Abstract
Objectives:
To examine the time required to suppress HIV in the genital tract with antiretroviral therapy (ART) in men with urethritis.
Design:
An observational cohort study.
Methods:
Men with HIV and urethritis not on ART were enrolled at an STI clinic in Malawi and offered to initiate ART. Blood and semen samples were collected pretreatment and at 1, 2, 4, 8, 12 and 24 weeks posturethritis treatment. Median viral loads (VLs) were calculated by ART initiation groups: ‘within 1 week’, ‘between 1 and 4 weeks’ and ‘no ART before 4 weeks’, based on the men's choice about whether or not to initiate ART. The presence of ART at each visit was confirmed by bioanalytical methods.
Findings:
Between January 2017 and November 2018, 74 men presented with urethritis and HIV and were confirmed ART naive. The median age was 32 years. Forty-one (55% of men) initiated ART within 1 week; 12 (16%) between 1 and 4 weeks; and 21 (28%) did not initiate ART by week 4. Within the 1 week group, median VL was suppressed within 4 weeks in both semen and blood. Among the 1–4 weeks group, VL was suppressed within 4 weeks in semen and 5 weeks in blood. Among the no ART before 4 weeks group, VL in semen declined within the first 4 weeks but remained unsuppressed through week 24, and there was no significant decline in blood HIV.
Conclusion:
Treatment of urethritis and prompt initiation of ART with counseling for safer sex for at least one month is a critical measure to reduce transmission of HIV.
Keywords: antiretroviral therapy, blood, HIV, semen, urethritis
Introduction
HIV concentration in blood [1] and semen [2] strongly correlate with the probability of HIV transmission. HIV concentration in semen is increased with the mucosal inflammation associated with urethritis [3,4], creating greater risk of viral transmission [2,3,5]. We recently demonstrated that treatment of gonococcal urethritis with an antibacterial agent among men already receiving antiretroviral therapy (ART) led to a decrease in transiently raised HIV viral load in semen to a baseline level over one month [6]. In the present study, we describe the velocity of reduction of HIV in blood and semen in men presenting with urethritis with initiation of ART. It is common to find untreated HIV infection among men who present with urethritis, and so understanding the timing and magnitude of benefit of initiation of ART is important for HIV prevention.
Methods
We conducted an observational cohort study of HIV-infected ART naïve men with acute urethritis, defined as presence of urethral discharge during clinical examination, at the Bwaila District Hospital STI clinic in Lilongwe, Malawi [6]. The Bwaila District Hospital is the largest public secondary care health facility under the Lilongwe District Health Office [6].
Men living with HIV who came for treatment of urethritis were recruited on the same day of their clinic visit. HIV diagnosis was confirmed using rapid point-of-care tests per Malawian HIV testing guidelines [7]. Blood and semen samples were collected for HIV viral load testing and syphilis serologies were performed (BD Macro-Vue RPR and Serodia TP-PA). Urethral swabs were collected at all episodes of urethritis for etiologic testing (Neisseria gonorrhea, Chlamydia trachomatis, and Trichomonas vaginalis) using GeneXpert (N. gonorrhoea and C. trachomatis), culture (N. gonorrhoeae) or OSOM-Trichomonas 105 Rapid Test (T. vaginalis). All patients were treated for urethritis with gentamicin 240 mg i.m. single dose, doxycycline 100 mg orally twice daily for 7 days, and metronidazole 2 g orally as a single dose, and referred for ART initiation per Malawian syndromic STI treatment guidelines [8].
Follow-up study visits occurred at 1, 2, 4, 8, 12, 24, 36, and 48 weeks. If a participant initiated ART after their week 1 visit, the calendar reset to week 1 to optimize visit frequency around the time of ART initiation. At each follow-up visit, blood and semen were collected for HIV viral loads and any incident cases of urethritis were assessed. Viral suppression was defined as <400 copies/ml. Detectable viral load was 20 copies/ml.
In this analysis, we included men living with HIV who enrolled in the study and completed at least one follow-up visit. Participants who had ART detected in their blood at enrollment were excluded from this analysis. For our longitudinal analyses, participant follow-up was analytically censored at any repeat case of urethritis after 4 weeks (urethral discharge within 4 weeks of enrollment was considered persistent urethritis) [6]. Follow-up was also analytically censored at ART interruption for those who started within 4 weeks of urethritis diagnosis, and at ART initiation for those who started after 4 weeks post-urethritis diagnosis. Pre-ART follow-up data from the latter group was then included in the ‘no ART before 4 weeks’ analyses. We used descriptive statistics to characterize participants, as well as Fishers exact test (categorical variables) and the Mann–Whitney U test (continuous variables) to assess differences by ART initiation status (α = 0.05). Median viral loads and interquartile ranges (IQRs) were calculated by visit and ART initiation groups.
To confirm ART use, blood plasma from venipuncture specimens from each visit were qualitatively screened for the presence of 10 different ART drugs by adapting a previously described LC–MS/MS approach [9] for the following drugs; efavirenz, atazanavir, lopinavir, ritonavir, tenofovir, lamivudine, emtricitabine, abacavir, zidovudine and nevirapine.
This study was approved by the Malawi National Health Science Research Ethics Committee and the University of North Carolina Institutional Review Board. All participants provided written informed consent for study participation. Study funders had no role in study.
Results
Between January 2017 and November 2018, 74 men presented to the STI clinic with acute urethritis and HIV who were confirmed to not be taking ART and had ≥1 follow-up visit. (Table 1). Participants reported having urethritis a median of 4 days (IQR: 3, 7) before seeking treatment. Patients had varying degrees of delay of ART (Table 1). Sixty participants initiated a combination of tenofovir, lamivudine and efavirenz and two participants initiated tenofovir, lamivudine and nevirapine. Detailed information about the cause of urethritis and the participants is presented in the Table 1.
Table 1.
Participant characteristics of men with acute urethritis living with HIV and not taking ART at enrollment.
| Characteristic | Total n (%) | Initiated ART n (%) | No ART n (%) |
| Total | 74 | 62 | 12 |
| Age (median, IQR) | 32 (27, 37) | 32 (27, 37) | 32 (26, 38) |
| Days of urethritis (median, IQR) | 4 (3, 7) | 4 (3, 7) | 4 (3, 11) |
| Urethritis etiologies at enrollment | |||
| Neisseria gonorrhoeae (NG) | 62 (84) | 51 (82) | 11 (92) |
| Neisseria gonorrhoeae (by NAAT) | 62 (84) | 51 (82) | 11 (92) |
| Neisseria gonorrhoeae (by culture)a | 53 (73) | 44 (71) | 9 (82) |
| Chlamydia trachomatis (CT) | 10 (14) | 8 (13) | 2 (17) |
| Trichomonas vaginalis (TV) | 2 (3) | 1 (2) | 1 (8) |
| Number of etiologies (of NG, CT, TV) | |||
| 0 | 8 (11) | 7 (11) | 1 (8) |
| 1 | 59 (80) | 50 (81) | 9 (75) |
| ≥2 | 7 (9) | 5 (8) | 2 (17) |
| Number of additional urethritis episodes (excluding enrollment) | |||
| 0 | 62 (84) | 53 (85) | 9 (75) |
| 1 | 9 (12) | 6 (10) | 3 (25) |
| 2 | 3 (4) | 3 (5) | 0 (0) |
| HIV diagnosis | |||
| New HIV diagnosis | 43 (58) | 37 (60) | 6 (50) |
| Previous HIV diagnosis | 31 (42) | 25 (40) | 6 (50) |
| Syphilisb | 7 (10) | 5 (8) | 2 (18) |
| ART use | |||
| ART by week 1, no interruptionc | 26 (35) | 26 (42) | 0 (0) |
| ART by week 1, interruptedc,d | 15 (20) | 15 (24) | 0 (0) |
| ART by weeks 2–4, no interruptionc | 6 (8) | 6 (10) | 0 (0) |
| ART by weeks 2–4, interruptedc | 6 (8) | 6 (10) | 0 (0) |
| ART after 4 weeks | 9 (12) | 9 (15) | 0 (0) |
| No ART initiation | 12 (16) | 0 (0) | 12 (100) |
CT, Chlamydia trachomatis; NAAT, Nucleic Acid Amplification Test; NG, Neisseria gonorrhoeae; TV, Trichomonas vaginalis.
n = 73; one N. gonorrhoeae culture result is missing. All participants with a positive N. gonorrhoeae culture tested positive for N. gonorrhoeae via NAAT.
n = 72; one syphilis result was unknown, one was indeterminate.
ART interruption was defined as a visit with no ART detected in the blood, after ART initiation.
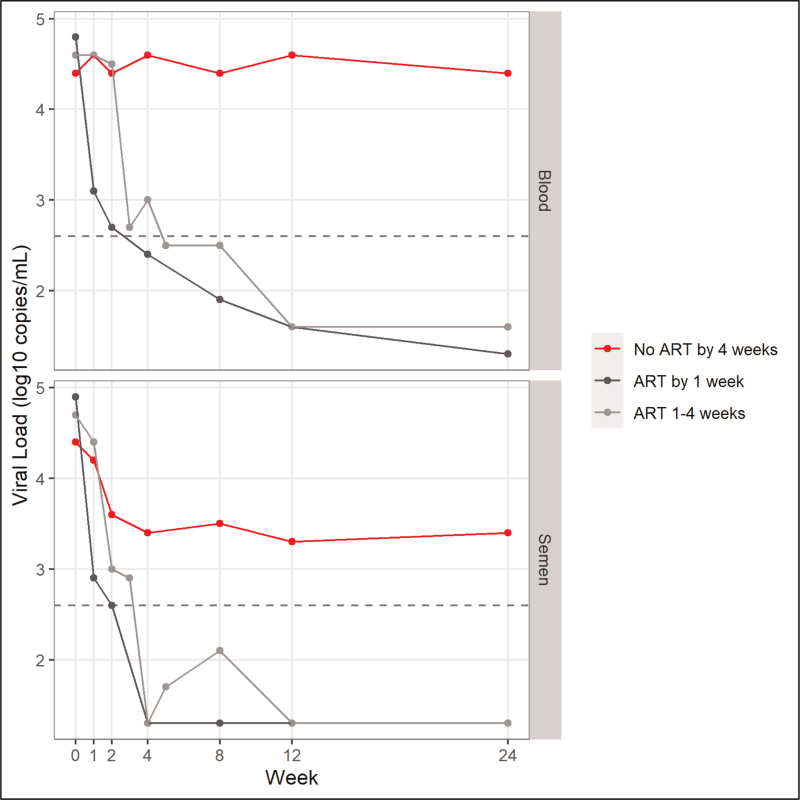
The concentration of HIV in blood and semen was measured at each visit and is summarized in Fig. 1. The time required to achieve viral suppression is provided in Table 2. Twenty-one subjects did not start treatment. Their median blood viral load at baseline (pretreatment) was 22 720 copies/ml (IQR: 9052–86 802) and the median semen viral load was 22 497 copies/ml (IQR: 3357–402 355; Fig. 1). During follow-up of this group, the median blood viral load remained relatively stable and unsuppressed at 4 weeks (median: 37 696; IQR: 21 928–101 154), 12 weeks (median: 44 398; IQR: 23 602–114 936) and 24 weeks (median: 23 814; IQR: 10 243–33 520). In the semen however, the median viral load decreased during the first 4 weeks (median: 2534; IQR: 366–61 117) and then remained relatively stable but unsuppressed through 12 weeks (median: 2062; IQR: 102–20 390) and 24 weeks (median: 2250; IQR: 470–12 723). One third of participants experienced suppression of HIV in semen in the absence of ART.
Fig. 1.
Median viral load in blood and semen by visit across ART initiation groups.
Group included if there were at least six data points in a given week.
Table 2.
Viral suppression (<400 copies/ml) by visit across ART group.
| Enroll | Week1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 8 | Week 12 | Week 24 | |
| Blood | |||||||||
| ART by 1 week | 1/41 (2) | 10/39 (26) | 17/41 (41) | – | 24/36 (67) | – | 22/31 (71) | 23/26 (88) | 21/25 (84) |
| ART 1–4 weeks | 0/12 (0) | 0/11 (0) | 1/12 (8) | 1/6 (17) | 2/8 (25) | 3/6 (50) | 3/6 (50) | 8/8 (100) | 6/7 (86) |
| No ART by 4 weeks | 2/21 (10) | 2/20 (10) | 2/20 (10) | – | 1/16 (6) | – | 1/14 (7) | 1/12 (8) | 0/13 (0) |
| Semen | |||||||||
| ART by 1 week | 7/40 (18) | 17/39 (44) | 19/39 (49) | – | 19/35 (54) | – | 27/31 (87) | 20/26 (77) | 21/25 (84) |
| ART 1–4 weeks | 2/12 (17) | 0/11 (0) | 4/11 (36) | 3/6 (50) | 4/7 (57) | 4/6 (67) | 3/6 (50) | 7/8 (88) | 7/7 (100) |
| No ART by 4 weeks | 4/21 (19) | 3/19 (16) | 5/20 (25) | – | 4/16 (25) | – | 3/13 (23) | 4/12 (33) | 3/13 (23) |
Because those who initiated ART after 1 week had visits added 1, 2, and 4 weeks after reported ART initiation, participants in the ART 1–4 week group were seen at different weeks than the other groups and had varying denominators on those weeks. Only those weeks with ≥5 participants are included (e.g. week 3 and week 5).
The remainder of subjects initiated treatment within one week or four weeks of urethritis.
Among participants who initiated ART within 1 week of acute urethritis diagnosis and treatment (n = 41), the baseline median blood viral load was 70 617 copies/ml (IQR: 22 995–271 400) and 82 071 copies/ml (IQR: 4926–233 022) in semen. The median blood viral load was suppressed by week 4 (<400 copies/ml) and remained suppressed through 24 weeks. The median seminal viral load experienced a rapid decrease to <1000 copies/ml by week 1 and was suppressed (<400 copies/ml) by week 4 and remained suppressed through week 24.
Among those who delayed ART and initiated treatment between 1 and 4 weeks (n = 12), the baseline median blood viral load was 44 258 copies/ml (IQR: 22 623–140 366) and the median semen viral load was 56 698 copies/ml (IQR: 3625–231 045). The seminal viral load remained above 10 000 copies/ml through the first week of treatment but was suppressed (<400 copies/ml) by 4 weeks and remained suppressed through 24 weeks. The median viral load in the blood reached suppression (<400 copies/ml) by week 5 after treatment and remained low through week 24. Assessment of adherence to treatment as demonstrated by detection of ART in blood samples is provided in Table 1.
Discussion
While there has been some reduction in HIV incidence worldwide [10], the burden of STIs remains very high [11] and STIs are frequently detected in people living with HIV [12–14]. ART leads to reduction of HIV transmission [15–18]. Untreated people living with HIV who develop an STI present increased risk for HIV transmission because of increased concentration of HIV in the genital tract [4]. Even on ART, urethritis causes transient increase in HIV in the male genital tract that is reversed over a month with additional antibiotic therapy that decreases inflammation and breakthrough of HIV [6]. The present study was undertaken to examine the time required to suppress HIV in the genital tract after first initiation of ART in men with urethritis [19–22].
Some men did not choose to initiate ART. While replication of gonorrhea abates only hours after antibiotic initiation [23] such treatment of urethritis reduces but generally does not eliminate HIV in semen [3,4]. In our earlier study of men with urethritis before ART was available, we found persistence of HIV of greater than104 copies/ml in semen 2 weeks after antibiotic treatment of urethritis [4], suggesting considerable risk for HIV transmission [5,16].
In the present study, most men started ART at or shortly after detection of urethritis. The treatment regimens used including agents initiated have been shown to be detected in seminal plasma and effective in suppressing HIV in blood and semen [15,20,21]. Participants with immediate or delayed initiation of ART experienced gradual reduction in HIV in blood and semen during recovery from urethritis.
ART can be expected to prevent HIV transmission when semen [5] and blood (Broyles et al., in press, Lancet 2023) viral load is reduced below 1000 copies/ml [5]. In the absence of a vaccine, treatment of HIV as prevention (TasP) is widely considered the most important strategy for HIV prevention [18,24]. HIV will be detected in a substantial number of men with urethritis, regardless of ART status [6]. The current study emphasizes the critical importance of rapid HIV testing for men with urethritis, and concomitant initiation of ART. The ability of ART to virtually eliminate HIV transmission [10,15–18] has rightly led to the U=U campaign [25], but it is worth noting that U=U is not instant as shown in this study and Mujugira et al.[26] that transmission risk persists for a period of time after ART initiation.
Acknowledgements
We would like to express our gratitude to the STI clinic staff at Bwaila District Hospital, and all study participants. This research was supported by University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410).
Author contributions: M.S.C., J.E.E., I.F.H., W.C.M., J.S.C., K.L. and M.M. were responsible for conceptualization, protocol development and design of the study. M.M., M.S.C., J.E.E., I.F.H., J.S.C. were responsible for data synthesis, analysis, and manuscript writing. C.M., B.N., N.B., E.M., E.J. and M.M. were responsible for data collection. J.S.C. was responsible for statistical analysis. A.K., M.L.C., A.P.S., B.V.H. and L.A.T. were responsible for antiretroviral drug testing and manuscript review. G.T., G.B., I.T. and A.J.L. were responsible for laboratory testing and manuscript review. All authors reviewed and approved the manuscript.
Conflicts of interest
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health [R01 DK108424 to M.S.C.]; the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [R01 AI114320 to W.C.M.; T32 AI070114 to J.S.C.]; and the Fogarty International Center at the National Institutes of Health [D43 TW010060 to M.M.]. The STI Clinic at Bwaila District Hospital is co-funded by the Lilongwe District Health Office, the Malawi Ministry of Health, and UNC Project, Malawi. A.K., M.L.C., A.P.S., B.V.H. and L.A.T. were supported by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR) Clinical Pharmacology/Analytical Chemistry Core, an NIH funded program P30AI050410. M.S.C. acts in an advisory role for Astra Zeneca, Gilead, Aerium and OPKO. The remaining authors declare no other conflicts of interest.
References
- 1.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med 2000; 342:921–929. [DOI] [PubMed] [Google Scholar]
- 2.Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, et al. Genital HIV-1 RNA quantity predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 2011; 3:77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadiq ST, Taylor S, Copas AJ, Bennett J, Kaye S, Drake SM, et al. The effects of urethritis on seminal plasma HIV-1 RNA loads in homosexual men not receiving antiretroviral therapy. Sex Transm Infect 2005; 81:120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 1997; 349:1868–1873. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty H, Sen PK, Helms RW, Vernazza PL, Fiscus SA, Eron JJ, et al. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS 2001; 15:621–627. [DOI] [PubMed] [Google Scholar]
- 6.Chen JS, Matoga M, Massa C, Tegha G, Ndalama B, Bonongwe N, et al. Effects of urethritis on human immunodeficiency virus (HIV) in semen: implications for HIV prevention and cure. Clin Infect Dis 2021; 73:e2000–e2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.2016 Malawi HIV Testing Services Guidelines. Lilongwe, Malawi: Malawi Ministry of Health; 2016. [Google Scholar]
- 8.Ministry of Health and Population Malawi. Malawi Guidelines for Syndromic Management of Sexually Transmitted Infections 2017. Lilongwe, Malawi: Malawi Ministry of Health; 2017. [Google Scholar]
- 9.Blazkova J, Gao F, Marichannegowda MH, Justement JS, Shi V, Whitehead EJ, et al. Distinct mechanisms of long-term virologic control in two HIV-infected individuals after treatment interruption of antiretroviral therapy. Nat Med 2021; 27:1893–1898. [DOI] [PubMed] [Google Scholar]
- 10.UNAIDS. Fact Sheet 2022. Geneva; 2022. [Google Scholar]
- 11.World Health Organization. Global Progress Report on HIV, HepB and STIs, 2021. Geneva; 2021. Available at: https://www.who.int/publications/i/item/9789240027077 [cited 2023 Jul 14]. [Google Scholar]
- 12.Rathod S, Padhiar B, Shah B. Sexually transmitted infections and human immunodeficiency virus coinfection: scenario in western India. Indian J Sex Transm Dis AIDS 2020; 41:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu W, Li YY, Kuang YQ, Xie RH, Dong XQ, Zhang D, et al. High prevalence of sexually transmitted infections and risk factors among HIV-positive individuals in Yunnan, China. Eur J Med Res 2022; 27:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tosato Boldrini NA, Bondi Volpini LP, Freitas LB, Spano LC, Musso C, Silva Santos MCLF, et al. Sexually transmitted infections among women living with HIV in a Brazilian city. Braz J Infect Dis 2021; 25:101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 6:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loutfy MR, Wu W, Letchumanan M, Bondy L, Antoniou T, Margolese S, et al. Systematic review of HIV transmission between heterosexual serodiscordant couples where the HIV-positive partner is fully suppressed on antiretroviral therapy. PLoS One 2013; 8:e55747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Van Lunzen J, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316:171–181. [DOI] [PubMed] [Google Scholar]
- 19.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–1404. [DOI] [PubMed] [Google Scholar]
- 20.Gupta P, Mellors J, Kingsley L, Riddler S, Singh MK, Schreiber S, et al. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol 1997; 71:6271–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vernazza PL, Troiani L, Flepp MJ, Cone RW, Schock J, Roth F, et al. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. AIDS 2000; 14:117–121. [DOI] [PubMed] [Google Scholar]
- 22.Pilcher PD, Shugars SA, Fiscus SA, Miller WC, Menezes P, Giner J, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS 2001; 15:837–845. [DOI] [PubMed] [Google Scholar]
- 23.Haizlip J, Isbey SF, Hamilton HA, Jerse AE, Leone PA, Davis RH, et al. Time required for elimination of Neisseria gonorrhoeae from the urogenital tract in men with symptomatic urethritis. Sex Transm Dis 1995; 22:145–148. [DOI] [PubMed] [Google Scholar]
- 24.Rodger AJ, Cambiano V, Phillips AN, Bruun T, Raben D, Lundgren J, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 2019; 393:2428–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Lancet HIV. U=U taking off in 2017. Lancet HIV. 2017;4. Available at: http://programme.ias2017. [Google Scholar]
- 26.Mujugira A, Celum C, Coombs RW, Campbell JD, Ndase P, Ronald A, et al. HIV transmission risk persists during the first 6 months of antiretroviral therapy. J Acquir Immune Defic Syndr 2016; 72:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]