Abstract
Purpose of review
Allergy and atopic features are now well recognized manifestations of many inborn errors of immunity (IEI), and indeed may be the hallmark in some, such as DOCK8 deficiency. In this review, we describe the current IEI associated with atopy, using a comprehensive literature search and updates from the IUIS highlighting clinical clues for underlying IEI such as very early onset of atopic disease or treatment resistance to enable early and accurate genetic diagnosis.
Recent findings
We focus on recently described genes, their categories of pathogenic mechanisms and the expanding range of potential therapies.
Summary
We highlight in this review that patients with very early onset or treatment resistant atopic disorders should be investigated for an IEI, as targeted and effective therapies exist. Early and accurate genetic diagnosis is crucial in this cohort to reduce the burden of disease and mortality.
Keywords: atopy, eosinophilia, hyper IgE, inborn errors of immunity, STAT6 Gain of function
INTRODUCTION
Inborn errors of immunity (IEI) associated with atopy provide valuable insights into the pathophysiology of the immune system and pathways responsible for atopic disease. Atopy is a recognized component of a growing number of IEI as wider phenotypes are defined, and in some may be the predominant manifestation. Given atopy-related manifestations of these diseases may present to a range of clinical specialists across infancy to adulthood, we set out to summarise recent developments in this field. We highlight novel genetic conditions that may present at this interface, including gain of function mutations in the IKAROS transcription factor [1▪▪], and autosomal dominant gain of functions (GOF) in signal transducer and activator of transcription 6 (STAT6) [2▪▪,3]. Finally, we propose an updated mechanistic framework for the development of atopy at the interface of IEI whilst highlighting pitfalls for associated complications, and opportunities for precision therapy.
Box 1.
no caption available
MATERIALS AND METHODS
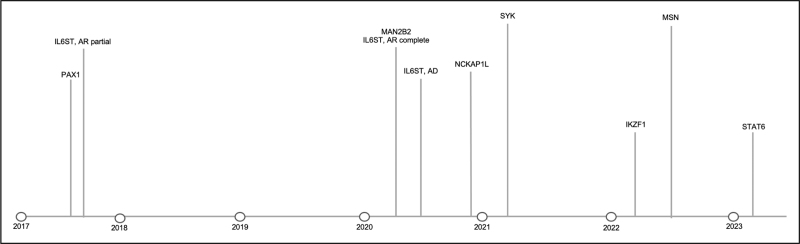
We conducted a rapid literature review using the search terms ‘Atopy AND primary immunodeficiency’ OR ‘atopy’ AND ‘inborn errors of immunity’ in PubMed, considering articles published between 1 January 2020 and 1 June 2023. Particular attention was given to monogenic disorders added to the International Union of Immunological Societies (IUIS) 2022 update of IEI [4▪]. We included disorders where case reports described clinically significant features of allergic rhinitis, asthma and atopic dermatitis (eczema), elevations in IgE or hypereosinophilia. Two independent reviewers classified each monogenic disorder within the predominant category of mechanism. Where disagreement arose regarding classification, a consensus was agreed with the wider team. We identified new genes with reported atopic presentations within the most recent 2022 IUIS IEI update combined with a rapid literature review. The dates these disorders were reported is shown in Fig. 1, and clinical phenotypes summarized in Table 1, adapted from Lyons et al. [5], and Nelson et al. [6▪]. Table 2 illustrates a comprehensive overview of all IEI associated with atopy, adapted from Lyons et al. [5], Nelson et al. [6▪] and IUIS update [4▪].
FIGURE 1.
Updated timeline of genes discovered responsible for inborn errors of immunity associated with atopy [1▪▪,2▪▪,4▪,17,19–27].
Table 1.
Novel IEIs with atopic manifestations – diagnostic features, atopic prevalence and clinical pitfalls
| References | Total cases described | Age of diagnosis (years) | Key features | Atopic features | Clinical pitfalls | |
| STAT6 GOF | Sharma et al.[2▪▪] | 16 | 3–60 | Early-onset atopy within the first year of life Treatment resistant atopy Recurrent viral infections Recurrent skin and respiratory infections |
Eczema Food allergies Asthma Eosinophilic gastrointestinal (GI) disease Anaphylaxis Eosinophilia Elevated IgE |
Lymphoma risk |
| RLTPR | Wang et al.[7]: six patients Shober [8]: four patients Yonkof [9]: two patients Sorte [10]: four patients Maccari [11]: one patient Anas M Alazami et al.[12]: seven patients Kurolap et al.[13]: one patient Magg et al.[14]: five patients Atschekzei [15]: three patients |
Greater than 10 | Not reported | Combined immunodeficiency (CID) Recurrent bacterial fungal and mycobacterial infections Skin infections e.g. molluscum, diffuse warts from Human papillomavirus (HPV) infection and abscesses Respiratory tract infections |
Eczema Eosinophilic oesophagitis High IgE Asthma Food allergy Cold urticaria |
Epstein–Barr virus (EBV) lymphoproliferation |
| IKZF1 | Hoshino et al.[1▪▪] | 8 | over 40 | Autoimmunity (diabetes, colitis, thyroiditis) Lymphoproliferation Plasma cell expansion Evans Syndrome Recurrent infections, Immune dysregulation |
Food allergy Asthma Rhinitis Dermatitis Eosinophilic oesophagitis |
IgG4-related disease (3/8) |
| NCKAP1L LOF | Cook et al.[16] Castro et al.[17] |
9 | 15 months – 11 years | Autoinflammatory Recurrent upper respiratory tract infection (URTI) Skin abscesses |
Eczema Elevated IgE |
|
| MSN | Lagresle-Peyrou et al.[18] and Fang et al.[19] | 16 | Not reported | Recurrent infections with bacteria and varicella and molluscum contagiosum Neutropenia Decreasing immunoglobulin over time |
Eczema Atopic dermatitis |
Very Early Onset Inflammatory Bowel Disease (VEOIBD) (1 case report) |
Table 2.
Atopy as a manifestation of IEI
| Mechanism of pathogenesis | Associated genes | Immunological features of presentation | Atopic features of presentation | Mode of inheritance |
| Impaired skin and mucosal barrier function | FLG | Skin infections | Atopic dermatitis Food allergy Allergic rhinitis Asthma Eosinophilia High IgE |
Autosomal recessive |
| SPINK5 | Skin infections | Atopic dermatitis Food allergy Allergic rhinitis Asthma Eosinophilia High IgE |
Autosomal recessive | |
| CDSN | Skin infections | Atopic dermatitis Food allergy Eosinophilia High IgE |
Autosomal recessive | |
| DSG1 | Skin infections | Atopic dermatitis Food allergy Eosinophilia High IgE |
Autosomal recessive | |
| DSP | Skin infections | Atopic dermatitis Food allergy Eosinophilia High IgE |
Autosomal recessive | |
| sIgA deficiency | Antibody deficiency Bacterial infections Autoimmunity |
Asthma, food allergy, allergic rhinitis and eczema | Unknown | |
| NEMO | Monocyte dysfunction Low immunoglobulins |
Atopic dermatitis Asthma Food allergies Allergic rhinitis |
X-linked | |
| Cytoskeletal abnormalities | WAS | CID | Atopic dermatitis Food allergy Eosinophilia High IgE |
X-linked |
| WIP | CID | Atopic dermatitis Food allergy Eosinophilia High IgE |
Autosomal recessive | |
| DOCK8 | CID Susceptibility to viral infections |
Atopic dermatitis Food allergy Eosinophilia High IgE |
Autosomal recessive | |
| STK4 | CID | Atopic dermatitis Food allergy Eosinophilia High IgE |
Autosomal recessive | |
| NCKAP1L deficiency | Autoinflammatory Recurrent URTI Skin abscesses |
Atopic dermatitis | Autosomal recessive LOF | |
| ARPC1B | CID Recurrent invasive infections |
Eosinophilia High IgE |
Autosomal recessive | |
| MSN Less than 10 reported cases to date |
Recurrent infections with bacteria and varicella Neutropenia Decreasing immunoglobulin over time |
Atopic dermatitis | X-linked | |
| Aberrant TCR signalling | CARD11 | CID/SCID | Eosinophilia High IgE |
Autosomal recessive |
| CARD11 | Cutaneous viral infections Recurrent respiratory tract infections |
Atopy Eosinophilia |
Autosomal dominant LOF (dominant negative) | |
| BCL10 | CID/SCID | Eosinophilia High IgE |
Autosomal recessive | |
| MALT1 | CID/SCID | Eosinophilia High IgE |
Autosomal recessive | |
| CARML2 | CID | Eosinophilia High IgE |
Autosomal recessive | |
| ZAP70 | CID/SCID | Eosinophilia High IgE |
Autosomal recessive | |
| LAT | CID/SCID | Eosinophilia High IgE |
Autosomal recessive | |
| RLTPR deficiency | CID Recurrent bacterial, fungal and mycobacterial infections Skin infections e.g. molluscum, diffuse warts from HPV infection, and abscesses Respiratory tract infections EBV lymphoproliferation |
Atopic dermatitis Eosinophilic oesophagitis High IgE Asthma Food allergy Cold urticaria |
Autosomal recessive | |
| Disrupted cytokine signalling | IL6RA | Skin infections Respiratory tract infections Recurrent pyogenic infections Abscesses |
Atopic dermatitis Eosinophilia High IgE |
Autosomal recessive |
| IL6ST | Skin infections Respiratory tract infections Bronchiectasis Boils Aspergillosis |
Atopic dermatitis Eosinophilia High IgE |
Autosomal recessive/autosomal dominant | |
| STAT3 | Skin infections Respiratory tract infections |
Atopic dermatitis Eosinophilia High IgE |
Autosomal dominant | |
| ZNF341 | Skin infections Respiratory tract infections |
Atopic dermatitis Eosinophilia High IgE |
Autosomal recessive | |
| IL21R Less than 10 reported cases to date |
CID Recurrent infections including PCP and cryptosporidium |
Increased IgE | Autosomal recessive | |
| TGFBR1/2 (Loeys – Dietz syndrome) | CID Recurrent respiratory tract infections |
Eczema Food allergies |
Autosomal dominant | |
| ERBB21P (ERBIN deficiency) One case/kindred been reported to date |
CID Recurrent respiratory tract infections Susceptibility to Staph aureus |
Atopic dermatitis Moderately increased IgE |
Autosomal dominant | |
| STAT5B | CID Hypergammaglobulinaemia Autoimmunity |
Atopic dermatitis High IgE |
Autosomal recessive/autosomal dominant | |
| STAT5B GOF | Normal immunoglobulin levels, T cells and B cells Diarrhoea |
Atopic dermatitis Urticaria Eosinophilia Hypereosinophilic syndrome |
Unknown | |
| PIK3CG Less than 10 reported cases to date |
Antibody deficiency Recurrent infections |
Eosinophilia | Autosomal recessive | |
| JAK1 (GOF) One case/kindred been reported to date |
Immune dysregulation Autoimmunity Viral infections |
Eosinophilic enteritis Eosinophilia |
Autosomal dominant | |
| TYK2 | Susceptibility to viruses Multiple cytokine signalling defects |
Elevated IgE | Autosomal recessive | |
| OTULIN Less than 10 reported cases to date |
Autoinflammatory Neonatal recurrent fever Neutrophilia |
Dermatitis | Autosomal recessive | |
| SYK Less than 10 reported cases to date |
Autoinflammatory Recurrent infections Multiorgan inflammatory disease Dysgammaglobulinaemia B-cell lymphoma |
Dermatitis | Autosomal dominant GOF | |
| Regulatory T cell Disorders | FOXP3 | Autoimmunity | Atopic dermatitis Food allergy Asthma Eosinophilia High IgE |
X-linked |
| IL2RA |
CID Autoimmunity |
Atopic dermatitis Food allergy Asthma Eosinophilia High IgE |
Autosomal recessive | |
| IKZF1 Less than 10 reported cases to date |
Autoimmunity Recurrent infections |
Allergy | Autosomal dominant GOF | |
| IL2RB (CD122 deficiency) 5 kindreds |
Immune dysregulation Autoimmunity Autoimmune haemolytic anaemia Hypergamma Viral infections – EBV, CMV |
Dermatitis | Autosomal recessive | |
| Innate cell effector mechanisms | PLCG2 | CVID Autoimmunity Autoinflammatory |
Temperature-sensitive mast cell degranulation | Autosomal dominant |
| NLRP3 | Autoinflammatory Fever Leukocytosis Conjunctivitis |
Urticaria | Autosomal dominant GOF | |
| Thymic development disorders | PAX1 Less than 10 reported cases to date |
SCID Omenn's-like syndrome Severe, recurrent infections Athymic |
Erythroderma Eosinophilia Normal to raised IgE |
Autosomal recessive |
| EXTL3 Less than 10 reported cases to date |
CID Low Immunoglobulins |
Eosinophilia | Autosomal recessive | |
| FOXN1 | CID Recurrent viral and bacterial respiratory tract infections |
Atopic dermatitis | Autosomal dominant | |
| 22q11 deletion syndrome | CID Normal or decreased immunoglobulins May have low TRECs at newborn screening |
Eczema Asthma |
Autosomal dominant | |
| Decreased T cell repertoire diversity | Multiple genes presenting as Omenn syndrome, such as RAG1/2, ADA, LIG4, ZAP70, etc. | Leaky SCID | Erythroderma Eosinophilia High IgE |
Autosomal recessive |
| BCL11B | CID | Severe atopic dermatitis Food allergies Allergic asthma Urticaria Eosinophilia Elevated IgE |
Autosomal dominant | |
| Metabolic | MAN2B2 One case/kindred reported to date |
CID Recurrent infections |
High IgE | Autosomal recessive |
| PGM3 | CID Recurrent pneumonia Recurrent skin abscesses Bacterial and viral infections |
Severe atopy High IgE Eosinophilia |
Autosomal recessive | |
| PEPD (prolidase deficiency) | Immune dysregulation Autoimmunity Autoantibodies Chronic skin ulcers Infections |
Atopic dermatitis | Autosomal recessive |
Genes are ordered into their associated pathway/mechanism of disease; however, there may be overlap between mechanisms of pathogenesis for the same gene (Adapted from Milner 2018, and Nelson 2022 and including recent IUIS updates). FLG and DSG1 are marked in italics, as they are not necessarily associated with IEI but are monogenic defects supporting the pathogenic category. We have grouped thymic development disorders and decreased T cell repertoire diversity [4▪,5,6▪,26,28–41].
CATEGORISATION OF MONOGENIC INBORN ERRORS OF IMMUNITY INTO MECHANISTIC PATHWAYS TO ATOPY
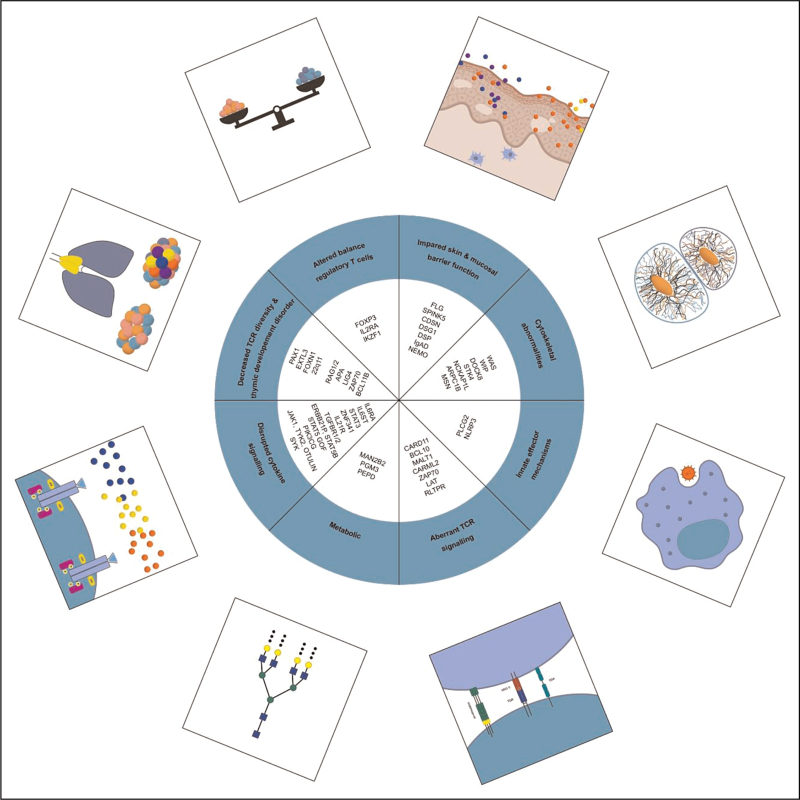
Lyons et al.[5] proposed seven broad categories of inborn errors of immunity favouring development of atopy. Our adaptation has been modified to include the following eight categories, summarised in Fig. 2: impaired skin and mucosal barrier function; cytoskeletal abnormalities; aberrant TCR signalling; disrupted cytokine signalling; decreased T cell repertoire diversity and thymic development disorders; regulatory T cell (Treg) disorders; innate cell effector mechanisms; and metabolic disorders.
FIGURE 2.
Categories of pathogenic mechanisms of atopy in inborn error of immunity.
We describe an expansion in both the number of IEI with associated atopic manifestations and in the mechanistic categories underpinning the pathogenesis. This highlights the importance of awareness and early recognition of atopy as a manifestation of a growing number of IEI.
Cytoskeletal abnormalities
Cytoskeletal disorders include Wiskott-Aldrich syndrome (WAS), Wiskott-Aldrich syndrome protein (WASP) WAP interacting protein (WIP), Dedicator of cytokinesis 8 (DOCK8) deficiency and Serine/threonine kinase 4 (STK4) deficiency. These cause a combined immunodeficiency with atopic features. WAS, DOCK8 and STK4 are also linked to a higher rate of autoimmunity and malignancy, illustrating the broad effects of immune dysregulation in IEI [4▪,5,6▪]. Deficiencies in the Nck-associated protein 1-like (NCKAP1L) gene, also known as hematopoietic protein 1 (HEM1), were first reported in humans by Castro et al.[17]. The gene encodes a haematopoietic lineage specific regulator of the actin cytoskeleton, vital for downstream signalling of activated Rac to stimulate F-actin polymerization in response to engagement of immune receptors [B cell receptor, TCR, Toll like receptor (TLR) and cytokine receptors] and is responsible for actin cytoskeleton reorganisation. Disruption to mechanistic Target of Rapamycin (mTOR) 2 and F-actin control results in immune dysregulation. Nine patients have been reported, for which a cohort of five patients from four unrelated families described by Cook et al.[16], had atopic and inflammatory diseases, chronic hepatosplenomegaly, lymphadenopathy with elevated IgE in 4 patients. Other features included recurrent bacterial and viral skin and respiratory infections and specific antibody deficiencies. Lymphoproliferation, cytokine overproduction, lymphadenopathy, hyperinflammation and autoimmune manifestations were also reported [17].
Variants in the MSN gene have recently been described as the cause of X-linked moesin-associated immunodeficiency (X-MAID). Sixteen cases have been reported worldwide. Patients with hemizygous mutations in the MSN gene present with lymphopenia, impaired T-cell proliferation, hypogammaglobulinemia, altered migration and adhesion capacities and susceptibility to bacterial and viral infections of the respiratory and gastrointestinal systems. Eight patients had skin manifestations mainly of eczema, molluscum contagiosum and atopic dermatitis [18,19]. MSN, ezrin and radixin are members of the ezrin-radixin-moesin (ERM) family which modulates the actin cytoskeleton and plasma membranes [42].
Aberrant TCR signalling
Defective TCR signalling is evident in CARD11, BCL10, MALT1, CARML2, ZAP70, LAT and RLTPR deficiencies. Presentations consist of CID/severe combined immunodeficiency (SCID) with atopic features such as eosinophilia and high IgE. TCR signalling can either be absent or of reduced strength. Low strength signals between the TCR and major histocompatibility complex (MHC) complex have previously been demonstrated to skew naive T cell differentiation toward a T helper cell (Th) 2 response, promoting atopy [4▪,5,6▪]. Depending on the type of defect in CARD11, presentation can differ. Dominant negative mutations are associated with atopy, including moderate to severe dermatitis, high IgE and CID, like MALT1 deficiencies [5,6▪]. ZAP70 deficiency may manifest as atopic disease before the immunodeficiency becomes apparent [6▪].
RLTPR deficiency causes aberrant TCR signalling, by interfering with CD28 stimulation in T-cells [7]. Patients present with CID, recurrent bacterial, fungal and mycobacterial infections, and skin manifestations such as diffuse and recurrent warts. Atopic features include dermatitis, eosinophilic oesophagitis, asthma, food allergy, cold urticaria and high IgE [4▪,5,6▪].
Disruption of cytokine signalling
Genetic defects causing ineffective cytokine signalling include IL6RA, IL6ST, STAT3 and ZNF341.
Patients with dominant negative loss of function mutations in STAT3, present with recurrent infections, atopic dermatitis, eosinophilia, food allergy and high IgE. ZNF341 is involved in STAT3 gene expression and presents in a similar fashion. This condition promotes atopy, as STAT3 phosphorylation leads to suppression of Th2 responses and favours Th17 responses, thereby reducing the propensity for atopy. Mutations in STAT3 diminish this effect, resulting in increasing Th2 responses [6▪].
Autosomal dominant STAT6 GOF variants associated with early-onset (<12 months) severe atopy have been reported by multiple groups [2▪▪,3,43–45]. Treatment-resistant atopic dermatitis and food allergies were most common, followed by asthma, eosinophilic gastrointestinal disease and anaphylaxis. Elevated IgE levels and eosinophilia were noted [2▪▪]. STAT6 is an intracellular transcription factor downstream of IL4 and IL4R/JAK-kinase signalling cascade and a central node of immune polarization and a key modulator for the risk of allergic disease in humans and mice [3,46]. Translocation of STAT6 to the nucleus, activates a pattern of gene expression mediating Th2 cell differentiation, M2 macrophage polarization, promotion of B cell survival and IgE class switching [47–50].
Seven kindreds were reported as sporadic, and three kindreds followed an autosomal dominant pattern of inheritance. Clinical features of wider immune dysregulation included recurrent nonfatal skin, respiratory, and viral infections identified in half of the cohort. Similar to characteristics of DN STAT3 LOF, short stature, pathologic fractures and generalised hypermobility were described. One patient died due to anaphylaxis at aged 20 and the other aged 35 secondary to a cerebral aneurysm, demonstrating the severity of the multisystem disease in this cohort [2▪▪]. It is notable that somatic activating mutations in STAT6 have been associated with B cell lymphoma [51–53]. The oldest patient in the cohort, experienced recurrent B cell lymphoma with follicular lymphoma aged 49 with subsequent relapse with a transformed follicular lymphoma (diffuse large B cell lymphoma) aged 60 [2▪▪].
Decreased T cell repertoire diversity
This mechanism manifests as Omenn syndrome, a type of leaky SCID, associated with multiple genetic defects including recombination activating gene (RAG)1, RAG2 and adenosine deaminase (ADA). Hypomorphic mutations in the responsible genes result in a limited number of T cells which undergo oligoclonal expansion. These T cells preferentially differentiate into the Th2 lineage, causing the classical presenting symptoms of hepatosplenomegaly, lymphadenopathy, erythroderma, eosinophilia and high IgE [4▪,5,6▪].
Two hypotheses exist to explain how a reduced diversity of T cells can result in atopy. The first suggests that reduced T cell diversity causes a lack of Tregs and loss of regulation of Th2 with subsequent atopy. The second hypothesis suggests low strength TCR signalling leading to skewing of Th2 differentiation. Due to reduced thymopoiesis, there is a lack of T cells with high affinity receptors which leads to a preferential expansion of T cells with low affinity receptors that differentiate into Th2 cells, thus promoting atopy [5].
Altered balance of conventional T cells and regulatory T cells
Reduced numbers of Tregs leads to a failure of tolerance and presents as autoimmunity and features of immune dysregulation such as atopy [5,6▪].
FOXP3 is the master transcription factor for Tregs, and its deficiency is responsible for immunodysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome. IPEX presents as autoimmunity with severe atopic dermatitis, food allergy, asthma, eosinophilia and high IgE [4▪,5,6▪].
IL2RA loss of function mutations lead to atopic features such as dermatitis, elevated IgE with autoimmunity and immunodeficiency. Tregs express the most IL2RA and fail to survive in its absence. IL-2 signalling through its receptor on Tregs promotes production of IL-10, promoting tolerance. Deficiencies in IL2RA result in loss of survival signals for Tregs and loss of suppressive function, favouring atopy [5,6▪].
IKAROS gain-of-function mutations
Germline heterozygous IKAROS GOF mutations presented with profound autoimmunity and immune dysregulation (75%, 6/8) with an age of onset of less than 1 to over 40 years. The regulation of IKZF1 is required for T helper cell, Treg and plasma cell differentiation [1▪▪].
Patients developed autoimmune diseases including type 1 diabetes mellitus, enteritis, autoimmune hepatitis, Hashimoto thyroiditis, leukocytoclastic vasculitis, vitiligo and alopecia with autoantibodies. GOF patients showed an absence of effector Treg and increased T follicular cell population, suggesting T-cell differentiation is compromised by abnormal IL-2 production. Autoimmune manifestations may be due to abnormal IL-2 production and effector Treg populations in these patients, as with other IEI patients with impaired Treg numbers and/or function IPEX syndrome and cytotoxic T-lymphocyte antigen 4 (CTLA-4) haploinsufficiency [54]. T cells expressing GOF mutations showed increased IL-4 (Th2) production, and decreased IL-2 and IFNγ production (Th1) [1▪▪,55].
Features also included atopy, lymphoproliferation and generally nonsevere bacterial infections. Whole-exome sequencing identified two patients with apparent autosomal dominant inheritance, as well as de novo occurrences. One patient harbouring a GOF mutation did not present with any clinical manifestations, demonstrating variable immunological penetrance.
Patients had mostly normal B-cell numbers, with normal to elevated immunoglobulin and IgE levels. Presentations of atopic disease included asthma, rhinitis, dermatitis, food allergy and eosinophilic oesophagitis. These are postulated to be due to increased Th2 differentiation with increased eosinophils, and production of IL-4 [56]. Increased IL-4 may result in Th2 and T follicular helper cell (TFH)2 skewing through negative regulation by IL-2 and/or hyper-IgE likely contributes to the development of allergic manifestations. Plasma cell hyper-proliferation was reported. Three patients had IgG4-related diseases demonstrated by an increased infiltration of the IgG4-positive plasma cells in the lymph nodes, intestine or bile duct [1▪▪].
Skin barrier defects
Multiple genes are associated with disrupted skin barrier function and infection, summarized in Table 2.
The ‘atopic march’ is characterized by early onset eczema predisposing to developing allergic rhinitis, then subsequently asthma and food allergies [6▪]. It is suggested that increased skin permeability from eczema, leads to cutaneous antigen-presenting cells (APCs) being exposed to increased amounts of usually innocuous environmental antigens. This leads to sensitisation, and production of Th2 associated pro-inflammatory cytokines, consequently initiating the allergic response [5,6▪]. Skin barrier disruption alongside downregulation of protective antimicrobial peptides, increases infection risk [6▪].
Pro-inflammatory type 2 cytokines also downregulate filaggrin, an important protein for skin barrier integrity [57], due to its role in producing natural moisturising factor, essential for hydration, during normal skin desquamation [58]. Therefore, disturbances in filaggrin production result in dry, flaky skin, increasing skin permeability, allowing increased exposure to antigens, and so the cycle continues [5]. This is observed in ichthyosis vulgaris, due to a homozygous LOF mutation in filaggrin, resulting in early onset (first months of life) severe atopy with elevated IgE [5,6▪].
Selective IgA deficiency
Selective IgA deficiency (sIgAD) has similarly been postulated to result in impaired mucosal barrier function resulting in greater sensitisation and propagation of allergy. Up to 40% of sIgAD patients have allergy as a presenting or only symptom [37,38], with up to 84% of patients having some form of allergic manifestation, asthma being the commonest [35], others include allergic rhinitis, eczema and food allergy [35].
Ectodermal dysplasia and NF-κB essential modulator
Atopic features have been described in ectodermal dysplasia, including scalp dermatitis, atopic dermatitis and elevated IgE with positive skin prick tests [59].
Children with ectodermal dysplasia syndromes experience atopic symptoms more frequently compared to the general paediatric population, including asthma, food allergies, allergic rhinitis and eczema [40] due to skin barrier disruption [60] and hypohidrosis or anhidrosis, fuelling their atopic march [61].
NEMO deficiency is associated with eczema and erythroderma [62].
Thymic development disorders
Atopy in chromosome 22q11.2 deletion syndrome (22q11.2del) is proposed to be related to T-cell lymphopenia and homeostatic pressure driving Th2 polarization [63]. Atopy has been associated with low T-cell receptor excision circles, with low T cells conferring nearly a three-fold increased risk of allergy [64,65], with patients presenting with asthma, rhinitis/conjunctivitis, food allergy and atopic dermatitis. Other IEI in this category are PAX1, EXTL3 and FOXN1.
Metabolic disorders
Mutations in MAN2B2 and PGM3 are congenital disorders of glycosylation (CDGs) [22,66].
Biallelic mutations in MAN2B2 have been shown to result in a CID, characterised by recurrent pneumonia, thrush, chronic diarrhoea and elevated IgE. Extra-immunological manifestations included small vessel vasculitis and thrombotic stroke [4▪,22].
PGM3 deficiency is regarded as a HIES [4▪]. Patients suffer from recurrent bacterial and viral infections, commonly affecting the skin and respiratory tract, low T cells and reduced memory B cells. Autoimmunity, along with severe atopy, including severe atopic dermatitis, food allergies and asthma have been reported, accompanied by marked eosinophilia and high IgE. Extra-immunological manifestations include neurological impairment, such as sensorineural hearing loss, low IQ, developmental delay and facial dysmorphism [4▪,66].
TREATMENT UPDATES – FOCUS ON PRECISION THERAPIES
Improvements in genetic analysis have facilitated early diagnosis and options for precision therapy to modulate these defects. An expanding range of biologics and small molecule drug inhibitors are available for asthma or eczema, such as Mepolizumab (anti-IL5), Dupilumab (anti-IL4Rα) and Tezepelumab (antithymic stromal lymphopoietin) with potential for translational repurposing to rare diseases.
Dupilumab has been shown to be well tolerated and effective in a number of atopic diseases, especially refractory eczema. The IL-4α receptor antagonist inhibits the IL-13/ IL-4/ STAT 6 axis, disrupting IL-4 signalling and the allergic type 2 cytokine signature [67].
Dupilumab was highly effective in the three patients with STAT6 GOF variants, demonstrating clinical and immunological biomarker and cutaneous improvement with increased growth velocity and weaning or discontinued oral corticosteroids. Preclinical data have suggested that Janus kinase (JAK) inhibitors such as Tofacitinib and Ruxolitinib may be beneficial [2▪▪]. Phase II studies are ongoing with Bruton's tyrosine kinase inhibitors (BTKi) in atopic dermatitis [68].
Dupilumab used in autosomal dominant AD STAT3 LOF showed improved atopic dermatitis, eosinophilic folliculitis and recurrent cutaneous infections [69]. Improvements to other manifestations such as asthma and allergic bronchopulmonary aspergillosis have been reported [70,71]. Dupilumab has also been used to successfully treat severe atopic dermatitis in a patient with CARD11-associated atopy with dominant interference of NF-kB signalling (CADINS) [72].
There are case and single-centre reports for the use of Omalizumab in IEI, such as in AD STAT3 LOF with concomitant respiratory manifestations; however, its role is still to be defined. Glutamine supplementation for dominant negative CARD11 variants has not yet translated to clinical therapy. Oral dietary supplementation is a research avenue for phosphoglucomutase 3 (PGM3) deficiency, with evidence suggesting in-vitro supplementation with the nondiabetogenic amino-sugar N-acetylglucosamine (GlcNAc) led to normalised intracellular UDP-GlcNAc, surface CTLA-4 expression and alterations in cellular glycosylation and immune pathways [66,73]. The use of lenalidomide has been shown to lead to degradation of IKZF1 and prevent some of the abnormal IKZF1 GOF using in vitro assays [1▪▪].
Role and effectiveness of allergen immunotherapy
Primary immunodeficiencies are described as a relative contraindication to commencing AIT; however, no controlled studies have investigated the effectiveness or associated risks. AIT is likely to have been performed in many cases of undiagnosed selective IgA deficiency [74,75].
The current European Academy of Allergy & Clinical Immunology (EAACI) guidelines state that careful consideration, on a case-by-case basis, with discussion between patient and the treating physician is required before deciding whether or not to commence AIT [76]. The British Society for Allergy & Clinical Immunology (BSACI) guidelines for venom immunotherapy (VIT) also state the effects of VIT in patients with disorders of the immune system such as immunodeficiency are not known and therefore the decision to offer treatment should be based on an individual ‘risk-benefit’ analysis [77]. We support individual consideration of patients with IEI for AIT, accepting that the efficacy remains unclear.
Haematopoietic stem cell transplantation
HSCT is curative for certain IEI and may lead to resolution of atopy, however the durability remains unknown. IgE levels substantially decreased post-HSCT in the majority of patients who underwent transplantation for DN STAT3 LOF and DOCK 8 deficiency [78–80] alongside resolution of eczema post-HSCT [78,79]. Allergen-specific IgE also declined post-HSCT in all patients tested with DOCK 8 deficiency. Al-Herz et al.[80] reported, in 10 patients (91%) with DOCK 8 deficiency who presented with food allergy and food allergen-specific IgE levels, that food allergies clinically resolved post-HSCT in eight out of 10 patients confirmed by oral challenges, although not all studies confirmed this [81].
CONCLUSION
Presentations of atopy should be considered as part of an underlying IEI and would warrant investigation particularly if early onset, refractive to treatment and with concurrent signs of autoimmunity, lymphoproliferation and recurrent infections. Patients who remain undiagnosed have a higher risk of morbidity and mortality. An initial immunological assessment proceeding to genetic testing aids early identification of specific genetic abnormalities enabling precision treatments improving outcomes for atopic disease in IEI.
Acknowledgements
L.S. and S.W. are joint first authors.
A.G. and S.J. joint last authors.
The authors would like to thank Harriet Gibson for her expert development of the illustrations.
Financial support and sponsorship
None.
Drug information
Mepolizumab: GlaxoSmithKline, North Carolina and Pennsylvania, USA.
Tezepelumab: Astrazeneca, Delaware, USA.
Dupilumab: jointly by Regeneron New York USA and Sanofi Paris France.
Tofacitinib: National Institutes of Health Maryland USA and Pfizer New York, USA.
Ruxolitinib by Incyte Corp Delaware USA and by Novartis Basel Switzerland elsewhere in the world.
Lenalomide: Bristol Myers Squibb, New York, USA.
Conflicts of interest
L.S., S.W., M.P., A.G. and S.J. have no conflicts of interest to declare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Hoshino A, Boutboul D, Zhang Y, et al. Gain-of-function IKZF1 variants in humans cause immune dysregulation associated with abnormal T/B cell late differentiation. Sci Immunol 2022; 7:eabi7160. [DOI] [PubMed] [Google Scholar]; This article describes the previously unidentified heterozygous IKZF1 variants (R183C/H) in eight individuals and demonstrates its relevance in atopic disease.
- 2▪▪.Sharma M, Leung D, Momenilandi M, et al. Human germline heterozygous gain-of-function STAT6 variants cause severe allergic disease. J Exp Med 2023; 220:e20221755. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the largest cohort of patients with STAT6 GOF, including the clinical presentations of very early onset atopy and possible treatments.
- 3.Minskaia E, Maimaris J, Jenkins P, et al. Autosomal dominant STAT6 gain of function causes severe Atopy associated with Lymphoma. J Clin Immunol 2023; 43:1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪.Bousfiha A, Moundir A, Tangye SG, et al. The 2022 Update of IUIS Phenotypical Classification for human inborn errors of immunity. J Clin Immunol 2022; 42:1508–1520. [DOI] [PubMed] [Google Scholar]; This provides an up-to-date version of classifications of IEIs including their clinical presentations of atopy.
- 5.Lyons JJ, Milner JD. Primary atopic disorders. J Exp Med 2018; 215:1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪.Nelson RW, Geha RS, McDonald DR. Inborn errors of the immune system associated with atopy. Front Immunol 2022; 13:860821. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides a comprehensive overview of atopy in relation to IEIs.
- 7.Wang Y, Ma CS, Ling Y, et al. Dual T cell– and B cell–intrinsic deficiency in humans with biallelic RLTPR mutations. J Exp Med 2016; 213:2413–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schober T, Magg T, Laschinger M, et al. A human immunodeficiency syndrome caused by mutations in CARMIL2. Nat Commun 2017; 8:14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yonkof JR, Gupta A, Rueda CM, et al. A novel pathogenic variant in CARMIL2 (RLTPR) causing CARMIL2 deficiency and EBV-associated smooth muscle tumors. Front Immunol 2020; 11:884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorte HS, Osnes LT, Fevang B, et al. A potential founder variant in CARMIL2/RLTPR in three Norwegian families with warts, molluscum contagiosum, and T-cell dysfunction. Mol Genet Genomic Med 2016; 4:604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maccari ME, Speckmann C, Heeg M, et al. Profound immunodeficiency with severe skin disease explained by concomitant novel CARMIL2 and PLEC1 loss-of-function mutations. Clin Immunol 2019; 208:108228. [DOI] [PubMed] [Google Scholar]
- 12.Alazami AM, Al-Helale M, Alhissi S, et al. Novel CARMIL2 mutations in patients with variable clinical dermatitis, infections, and combined immunodeficiency. Front Immunol 2018; 9:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regeneron Genetics Center, Kurolap A, Eshach Adiv O, Konnikova L, et al. A unique presentation of infantile-onset colitis and eosinophilic disease without recurrent infections resulting from a novel homozygous CARMIL2 variant. J Clin Immunol 2019; 39:430–439. [DOI] [PubMed] [Google Scholar]
- 14.Magg T, Shcherbina A, Arslan D, et al. CARMIL2 deficiency presenting as very early onset inflammatory bowel disease. Inflamm Bowel Dis 2019; 25:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atschekzei F, Jacobs R, Wetzke M, et al. A Novel CARMIL2 mutation resulting in combined immunodeficiency manifesting with dermatitis, fungal, and viral skin infections as well as selective antibody deficiency. J Clin Immunol 2019; 39:274–276. [DOI] [PubMed] [Google Scholar]
- 16.Cook SA, Comrie WA, Poli MC, et al. HEM1 deficiency disrupts mTORC2 and F-actin control in inherited immunodysregulatory disease. Science 2020; 369:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro CN, Rosenzwajg M, Carapito R, et al. NCKAP1L defects lead to a novel syndrome combining immunodeficiency, lymphoproliferation, and hyperinflammation. J Exp Med 2020; 217:e20192275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagresle-Peyrou C, Luce S, Ouchani F, et al. X-linked primary immunodeficiency associated with hemizygous mutations in the moesin (MSN) gene. J Allergy Clin Immunol 2016; 138:1681–1689. e8. [DOI] [PubMed] [Google Scholar]
- 19.Fang Y, Luo Y, Liu Y, Chen J. A novel variant of X-linked Moesin gene in a boy with inflammatory bowel disease like disease: a case report. Front Genet 2022; 13:873635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponsford MJ, Rae W, Klocperk A. What is new in HIES? Recent insights from the interface of primary immune deficiency and atopy. Curr Opin Allergy Clin Immunol 2018; 18:445–452. [DOI] [PubMed] [Google Scholar]
- 21.Paganini I, Sestini R, Capone GL, et al. A novel PAX1 null homozygous mutation in autosomal recessive otofaciocervical syndrome associated with severe combined immunodeficiency: PAGANINI et al. Clin Genet 2017; 92:664–668. [DOI] [PubMed] [Google Scholar]
- 22.Verheijen J, Wong SY, Rowe JH, et al. Defining a new immune deficiency syndrome: MAN2B2-CDG. J Allergy Clin Immunol 2020; 145:1008–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Béziat V, Tavernier SJ, Chen YH, et al. Dominant-negative mutations in human IL6ST underlie hyper-IgE syndrome. J Exp Med 2020; 217:e20191804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YH, Grigelioniene G, Newton PT, et al. Absence of GP130 cytokine receptor signaling causes extended Stüve-Wiedemann syndrome. J Exp Med 2020; 217:e20191306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwerd T, Twigg SRF, Aschenbrenner D, et al. A biallelic mutation in IL6ST encoding the GP130 co-receptor causes immunodeficiency and craniosynostosis. J Exp Med 2017; 214:2547–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redmond MT, Scherzer R, Prince BT. Novel genetic discoveries in primary immunodeficiency disorders. Clin Rev Allergy Immunol 2022; 63:55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Aschenbrenner D, Zeng Z, et al. Gain-of-function variants in SYK cause immune dysregulation and systemic inflammation in humans and mice. Nat Genet 2021; 53:500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki Y, Urrutia R, Franco LM, et al. PAX1 is essential for development and function of the human thymus. Sci Immunol 2020; 5:eaax1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotlarz D, Ziętara N, Uzel G, et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J Exp Med 2013; 210:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volpi S, Yamazaki Y, Brauer PM, et al. EXTL3 mutations cause skeletal dysplasia, immune deficiency, and developmental delay. J Exp Med 2017; 214:623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosticardo M, Yamazaki Y, Cowan J, et al. Heterozygous FOXN1 variants cause low TRECs and severe T cell lymphopenia, revealing a crucial role of FOXN1 in supporting early thymopoiesis. Am J Hum Genet 2019; 105:549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alrumayyan N, Slauenwhite D, McAlpine SM, et al. Prolidase deficiency, a rare inborn error of immunity, clinical phenotypes, immunological features,;1; and proposed treatments in twins. Allergy Asthma Clin Immunol 2022; 18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staple L, Andrews T, McDonald-McGinn D, et al. Allergies in patients with chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) and patients with chronic granulomatous disease. Pediatr Allergy Immunol 2005; 16:226–230. [DOI] [PubMed] [Google Scholar]
- 34.Delmonte OM, Biggs CM, Hayward A, et al. First case of X-linked Moesin deficiency identified after newborn screening for SCID. J Clin Immunol 2017; 37:336–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cinicola BL, Pulvirenti F, Capponi M, et al. Selective IgA deficiency and allergy: a fresh look to an old story. Medicina (Mex) 2022; 58:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morawska I, Kurkowska S, Bębnowska D, et al. The epidemiology and clinical presentations of atopic diseases in selective IgA deficiency. J Clin Med 2021; 10:3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yazdani R, Latif A, Tabassomi F, et al. Clinical phenotype classification for selective immunoglobulin A deficiency. Expert Rev Clin Immunol 2015; 11:1245–1254. [DOI] [PubMed] [Google Scholar]
- 38.Aghamohammadi A, Cheraghi T, Gharagozlou M, et al. IgA deficiency: correlation between clinical and immunological phenotypes. J Clin Immunol 2009; 29:130–136. [DOI] [PubMed] [Google Scholar]
- 39.Castagnoli R, Lougaris V, Giardino G, et al. Inborn errors of immunity with atopic phenotypes: a practical guide for allergists. World Allergy Organ J 2021; 14:100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mark BJ, Becker BA, Halloran DR, et al. Prevalence of atopic disorders and immunodeficiency in patients with ectodermal dysplasia syndromes. Ann Allergy Asthma Immunol 2012; 108:435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu HY, Sertori R, Contreras AV, et al. A novel germline heterozygous BCL11B variant causing severe atopic disease and immune dysregulation. Front Immunol 2021; 12:788278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burns SO, Zarafov A, Thrasher AJ. Primary immunodeficiencies due to abnormalities of the actin cytoskeleton. Curr Opin Hematol 2017; 24:16–22. [DOI] [PubMed] [Google Scholar]
- 43.Suratannon N, Ittiwut C, Dik WA, et al. A germline STAT6 gain-of-function variant is associated with early-onset allergies. J Allergy Clin Immunol 2023; 151:565–571. e9. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi I, Yanagi K, Takada S, et al. STAT6 gain-of-function variant exacerbates multiple allergic symptoms. J Allergy Clin Immunol 2023; 151:1402–1409. e6. [DOI] [PubMed] [Google Scholar]
- 45.Baris S, Benamar M, Chen Q, et al. Severe allergic dysregulation due to a gain of function mutation in the transcription factor STAT6. J Allergy Clin Immunol 2023; 152:182–194. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith TD, Doherty TA. Learning while treating: gain-of-function STAT6 variants in severe allergic disease. Cell Rep Med 2023; 4:101040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res 2011; 50:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeda K, Tanaka T, Shi W, et al. Essential role of Stat6 in IL-4 signalling. Nature 1996; 380:627–630. [DOI] [PubMed] [Google Scholar]
- 49.Villarino AV, Kanno Y, O'Shea JJ. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat Immunol 2017; 18:374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villarino AV, Gadina M, O'Shea JJ, Kanno Y. SnapShot: Jak-STAT Signaling II. Cell 2020; 181:1696–11696. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritz O, Guiter C, Castellano F, et al. Recurrent mutations of the STAT6 DNA binding domain in primary mediastinal B-cell lymphoma. Blood 2009; 114:1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tate JG, Bamford S, Jubb HC, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res 2019; 47:D941–D947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yildiz M, Li H, Bernard D, et al. Activating STAT6 mutations in follicular lymphoma. Blood 2015; 125:668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tangye SG, Al-Herz W, Bousfiha A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol 2020; 40:24–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuehn HS, Boast B, Rosenzweig SD. Inborn errors of human IKAROS: LOF and GOF variants associated with primary immunodeficiency. Clin Exp Immunol 2023; 212:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 2019; 50:1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2009; 124:R7–12. [DOI] [PubMed] [Google Scholar]
- 58.Sandilands A, Sutherland C, Irvine AD, McLean WHI. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci 2009; 122:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trüeb RM, Bruckner-Tuderman L, Wyss M, et al. Scalp dermatitis, distinctive hair abnormalities and atopic disease in the ectrodactyly-ectodermal dysplasia-clefting syndrome. Br J Dermatol 2006; 132:621–625. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki T, Tajima H, Migita M, et al. A case of anhidrotic ectodermal dysplasia presenting with pyrexia, atopic eczema, and food allergy. Asia Pac Allergy 2019; 9:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koguchi-Yoshioka H, Wataya-Kaneda M, Yutani M, et al. Atopic diathesis in hypohidrotic/anhidrotic ectodermal dysplasia. Acta Derm Venereol 2015; 95:476–479. [DOI] [PubMed] [Google Scholar]
- 62.Kohn LL, Braun M, Cordoro KM, et al. Skin and mucosal manifestations in NEMO syndrome: a case series and literature review. Pediatr Dermatol 2022; 39:84–90. [DOI] [PubMed] [Google Scholar]
- 63.Crowley TB, Campbell IM, Liebling EJ, et al. Distinct immune trajectories in patients with chromosome 22q11.2 deletion syndrome and immune-mediated diseases. J Allergy Clin Immunol 2022; 149:445–450. [DOI] [PubMed] [Google Scholar]
- 64.Morsheimer M, Brown Whitehorn TF, Heimall J, Sullivan KE. The immune deficiency of chromosome 22q11.2 deletion syndrome. Am J Med Genet A 2017; 173:2366–2372. [DOI] [PubMed] [Google Scholar]
- 65.Froňková E, Klocperk A, Svatoň M, et al. The TREC/KREC assay for the diagnosis and monitoring of patients with DiGeorge Syndrome. Speletas M, editor. PLoS One 2014; 9:e114514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Yu X, Ichikawa M, et al. Autosomal recessive phosphoglucomutase 3 (PGM3) mutations link glycosylation defects to atopy, immune deficiency, autoimmunity, and neurocognitive impairment. J Allergy Clin Immunol 2014; 133:1400–1409. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harb H, Chatila TA. Mechanisms of dupilumab. Clin Exp Allergy 2020; 50:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. clinicaltrials.gov. A study of BMS-986166 or Branebrutinib for the treatment of participants with atopic dermatitis [Internet]. https://classic.clinicaltrials.gov/ct2/show/NCT05014438. [Accessed 4 July 2023] [Google Scholar]
- 69.Nihal A, Comstock JR, Holland KE, et al. Clearance of atypical cutaneous manifestations of hyper-IgE syndrome with dupilumab. Pediatr Dermatol 2022; 39:940–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Igelman S, Kurta AO, Sheikh U, et al. Off-label use of dupilumab for pediatric patients with atopic dermatitis: a multicenter retrospective review. J Am Acad Dermatol 2020; 82:407–411. [DOI] [PubMed] [Google Scholar]
- 71.James AE, West L, Schloss K, et al. Treatment of STAT3-deficient hyper–immunoglobulin E syndrome with monoclonal antibodies targeting allergic inflammation. J Allergy Clin Immunol Pract 2022; 10:1367–1370. e1. [DOI] [PubMed] [Google Scholar]
- 72.Charvet E, Bourrat E, Hickman G, et al. Efficacy of dupilumab for controlling severe atopic dermatitis with dominant-negative CARD11 variant. Clin Exp Dermatol 2021; 46:1334–1335. [DOI] [PubMed] [Google Scholar]
- 73. clinicaltrials.gov. Immunologic effects of supplemental monosaccharide and nucleoside derivatives in patients with inherited disorders of glycosylation [Internet]. 2017. https://classic.clinicaltrials.gov/ct2/show/NCT02511041?term=PGM3+deficiency. [Accessed 4 July 2023] [Google Scholar]
- 74.Pitsios C, Demoly P, Bilò MB, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy 2015; 70:897–909. [DOI] [PubMed] [Google Scholar]
- 75.Pitsios C, Tsoumani M, Bilò MB, et al. Contraindications to immunotherapy: a global approach. Clin Transl Allergy 2019; 9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roberts G, Pfaar O, Akdis CA, et al. EAACI Guidelines on Allergen Immunotherapy: allergic rhinoconjunctivitis. Allergy 2018; 73:765–798. [DOI] [PubMed] [Google Scholar]
- 77.Krishna MT, Ewan PW, Diwakar L, et al. Diagnosis and management of hymenoptera venom allergy: British Society for Allergy and Clinical Immunology (BSACI) guidelines: BSACI venom allergy guidelines. Clin Exp Allergy 2011; 41:1201–1220. [DOI] [PubMed] [Google Scholar]
- 78.Harrison SC, Tsilifis C, Slatter MA, et al. Hematopoietic stem cell transplantation resolves the immune deficit associated with STAT3-dominant-negative Hyper-IgE syndrome. J Clin Immunol 2021; 41:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pillay BA, Avery DT, Smart JM, et al. Hematopoietic stem cell transplant effectively rescues lymphocyte differentiation and function in DOCK8-deficient patients. JCI Insight 2019; 4:e127527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Al-Herz W, Chu JI, Van Der Spek J, et al. Hematopoietic stem cell transplantation outcomes for 11 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol 2016; 138:852–859. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Happel CS, Stone KD, Freeman AF, et al. Food allergies can persist after myeloablative hematopoietic stem cell transplantation in dedicator of cytokinesis 8–deficient patients. J Allergy Clin Immunol 2016; 137:1895–1898. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]