Abstract
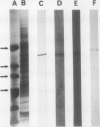
Plant microsomes contain a protein clearly related to a calcium-binding protein, calsequestrin, originally found in the sarcoplasmic reticulum of muscle cells, responsible for the rapid release and uptake of Ca2+ within the cells. The location and role of calsequestrin in plant cells is unknown. To generate monoclonal antibodies specific to plant calsequestrin, mice were immunized with a microsomal fraction from cultured cells of Streptanthus tortuosus (Brassicaceae). Two clones cross-reacted with one protein band with a molecular weight equal to that of calsequestrin (57 kilodaltons) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting. This band is able to bind 45Ca2+ and can be recognized by a polyclonal antibody against the canine cardiac muscle calsequestrin. Rabbit skeletal muscle calsequestrin cross-reacted with the plant monoclonal antibodies. The plant monoclonal antibodies generated here are specific to calsequestrin protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell K. P., MacLennan D. H., Jorgensen A. O. Staining of the Ca2+-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye "Stains-all". J Biol Chem. 1983 Sep 25;258(18):11267–11273. [PubMed] [Google Scholar]
- Damiani E., Spamer C., Heilmann C., Salvatori S., Margreth A. Endoplasmic reticulum of rat liver contains two proteins closely related to skeletal sarcoplasmic reticulum Ca-ATPase and calsequestrin. J Biol Chem. 1988 Jan 5;263(1):340–343. [PubMed] [Google Scholar]
- Drøbak B. K., Ferguson I. B. Release of Ca2+ from plant hypocotyl microsomes by inositol-1,4,5-trisphosphate. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1241–1246. doi: 10.1016/0006-291x(85)91747-4. [DOI] [PubMed] [Google Scholar]
- Fliegel L., Ohnishi M., Carpenter M. R., Khanna V. K., Reithmeier R. A., MacLennan D. H. Amino acid sequence of rabbit fast-twitch skeletal muscle calsequestrin deduced from cDNA and peptide sequencing. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1167–1171. doi: 10.1073/pnas.84.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini J. L., Gildensoph L. H., Reynolds-Niesman I., Briskin D. P. Calcium Transport in Sealed Vesicles from Red Beet (Beta vulgaris L.) Storage Tissue : I. Characterization of a Ca-Pumping ATPase Associated with the Endoplasmic Reticulum. Plant Physiol. 1987 Dec;85(4):1129–1136. doi: 10.1104/pp.85.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Bruno B., Lew D. P., Pozzan T., Volpe P., Meldolesi J. Immunocytochemistry of calciosomes in liver and pancreas. J Cell Biol. 1988 Dec;107(6 Pt 2):2523–2531. doi: 10.1083/jcb.107.6.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Krause K. H., Chou M., Thomas M. A., Sjolund R. D., Campbell K. P. Plant cells contain calsequestrin. J Biol Chem. 1989 Mar 15;264(8):4269–4272. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine B. A., Dalgarno D. C. The dynamics and function of calcium-binding proteins. Biochim Biophys Acta. 1983 Sep 15;726(3):187–204. doi: 10.1016/0304-4173(83)90005-8. [DOI] [PubMed] [Google Scholar]
- Lew R. R., Briskin D. P., Wyse R. E. Ca uptake by endoplasmic reticulum from zucchini hypocotyls : the use of chlorotetracycline as a probe for ca uptake. Plant Physiol. 1986 Sep;82(1):47–53. doi: 10.1104/pp.82.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Mikawa T., Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984 Feb;95(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Oberdorf J. A., Lebeche D., Head J. F., Kaminer B. Identification of a calsequestrin-like protein from sea urchin eggs. J Biol Chem. 1988 May 15;263(14):6806–6809. [PubMed] [Google Scholar]
- Paliyath G., Thompson J. E. Senescence-Related Changes in ATP-Dependent Uptake of Calcium into Microsomal Vesicles from Carnation Petals. Plant Physiol. 1988 Oct;88(2):295–302. doi: 10.1104/pp.88.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. T., Simmerman H. K., Collins J. H., Nadal-Ginard B., Jones L. R. Complete amino acid sequence of canine cardiac calsequestrin deduced by cDNA cloning. J Biol Chem. 1988 Jun 25;263(18):8958–8964. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]