Zhang JX, Rabbani A, Meltzer DO, Alexander GC, 2008. The effect of the Medicare Part D prescription benefit on drug utilization and expenditures. Annals of Internal Medicine 148 (3), 169–177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


