Summary
Interferon regulatory factor 8 (IRF8) is a member of the IRF family that is specific to the hematopoietic cell and is involved in regulating the development of human monocytic and dendritic-lineage cells, as well as B-cells. Because its utility as a sensitive and specific monoblast marker in the context of acute monocytic leukemias has been recently demonstrated, we hypothesized that it may also be useful as a novel immunohistochemical marker in myeloid sarcomas and blastic plasmacytoid dendritic cell neoplasms (BPDCNs) with respect to their differential diagnoses. In this retrospective study, we analyzed the IHC expression pattern of IRF8 in 385 patient samples across 30 types of cancers, referenced to their mRNA expression data available through The Cancer Genome Atlas. In addition, we assessed IRF8 in 35 myeloid sarcomas and 15 BPDCNs. Twenty-four of 35 cases of myeloid sarcomas (68.5%) showed positivity for IRF8, with six cases (17.1%) demonstrating IRF8 expression in the absence of CD34 and MPO. All 15 of 15 BPDCNs (100%) showed strong uniform expression of IRF8 and were occasionally more definitive than CD123. IRF8 was negative in all desmoplastic small round cell tumors, Ewing sarcomas, synovial sarcomas, and undifferentiated pleomorphic sarcomas, as well as all epithelial malignancies tested except for 2 triple negative breast cancers that showed subset weak staining. In conclusion, IRF8 is a novel marker helpful in identifying extranodal hematopoietic tumors that can otherwise be difficult to diagnose given the broad differential diagnoses and frequent loss of more common lineage-defining markers.
Keywords: Myeloid sarcoma, Blastic plasmacytoid dendritic neoplasms, IRF8, Cancer tissue microarrays
1. Introduction
Interferon regulatory factor 8 (IRF8) is a hematopoietic-specific member of the IRF family that is essential in the commitment of myeloid progenitors to monocyte, macrophage, and dendritic cell lineages [1,2]. IRF8 expression is significantly elevated in monocyte-dendritic cell progenitors in mice, and conversely, Irf8−/− mice lack bone marrow–resident macrophages, dendritic cells, and plasmacytoid dendritic cells in lymphatic tissue [3-5]. The role of IRF8 in the generation of monocyte and dendritic cell progenitors is conserved in humans, as IRF8 mutations that impair IRF8 transcriptional activity are associated with immunodeficiency via decreased monocyte and dendritic cell populations [6]. Past work has shown that IRF8 is upregulated in subsets of acute myeloid leukemia (AML) and correlates with poor prognosis in patients with AML [7]. Moreover, we recently demonstrated that IRF8 immunohistochemistry (IHC) stains monoblasts in cases of acute monocytic leukemia and advanced chronic myelomonocytic leukemia, but is negative in mature monocytes and granulocytes [8]. Given this observation, IRF8 may hold promise as a biomarker for additional malignancies with monocytic and dendritic cell differentiation.
Myeloid sarcoma is an uncommon hematologic malignancy of immature monocyte and/or granulocyte populations that manifests as an extramedullary soft-tissue mass [9]. It is defined by the World Health Organization as a tumor of myeloid blasts that occurs at an anatomic location beyond the bone marrow [10]. Myeloid sarcoma typically develops secondary to AML, although rare cases of isolated disease without bone marrow involvement or history of myeloid malignancy have been described [11]. These tumors most commonly arise at the lymph nodes, skin, soft tissues, and bone and must cause effacement of local tissue to render a myeloid sarcoma diagnosis [10]. Owing to the disparate sites of disease involvement, clinical presentation is varied and pathology evaluation is critical for diagnosis. However, myeloid sarcoma is notoriously difficult to diagnose, with an estimated misdiagnosis rate of 25–47% [12-14]. This diagnostic inaccuracy is a product of disease rarity, particularly in the absence of a known myeloid disorder, as well as a dearth of markers for staining [15]. Histologically, the tumor demonstrates myeloid cell infiltration with myeloblasts or monoblasts [16]. Of note, lesions with granulocytic differentiation have features such as eosinophilic metamyelocytes that aid in a myeloid sarcoma diagnosis, whereas tumors with monocytic differentiation lack these characteristics and are typically more difficult to diagnose [12]. Moreover, although monocytic markers such as lysozyme and CD14 are available to phenotype AML with monocytic differentiation, they are not specific to blast populations, limiting their utility for differentiating myeloid sarcoma [15]. Consequently, additional biomarkers are needed to ameliorate the diagnostic challenges of this extramedullary malignancy.
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is an aggressive hematopoietic disorder of plasmacytoid dendritic cells [17]. BPDCN is a rare cancer, estimated to account for less than 1% of all hematologic malignancies, or roughly 700 cases annually in the United States [18]. In 90% of patients, BPDCN presents with skin manifestations and is later accompanied by involvement of bone marrow, peripheral blood, and lymph nodes [19,20]. Progression to systemic disease is rapid, and median survival is estimated to be between 12 and 14 months [21]. Diagnosis of BPDCN relies on clinical presentation, histological evaluation of the lesion, and immunophenotyping [22]. Owing to cutaneous involvement that presents with blast cells in the dermis, suspicion is often high for soft-tissue tumors, T-cell leukemias/lymphomas, and NK-cell leukemias/lymphomas [10]. It can be difficult to differentiate these malignancies from BPDCN, and immunophenotyping is critical to validate the observed skin lesions are comprised of plasmacytoid dendritic cells rather than myeloid or lymphoid blasts [23]. Additionally, expression of antigens expressed by other cell lineages, such as CD56 on NK/T-cell leukemia/lymphomas, and CD4 and CD123 on AMLs, can make it difficult to render a definitive diagnosis [24]. Thus, additional IHC markers in the diagnostic panel of BPDCN can help differentiate this malignancy from others with similar morphologic characteristics and clinical presentations.
We hypothesized that IRF8 may serve as a useful biomarker for myeloid sarcoma and BPDCN owing to their prominent monoblastic and dendritic cell differentiation, respectively. Herein, we demonstrate that IRF8 is able to stain neoplastic cells of both myeloid sarcoma and BPDCN, even in cases lacking traditional markers for these malignancies. Importantly, IRF8 expression is absent in a panel of soft-tissue cancers that could potentially mimic myeloid sarcoma and BPDCN, as well as solid tumors. Our work demonstrates the utility for IRF8 in the identification of these rare hematologic malignancies that are in need of additional markers to aid in diagnosis.
2. Methods
2.1. Human tumor samples and tissue microarray (TMA) creation
Thirty-five cases of myeloid sarcoma and 15 cases of BPDCN were obtained following approval by the Yale Institutional Review Board. All cases were diagnosed by board-certified pathologists at the Yale School of Medicine according to WHO criteria and validated by a second reviewer at outside institutions. All of the myeloid sarcoma cases were obtained from patients with a prior or concurrent AML diagnosis that demonstrated blast-like morphology on either peripheral blood or aspirate smear. Both a pan-cancer tissue microarray of 150 cases spanning 25 tumor subtypes and 27 tissue-matched normal samples as well as a triple-negative breast cancer—specific TMA of 97 cases were made in the Department of Pathology at the Yale School of Medicine. All cases were previously diagnosed at Yale, and use of these biopsies in this study was approved by the Yale Institutional Review Board. In addition, we used specimens from select sections of various previously published sarcoma-specific TMAs, including desmoplastic small round cell tumor (n = 30), Ewing sarcoma (n = 24), synovial sarcoma (n = 45), and undifferentiated pleomorphic sarcoma (n = 12) (Table 3).
Table 3.
IRF8 expression in different types of sarcoma included in sarcoma-specific TMAs.
| Sarcoma Type | Negative | Positive | Total |
|---|---|---|---|
| Desmoplastic small round cell tumor | 30 (100%) |
0 | 30 |
| Ewing sarcoma | 24 (100%) |
0 | 24 |
| Synovial sarcoma | 45 (100%) |
0 | 45 |
| Undifferentiated pleomorphic sarcoma | 12 (100%) |
0 | 12 |
2.2. IHC staining and scoring
IHC of all aforementioned samples and TMAs was performed using a rabbit monoclonal IRF8 antibody (Anti-IRF8 ab207418 1:900 dilution from Abcam, Waltham, MA, USA) as previously described [8]. We additionally stained myeloid sarcoma samples with both myeloperoxidase (MPO rabbit polyclonal concentrate 1:10 000 dilution from Dako, Carpinteria, CA, USA) and CD34 (QBEND Neat from Ventana Medical Systems, Tucson, AZ, USA). BPDCN samples were stained with MPO and CD123 (9F5 1:50 dilution from BD Biosciences, San Jose, CA, USA), CD4, CD56, TdT, TCL1, and TIA-1. Cases that were double stained with IRF8 and lysozyme utilized the double-staining protocol on Bond with lysozyme (polyclonal Neat from Agilent, Santa Clara, CA, USA). To perform staining, formalin-fixed, paraffin-embedded tumor tissue sections were deparaffinized and rehydrated prior to antigen retrieval and primary antibody incubation. Afterward, specimens were incubated with diaminobenzidine (DAB) chromagen for primary antibody detection and counterstained with hematoxylin. All stained samples were evaluated for both the percentage of immunoreactive cells and staining intensity (0, negative; 1+, weak; 2+, moderate; 3+, strong) ([25], Supplemental Fig. 1). When possible for both TMAs and whole-tissue sections, at least 3 nonadjacent fields containing tumor cells were evaluated for IRF8 staining. A subset (n = 12) of the cases on the TMA were also stained and evaluated on the whole slide of resection specimens to confirm that the sampling provided adequate representation. Only IRF8 nuclear staining of neoplastic cells was considered positive, as several samples demonstrated cytoplasmic staining of cancerous cells or nuclear staining of infiltrating lymphocytes. Nuclear reactivity at any intensity was denoted as positive for IRF8 and given as the percentage of overall tumor cells in a sample. The percentage of positive cells within tumor was also scored for CD34 and MPO in all myeloid sarcoma cases.
2.3. TCGA database analysis
Expression levels of IRF8 across different cancer types and their matched normal tissue were analyzed using Gene Expression Profiling Interactive Analysis (GEPIA), which utilizes global RNA-Seq data from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases. While TCGA contains expression data from 33 types of cancer, we collated this dataset to only analyze subtypes that were represented in our pan-cancer and sarcoma TMAs. We then determined the log2 fold-change of IRF8 expression between cancerous and normal tissue, utilizing a fold-change threshold of 1 and a p-value cutoff of 0.01 (as determined by a one-way analysis of variance) to identify significantly different IRF8 transcript abundance between these two groups.
3. Results
3.1. IRF8 abundance is elevated in monocyte-differentiated myeloid sarcoma
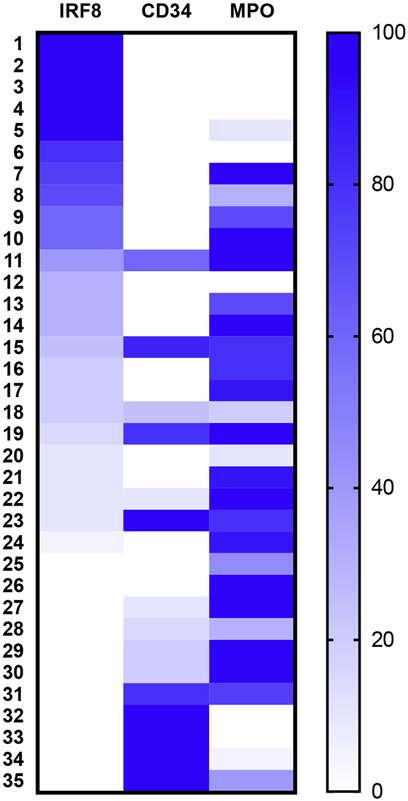
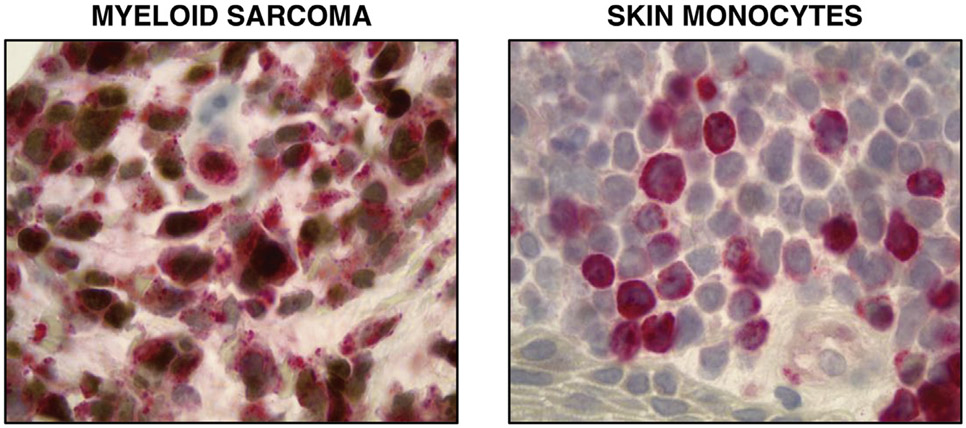
To investigate IRF8 expression in tumors of monocytic differentiation, we evaluated its abundance in myeloid sarcoma, an extramedullary malignancy of neoplastic immature monocytes and/or granulocytes [9]. We performed IHC staining of 35 myeloid sarcoma patient samples and concomitantly stained for CD34, a hematopoietic stem cell marker, and MPO [26], a myeloid marker. IRF8 was detectable in 24 of 35 (68.5%) of these cases, and CD34 was often undetectable in cases with the greatest degree of IRF8 positivity (Fig. 1). Importantly, there were six cases for which IRF8 positivity was observed in the absence of CD34 or MPO expression (Fig. 2). To confirm monocytic differentiation of these blasts, double staining with lysozyme was performed on seven MPO-negative specimens that had sufficient remaining tissue. In each case, there were IRF8+ lysozyme + blasts as well as background scattered mature monocytes that were IRF8-lysozyme+ (Fig. 3).
Fig. 1. IRF8 expression in myeloid sarcoma.
Heat map of myeloid sarcoma cases based on percent positivity of tumor cells for IRF8, CD34, or MPO by IHC.
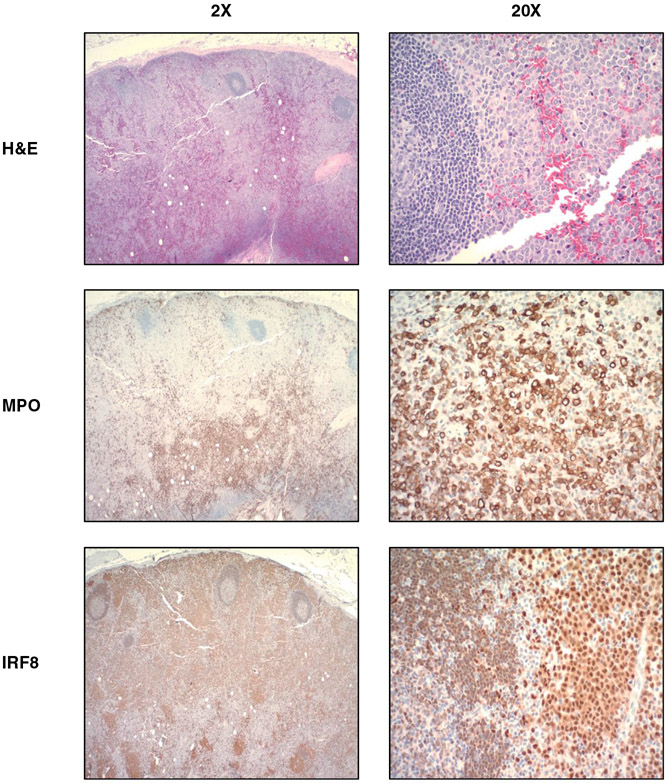
Fig. 2. Representative case of myeloid sarcoma at ×2, ×20 magnification.
This tumor shows strong IRF8 expression in the monoblastic population of tumor cells and MPO staining in the granulocytic population (MS Case 8 in Fig. 1). IRF8 expression is dimly observed in the mantle zone B cells adjacent to neoplastic cells.
Fig. 3. IRF8 and lysozyme staining in monocytic malignancies.
Double stain with IRF8 (brown) and lysozyme (red) highlights double-positive monoblasts in a case of myeloid sarcoma (left) and lysozyme single-positive monocytes in the skin (right).
3.2. IRF8 is expressed in blastic plasmacytoid dendritic cell neoplasm
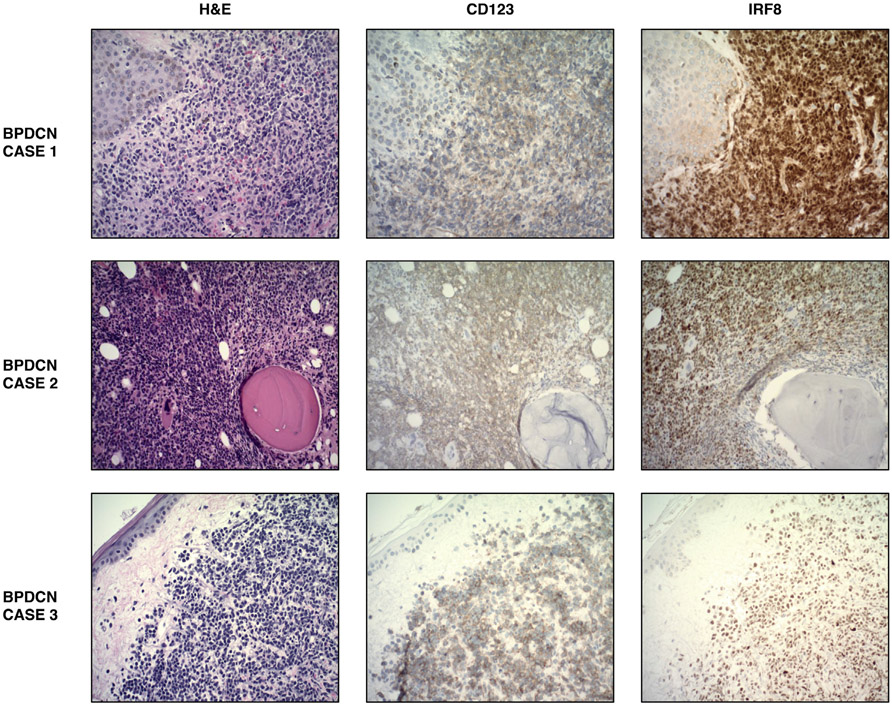
We next investigated IRF8 abundance in BPDCN, a rare hematopoietic malignancy arising from immature plasmacytoid dendritic cells [17]. Staining of 15 BPDCN cases demonstrated uniform IRF8 expression, with all (100%) samples showing positive IRF8 staining by IHC. The diagnosis of each of these BPDCN cases was validated by a panel of IHC that included CD4, CD56, and CD123 (Table 1). Importantly, there was one case that stained negatively and three cases that stained dimly for CD123, a marker commonly used in the diagnosis of BPDCN, but stained positively for IRF8 (Fig. 4, Table 1).
Table 1.
Immunophenotyping of 15 BPDCN cases.
| BPDCN | IRF8 | CD4 | CD56 | CD123 | TdT | TCL1 | MPO | TIA-1 |
|---|---|---|---|---|---|---|---|---|
| Case # | ||||||||
| 1 | + | + | + | + | − | N/A | − | + |
| 2 | + | + | + | Dim | + | + | − | N/A |
| 3 | + | + | + | Weak | N/A | N/A | N/A | N/A |
| 4 | + | + | + | + | Variable | N/A | N/A | N/A |
| 5 | + | + | + | + | Variable | + | N/A | N/A |
| 6 | + | + | Dim | + | Rare | N/A | − | N/A |
| 7 | + | + | − | + | − | + | − | + |
| 8 | + | Dim | Subset | − | − | + | − | N/A |
| 9 | + | + | − | + | − | + | − | N/A |
| 10 | + | + | + | + | N/A | + | N/A | N/A |
| 11 | + | + | + | Dim | + | + | − | N/A |
| 12 | + | + | Dim | + | Few | − | − | − |
| 13 | + | + | + | + | Variable | N/A | − | − |
| 14 | + | + | + | + | − | − | N/A | N/A |
| 15 | + | + | − | + | Rare | − | − | − |
+ denotes positive staining, − denotes negative staining, N/A denotes staining not performed.
Fig. 4. Expression patterns of BPDCN in three representative patients.
BPDCN case 1 shows a mid-chest skin biopsy. BPDCN case 2 shows a bone marrow biopsy. BPDCN case 3 shows a left chest skin biopsy. All images taken at ×200 magnification.
3.3. Assessment of IRF8 expression across different cancer types
IRF8 expression across additional tumor types and normal tissues was evaluated to validate its specificity for hematopoietic malignancies. A pan-cancer TMA comprising 177 patient samples of both normal and cancerous tissue across 11 tissue types and 25 cancer subtypes revealed that IRF8 expression was negative in all normal tissue samples and all but one tumor sample, which was a diffuse large B-cell lymphoma of the testis (Table 2, Fig. 5). Because our small cohort of breast cancers in the initial pan-cancer TMA were devoid of triple-negative breast cancers (TNBCs), we assessed IRF8 expression on a TMA of 97 TNBCs and observed that 2 (2.1%) of these tumors were IRF8-positive. The staining pattern for these cases was weak and showed subset reactivity (Fig. 5). Further staining of 2 breast cancer cases of whole slides showed identical focal, weak staining. In many samples, we observed IRF8 staining of background lymphocytes that spared large, neoplastic cells.
Table 2.
IRF8 expression in normal tissues and malignancies of different differentiation included in a pan-cancer TMA.
| Tissue | Subtype | Negative | Positive | Total |
|---|---|---|---|---|
| Bladder | Normal bladder | 3 (100%) |
0 | 3 |
| Papillary transitional cell carcinoma | 1 (100%) |
0 | 1 | |
| Papillary urothelial cell carcinoma | 4 (100%) |
0 | 4 | |
| Serous adenocarcinoma | 1 (100%) |
0 | 1 | |
| Urothelial carcinoma | 10 (100%) |
0 | 10 | |
| Breast | Ductal carcinoma | 9 (100%) |
0 | 9 |
| Lobular carcinoma | 1 (100%) |
0 | 1 | |
| Colon | Normal colon | 2 (100%) |
0 | 2 |
| Adenocarcinoma | 13 (100%) |
0 | 13 | |
| Kidney | Normal kidney | 3 (100%) |
0 | 3 |
| Papillary renal cell carcinoma | 10 (100%) |
0 | 10 | |
| Renal cell carcinoma | 4 (100%) |
0 | 4 | |
| Liver | Normal liver | 4 (100%) |
0 | 4 |
| Hepatocellular carcinoma | 14 (100%) |
0 | 14 | |
| Mixed hepatocholangiocarcinoma | 1 (100%) |
0 | 1 | |
| Lung | Normal lung | 2 (100%) |
0 | 2 |
| Adenocarcinoma | 7 (100%) |
0 | 7 | |
| Neuroendocrine carcinoma | 1 (100%) |
0 | 1 | |
| Squamous cell carcinoma | 8 (100%) |
0 | 8 | |
| Ovary | Normal ovary | 4 (100%) |
0 | 4 |
| Adenocarcinoma | 12 (100%) |
0 | 12 | |
| Pancreas | Normal pancreas | 3 (100%) |
0 | 3 |
| Adenocarcinoma | 1 (100%) |
0 | 1 | |
| Endocrine carcinoma | 12 (100%) |
0 | 12 | |
| Neuroendocrine carcinoma | 2 (100%) |
0 | 2 | |
| Skin | Normal skin | 2 (100%) |
0 | 2 |
| Squamous cell carcinoma | 14 (100%) |
0 | 14 | |
| Stomach | Normal stomach | 2 (100%) |
0 | 2 |
| Adenocarcinoma | 10 (100%) |
0 | 10 | |
| Testis | Normal testis | 2 (100%) |
0 | 2 |
| Embryonal carcinoma | 1 (100%) |
0 | 1 | |
| Leydig cell carcinoma | 2 (100%) |
0 | 2 | |
| Lymphoma | 0 | 1 (100%) |
1 | |
| Mixed germ cell carcinoma | 6 (100%) |
0 | 6 | |
| Seminoma | 5 (100%) |
0 | 5 |
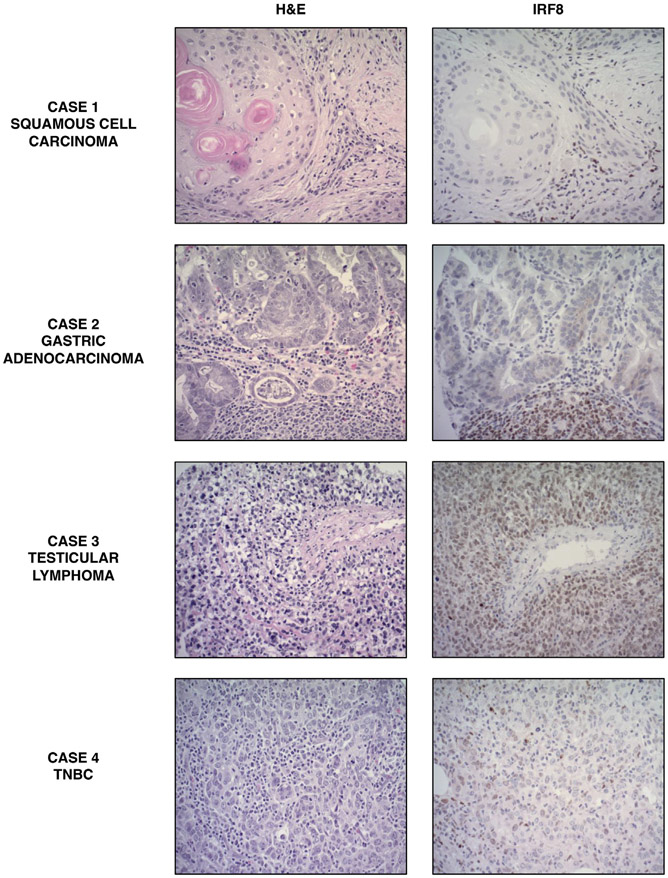
Fig. 5. Representative cases of IRF8 IHC from pan-cancer TMA.
Case 1 shows a squamous cell skin carcinoma with IRF8 staining of infiltrating lymphocytes but not neoplastic cells. Case 2 shows a gastric adenocarcinoma with a similar staining pattern to Case 1. Case 3 shows a testicular lymphoma with IRF8 staining of large, polymorphic tumor cells. Case 4 shows a representative case of an IR8-positive TNBC.
We also performed IRF8 staining on sarcoma TMAs of subtypes that may be considered in the differential diagnosis of myeloid sarcoma or BPDCN. IRF8 expression was not observed in any of the 111 soft-tissue tumors belonging to 4 subtypes of sarcomas in these TMAs, which included desmoplastic small round cell tumor, Ewing sarcoma, synovial sarcoma, and undifferentiated pleomorphic sarcoma (Table 3).
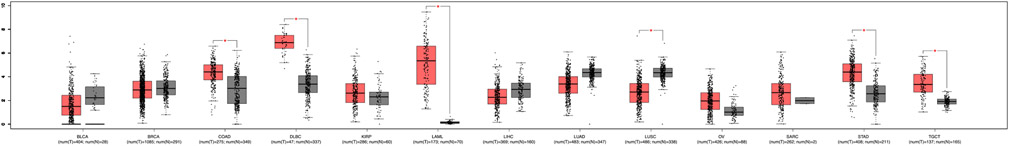
We next analyzed publicly available expression data from TCGA to further assess IRF8 expression between normal and cancerous tissues for malignancies included in the TMA. In line with previous reports, IRF8 was significantly upregulated in acute myeloid leukemia and diffuse large B-cell lymphoma samples relative to normal tissue [7]. However, IRF8 mRNA expression was also increased in colon adenocarcinoma, stomach adenocarcinoma, and testicular germ cell tumors (Fig. 6). Although these tumors were represented in our pan-cancer TMA, we did not observe a similar upregulation of IRF8 protein in the neoplastic cells, but rather in infiltrating lymphocytes (Table 2).
Fig. 6. IRF8 expression in cancerous tissues (red) and normal tissues (gray).
Box plots marked with a red asterisk indicate a statistically significant difference (p < .05) between tumor and normal tissue. BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B-cell lymphoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute myeloid leukemia; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma, LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; SARC, sarcoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors.
4. Discussion
Given our recent work demonstrating IRF8 as a reliable monoblast marker for acute monocytic leukemias [8], we sought to evaluate the utility of IRF8 in staining monocyte-differentiated hematopoietic cancers for which clinical diagnosis is challenging and helpful tissue markers are lacking. In this separate, nonoverlapping case series, we stained for IRF8 in samples of myeloid sarcoma, BPDCN, and nonhematopoietic tumors that may enter into the differential diagnosis.
IRF8 was positive in a significant proportion of myeloid sarcoma cases, including those negative for CD34 and MPO. As expected, the IRF8-positive cases had previous/concurrent marrows that demonstrated monocytic differentiation by aspirate morphology with or without flow immunophenotypic support. Importantly, IRF8 did not stain any of the soft-tissue sarcomas that represent some of the differential diagnoses of myeloid sarcoma. These include undifferentiated pleomorphic sarcoma and small round blue cell tumors, such as Ewing sarcoma, desmoplastic small round cell tumor, and synovial sarcoma, as well as its poorly differentiated variant [27]. Thus, IRF8 can be utilized as a biomarker for myeloid sarcoma and has the potential to improve diagnostic accuracy of this hematologic malignancy, particularly for cases which are primarily of monocytic differentiation. Because our institution had only one confirmed histiocytic sarcoma with adequate remaining tissue, a confident conclusion cannot be made from its positive staining with IRF8.
BPDCN diagnosis is made on the basis of clinical features, morphological findings, and immunophenotype. Diagnosis is challenging, and BPDCN is often difficult to distinguish from soft-tissue tumors, AML, and T-cell leukemias/lymphomas owing to both an overlap in immunophenotyping as well as the common lymphoid-like morphology of BPDCN blasts that leads to a false suspicion of lymphoma [26]. Although our study is limited by the relatively few cases of BPDCN, all 15 samples were strongly positive for IRF8 staining. Additionally, 4 of these IRF8-positive cases were dim to negative for CD123, a marker commonly used for BPDCN, suggesting that IRF8 may be a useful marker to add to the diagnostic panel for this aggressive hematopoietic malignancy [28]. As TCF4 has recently been found to be a very sensitive and specific marker for BPDCN when used as a costain with CD123 [29], it is of great interest to investigate the diagnostic utility of IRF8/TCF4 coexpression, particularly in cases of diminished CD123 expression.
To confirm that IRF8 expression is specific to cancers of hematologic origin, as poorly differentiated carcinomas can occasionally enter the differential diagnosis of hematopoietic malignancies, we showed that IRF8 did not stain any solid tumors or normal tissue on a pan-cancer TMA comprising 25 cancer subtypes. Although a recent study documented increased IRF8 expression in estrogen receptor (ER)–negative breast cancers [30], only rare cases (2.1%) of our breast cancer cohort stained weakly positive for IRF8. This difference is potentially due to a decreased representation of early-stage ER-negative cancers on the TMA. Our TMA also highlighted a discrepancy with TCGA global expression data, which suggested that IRF8 is transcriptionally upregulated in several carcinomas relative to normal tissue, particularly colon adenocarcinoma, stomach adenocarcinoma, and testicular germ cell tumors, all of which were represented in our TMA. In contrast to this TCGA data, prior reports have indicated that IRF8 expression is preferentially decreased in both colon and gastric cancer samples relative to noncancerous tissue, through mechanisms such as increased promoter methylation [31,32]. This discrepancy likely arises from a lymphocytic infiltrate in these carcinomas.
In the TCGA dataset, IRF8 upregulation was most significantly associated with AML and diffuse large B-cell lymphoma, further supporting its utility as a marker of hematopoietic neoplasms [7]. In fact, the only sample positive for IRF8 on the carcinoma TMA turned out to be a testicular diffuse large B-cell lymphoma mistakenly added. Diagnosis was confirmed by additional B-cell markers on the whole slide of this particular case. These observations, in conjunction with reports of IRF8 as a B-cell transcription factor [33], support the possibility that IRF8 may also be used as a marker for B-cell lymphomas. In preliminary work for a separate report, we find that IRF8 positively stained a cohort of 9 diffuse large B-cell lymphomas, but was undetectable in 15 T-cell lymphomas, suggesting its utility in differentiating between B cell– and T cell–derived lymphomas (data not shown).
In this study, we demonstrate that IRF8 can be used to identify monocyte-differentiated and dendritic cell–differentiated malignancies that are prone to misdiagnosis due to a scarcity of specific markers. Further validation of these findings by placing them in broader immunophenotypic, molecular, and clinical contexts will be critical to integrate IRF8 staining into pathologists’ workflow for diagnosis of both myeloid sarcoma and BPDCN. Additionally, future studies should build upon this work by determining if differential IRF8 expression correlates with poor prognosis or response to therapy in the diseases it stains. Although this will likely prove challenging owing to the rarity of both myeloid sarcoma and BPDCN, identifying additional prognostic markers for these diseases is crucial, particularly given the lack of data regarding survival differences between granulocyte-predominant and monocyte-predominant myeloid sarcoma [34]. IRF8 thus may hold promise not only as a marker for diagnosis but also as a prognostic indicator that guides treatment intervention for these rare hematopoietic neoplasms.
Supplementary Material
Acknowledgments
We thank Amos Brooks and Lori Charette from Yale Pathology Tissue Services for their invaluable experience and aid with immunohistochemical studies as well as tissue procurement and processing.
Footnotes
Disclosures: None.
Ethics approval and consent to participate: The study was performed in accordance with the Declaration of Helsinki. The study was approved by an internal review board (local ethics committee HIC protocol #2000023891).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.humpath.2022.01.004.
Data availability:
The tissue sections analyzed during this study are available from the corresponding author upon request. TCGA data analyzed during this study are publicly available using the GEPIA online resource.
References
- [1].Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity 2000;13:155–65. [DOI] [PubMed] [Google Scholar]
- [2].Kurotaki D, Osato N, Nishiyama A, Yamamoto M, Ban T, Sato H, et al. Essential role of the IRF8-KLF4 transcription factor cascade in murine monocyte differentiation. Blood 2013;121:1839–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kurotaki D, Yamamoto M, Nishiyama A, Uno K, Ban T, Ichino M, et al. IRF8 inhibits C/EBPα activity to restrain mononuclear phagocyte progenitors from differentiating into neutrophils. Nat Commun 2014;5:4978. [DOI] [PubMed] [Google Scholar]
- [4].Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J Immunol 2003;170:1131–5. [DOI] [PubMed] [Google Scholar]
- [5].Yamamoto M, Kato T, Hotta C, Nishiyama A, Kurotaki D, Yoshinari M, et al. Shared and distinct functions of the transcription factors IRF4 and IRF8 in myeloid cell development. PLoS One 2011;6:e25812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med 2011;365:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liss F, Frech M, Wang Y, Giel G, Fischer S, Simon C, et al. IRF8 is an AML-specific susceptibility factor that regulates signaling pathways and proliferation of AML cells. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Katz SG, Edappallath S, Xu ML. IRF8 is a reliable monoblast marker for acute monocytic leukemias. Am J Surg Pathol 2021;45:1391–8. [DOI] [PubMed] [Google Scholar]
- [9].Alexiev BA, Wang W, Ning Y, Chumsri S, Gojo I, Rodgers WH, et al. Myeloid sarcomas: a histologic, immunohistochemical, and cytogenetic study. Diagn Pathol 2007;2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Swerdlow SH, World Health Organization, International Agency for Research on Cancer. AML and related precursor neoplasms. In: WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon: International Agency for Research on Cancer; 2017. p. 167–9. [Google Scholar]
- [11].Shallis RM, Gale RP, Lazarus HM, Roberts KB, Xu ML, Seropian SE, et al. Myeloid sarcoma, chloroma, or extramedullary acute myeloid leukemia tumor: a tale of misnomers, controversy and the unresolved. Blood Rev 2021;47:100773. [DOI] [PubMed] [Google Scholar]
- [12].Almond LM, Charalampakis M, Ford SJ, Gourevitch D, Desai A. Myeloid sarcoma: presentation, diagnosis, and treatment. Clin Lymphoma Myeloma Leuk 2017;17:263–7. [DOI] [PubMed] [Google Scholar]
- [13].Antic D, Elezovic I, Milic N, Suvajdzic N, Vidovic A, Perunicic M, et al. Is there a "gold" standard treatment for patients with isolated myeloid sarcoma? Biomed Pharmacother 2013;67:72–7. [DOI] [PubMed] [Google Scholar]
- [14].Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer 2002;94:1739–46. [DOI] [PubMed] [Google Scholar]
- [15].Magdy M, Abdel Karim N, Eldessouki I, Gaber O, Rahouma M, Ghareeb M. Myeloid sarcoma. Oncol Res Treat 2019;42:224–9. [DOI] [PubMed] [Google Scholar]
- [16].Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007;21:340–50. [DOI] [PubMed] [Google Scholar]
- [17].Sapienza MR, Fuligni F, Agostinelli C, Tripodo C, Righi S, Laginestra MA, et al. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia 2014;28:1606–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pagano L, Valentini CG, Pulsoni A, Fisogni S, Carluccio P, Mannelli F, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica 2013;98:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cota C, Vale E, Viana I, Requena L, Ferrara G, Anemona L, et al. Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm-morphologic and phenotypic variability in a series of 33 patients. Am J Surg Pathol 2010;34:75–87. [DOI] [PubMed] [Google Scholar]
- [20].Julia F, Petrella T, Beylot-Barry M, Bagot M, Lipsker D, Machet L, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol 2013;169:579–86. [DOI] [PubMed] [Google Scholar]
- [21].Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med 2014;138:564–9. [DOI] [PubMed] [Google Scholar]
- [22].Sullivan JM, Rizzieri DA. Treatment of blastic plasmacytoid dendritic cell neoplasm. Hematology Am Soc Hematol Educ Program 2016;2016:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Deotare U, Yee KW, Le LW, Porwit A, Tierens A, Musani R, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: 10-Color flow cytometry diagnosis and HyperCVAD therapy. Am J Hematol 2016;91:283–6. [DOI] [PubMed] [Google Scholar]
- [24].Garnache-Ottou F, Feuillard J, Ferrand C, Biichle S, Trimoreau F, Seilles E, et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol 2009;145:624–36. [DOI] [PubMed] [Google Scholar]
- [25].Parris TZ, Aziz L, Kovács A, Hajizadeh S, Nemes S, Semaan M, et al. Clinical relevance of breast cancer-related genes as potential biomarkers for oral squamous cell carcinoma. BMC Cancer 2014;14:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garnache-Ottou F, Vidal C, Biichlé S, Renosi F, Poret E, Pagadoy M, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv 2019;3:4238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Marwah N, Bhutani N, Budhwar A, Sen R. Isolated myeloid sarcoma of the temporal bone: as the first clinical manifestation of acute myeloid leukemia in a patient of down’s syndrome. Int J Surg Case Rep 2019;58:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Laribi K, Denizon N, Besançon A, Farhi J, Lemaire P, Sandrini J, et al. Blastic plasmacytoid dendritic cell neoplasm: from origin of the cell to targeted therapies. Biol Blood Marrow Transplant 2016;22:1357–67. [DOI] [PubMed] [Google Scholar]
- [29].Sukswai N, Aung PP, Yin CC, Li S, Wang W, Wang SA, et al. Dual expression of TCF4 and CD123 is highly sensitive and specific for blastic plasmacytoid dendritic cell neoplasm. Am J Surg Pathol 2019;43:1429–37. [DOI] [PubMed] [Google Scholar]
- [30].Gatti G, Betts C, Rocha D, Nicola M, Grupe V, Ditada C, et al. High IRF8 expression correlates with CD8 T cell infiltration and is a predictive biomarker of therapy response in ER-negative breast cancer. Breast Cancer Res 2021;23:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ibrahim Ml l K. IRF8 deficiency in colonic epithelial cells promotes inflammation-mediated colon tumorigenesis and myeloid-derived suppressor cell differentiation. J Immunol 2017;198. 66.20. [Google Scholar]
- [32].Yamashita M, Toyota M, Suzuki H, Nojima M, Yamamoto E, Kamimae S, et al. DNA methylation of interferon regulatory factors in gastric cancer and noncancerous gastric mucosae. Cancer Sci 2010;101:1708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang H, Lee CH, Qi C, Tailor P, Feng J, Abbasi S, et al. IRF8 regulates B-cell lineage specification, commitment, and differentiation. Blood 2008;112:4028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol 2011;2:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The tissue sections analyzed during this study are available from the corresponding author upon request. TCGA data analyzed during this study are publicly available using the GEPIA online resource.