Abstract
Purpose: To report a case of painless posterior scleritis presenting as a choroidal nodule in a patient with history of a tumor being treated with pembrolizumab. Methods: A case and its findings were analyzed, and a relevant literature review was performed. Results: A 20-year-old woman with a history of ependymoma presented with painless blurred vision in the right eye after being started on pembrolizumab for a tumor recurrence. Fundoscopy showed a solitary amelanotic choroidal lesion with surrounding subretinal fluid in the affected eye. Ultrasonography showed moderate internal reflectivity and fluid in Tenon capsule consistent with nodular posterior scleritis. After a course of systemic steroids and discontinuation of the pembrolizumab, the choroidal lesion completely resolved. Conclusions: Clinicians should be aware of posterior scleritis as an ocular complication of this class of medications.
Keywords: checkpoint inhibitors, pembrolizumab, scleritis, uveitis
Introduction
Pembrolizumab was the first anti-programmed cell death receptor protein 1 (PD-1) agent approved by the US Food and Drug Administration and is being used worldwide in the treatment of various malignancies. 1 This class of medications has been associated with several inflammatory side effects, both systemic and ophthalmic. Dry eye is the most common ocular side effect, although cases of uveitis, including sarcoid-like granulomas and scleritis, have occurred.1–5 Although rare, ocular immune-related adverse events (irAEs) may warrant cessation of the medication. Providers should be aware of these potential ocular reactions to better care for patients.
Here, we present a case of nodular posterior scleritis resulting from pembrolizumab administration.
Case Report
A 20-year-old Black woman with a medical history of ependymoma was referred to an ocular oncology clinic for decreased vision and photopsias in the right eye. She previously had several treatments for recurrent ependymoma, including surgical excision and radiation as a child. Two months before her presentation to the ophthalmology clinic, the patient’s medical oncologist started pembrolizumab for intracranial tumor recurrence.
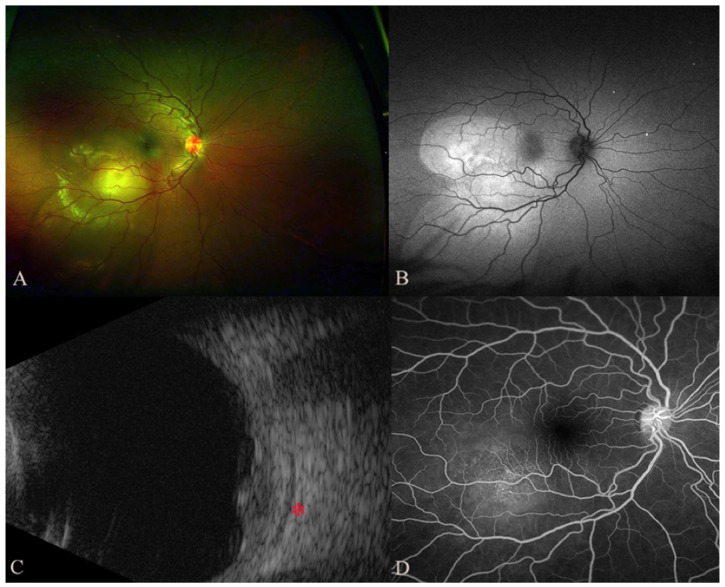
On examination, the Snellen visual acuity (VA) was 20/640 OD and 20/20 OS. Fundoscopic examination of the right eye showed a solitary amelanotic choroidal lesion measuring 12.0 mm × 8.0 mm × 5.2 mm in the inferotemporal macula with surrounding subretinal fluid (Figure 1, A and B). Ultrasonography of the lesion showed moderate heterogeneous internal reflectivity with subtle adjacent choroidal thickening and fluid in Tenon capsule (Figure 1C). The late arteriovenous phase of the fluorescein angiogram showed multiple pinpoint spots of leakage (Figure 1D). Optical coherence tomography through the macula showed an elevated choroidal lesion with overlying subretinal fluid that involved the fovea (Figure 2A).
Figure 1.
On initial presentation, the color fundus photograph (A) shows an amelanotic choroidal elevation with surrounding subretinal fluid that was highlighted on the autofluorescence image (B) as an area of hyperautofluorescence that was most pronounced over the lesion with areas of inferior, gravity-dependent guttering. (C) Ultrasonography shows a choroidal lesion with moderate internal reflectivity and associated fluid in Tenon space (red asterisk). (D) Fluorescein angiogram from the late arteriovenous phase shows multiple pinpoint spots of leakage overlying the lesion.
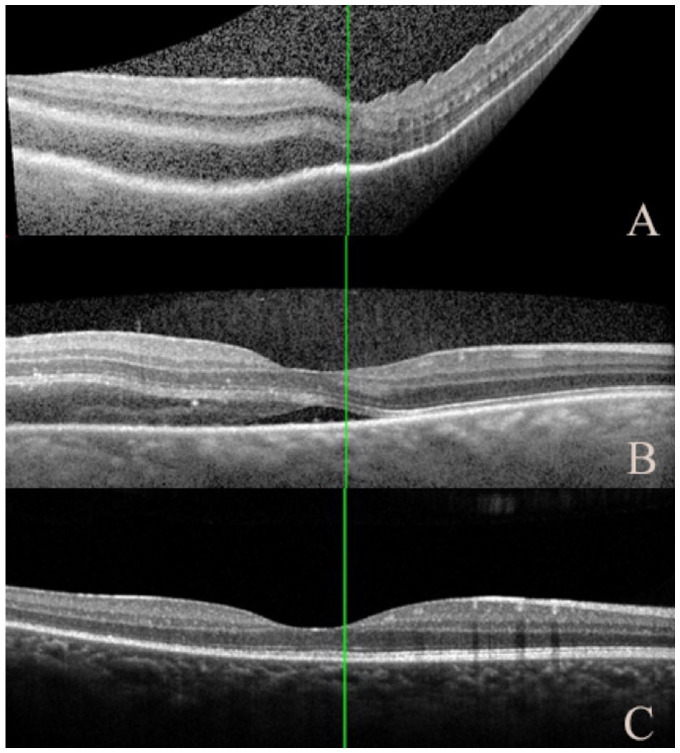
Figure 2.
(A) Optical coherence tomography shows choroidal thickening with subretinal fluid involving the fovea on presentation. Dramatic improvement was noted 2 days after pembrolizumab discontinuation (B) and resolution of fluid with minimal residual changes remaining in the retinal layers on follow-up 14 days later (C).
Initial concern for possible metastasis prompted a metastatic workup by the patient’s oncologist, which was negative. Given the constellation of findings, and notably the fluid in Tenon capsule, a diagnosis of nodular posterior scleritis was made. In collaboration with oncology, the patient was initiated on oral dexamethasone 8 mg twice daily and the pembrolizumab was discontinued. Dramatic improvement in VA and lesion size was noted 2 days later (Figures 2B and 3B). Complete resolution of the lesion was observed 14 days after pembrolizumab discontinuation (Figures 2C, 3C, and 4, A and B). Dexamethasone was tapered slowly over 3 weeks. Two months after pembrolizumab discontinuation, the VA was 20/25 OD and no known tumor recurrence had occurred.
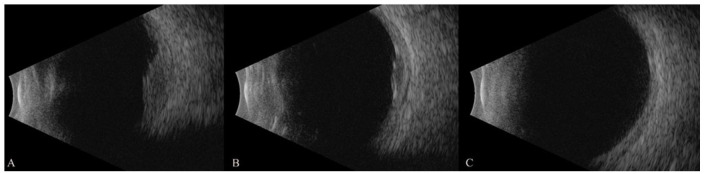
Figure 3.
Serial ultrasound images show progressive resolution of the choroidal lesion. On initial evaluation (A) the choroidal lesion with fluid in Tenon space was 5.2 mm in maximum thickness, decreasing to 2.5 mm 2 days after pembrolizumab discontinuation (B), and complete resolution of lesion and fluid in Tenon space was noted 14 days later (C).
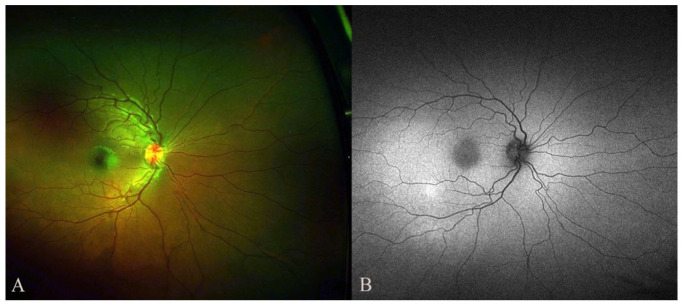
Figure 4.
Color fundus photograph (A) and autofluorescence (B) of the right eye show complete resolution of the choroidal lesion on follow-up examination 2 months after pembrolizumab discontinuation.
Conclusions
Immune checkpoint inhibitors are playing an increasingly central role in the modern treatment of various cancers. However, they have been associated with an array of ocular side effects, most commonly dry eye.1–5 Lupus-like reactions, sarcoid choroidal granulomas, and other forms of systemic and ocular inflammation have been described.2–6 To our knowledge, this case of painless nodular posterior scleritis presents a unique reaction to this medication that has not been previously noted in the literature.
Pembrolizumab is a monoclonal antibody against PD-1. Initially approved for use against advanced melanoma, it is now used for many types of cancer. PD-1 is expressed on subtypes of many immune cells, most notably T cells, and serves an important immune checkpoint function resulting in self-tolerance and immune suppression.6,7 PD-L1 (ligand) is expressed by antigen-presenting cells as well as some tumors, including ependymoma. 8 When PD-L1 binds PD-1, an inhibitory pathway is activated that inhibits the anti-cancer activity of T cells. Pembrolizumab blocks cellular binding of the PD-L1/PD-1 complex, in turn preventing tumor evasion from host immune cells. As a result of this drug-induced immune disinhibition, loss of self-tolerance may develop, leading to an array of irAEs to pembrolizumab and other immune checkpoint inhibitors. 6
When irAEs occur, the typical onset is within a few weeks to months of administration, although they may occur later. 9 Best treatment patterns of irAEs are still being explored. Options include localized or systemic steroid administration, addition of systemic immunosuppressive agents, or withdrawal of the offending agent if the reaction is severe.9–11 For moderate to severe ocular reactions requiring systemic treatment, permanent discontinuation of the offending drug is often warranted. In this case, collaboration with the patient’s medical oncologist allowed for safe withdrawal of the medication and initiation of systemic steroids, which prompted rapid resolution of the ocular findings and visual complaints.
Posterior scleritis can pose a diagnostic challenge, in particular when it presents as a choroidal nodule, and it may share signs and symptoms with infectious or malignant etiologies. 12 Posterior scleritis typically presents unilaterally with eye pain and redness, is more common in young females, and is frequently associated with anterior scleral involvement. 13 A subretinal localized granuloma can be the presenting sign in approximately 13% of patients with posterior scleritis. 13 Our case is unique in that the patient presented in the setting of recurrence of a malignancy and without pain. Although an ependymoma has rarely been reported to metastasize, the examination, history, and imaging were vital to elucidate the correct diagnosis in our patient and rule out this possibility. Discontinuing the offending agent and treatment with systemic steroids resulted in prompt resolution of the lesion and visual recovery.
Pembrolizumab and other checkpoint inhibitors are increasingly being used for a broad range of malignancies. Clinicians should be aware of posterior scleritis and other ocular side effects of these medications to better care for patients.
Footnotes
Ethical Approval: This case report was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a US Health Insurance Portability and Accountability Act–compliant manner.
Statement of Informed Consent: In accordance with the institutional review board, patient consent was not needed for this case report given the deidentified nature of all information herein.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Calvin L. McNelly  https://orcid.org/0000-0002-7068-9048
https://orcid.org/0000-0002-7068-9048
References
- 1. Khoja L, Butler MO, Kang SP, Ebbinghaus S, Joshua AM. Pembrolizumab. J Immunother Cancer. 2015;18(3):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fortes BH, Liou H, Dalvin LA. Ophthalmic adverse effects of immune checkpoint inhibitors: the Mayo Clinic experience. Br J Ophthalmol. 2021;105(9):1263-1271. [DOI] [PubMed] [Google Scholar]
- 3. Ung C, Gragoudas E. Checkpoint inhibitor-induced sarcoid choroidal granulomas. Am J Ophthalmol Case Rep. 2020;18:100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu X, Wang Z, Zhao C, et al. Clinical diagnosis and treatment recommendations for ocular toxicities of targeted therapy and immune checkpoint inhibitor therapy. Thorac Cancer. 2020;11(3):810-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young L, Finnigan S, Streicher H, et al. Ocular adverse events in PD-1 and PD-L1 inhibitors. J Immunother Cancer. 2021;9(7):e002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerasimova EV, Tabakov DV, Gerasimova DA, Popkova TV. Activation markers on B and T cells and immune checkpoints in autoimmune rheumatic diseases. Int J Mol Sci. 2022;23(15):8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141-151. [DOI] [PubMed] [Google Scholar]
- 8. Witt DA, Donson AM, Amani V, et al. Specific expression of PD-L1 in RELA-fusion supratentorial ependymoma: implications for PD-1-targeted therapy. Pediatr Blood Cancer. 2018;65:e26960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119-iv142. [DOI] [PubMed] [Google Scholar]
- 10. Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Velasco G, Bermas B, Choueiri TK. Autoimmune arthropathy and uveitis as complications of programmed death 1 inhibitor treatment. Arthritis Rheumatol. 2016;68(2):556-557. [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez-Gonzalez LA, Molina-Prat N, Doctor P, Tauber J, Sainz de la Maza M, Foster CS. Clinical features and presentation of posterior scleritis: a report of 31 cases. Ocul Immunol Inflamm. 2014;22(3):203-207. [DOI] [PubMed] [Google Scholar]
- 13. McCluskey PJ, Watson PG, Lightman S, Haybittle J, Restori M, Branley M. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology. 1999;106(12):2380-2386. [DOI] [PubMed] [Google Scholar]