Abstract
Neoadjuvant chemotherapy (NAC) prior to radical cystectomy is associated with improved survival in patients with muscle-invasive conventional urothelial carcinoma (CUC). Sarcomatoid urothelial carcinoma (SUC) is a rare histologic variant associated with aggressive tumor behavior and poor 28 prognosis, however the role of NAC is unclear. In this study, we examined survival outcomes and response to NAC in patients with SUC treated with radical cystectomy.
Introduction:
To examine oncologic outcomes and response to neoadjuvant chemotherapy (NAC) in patients with sarcomatoid urothelial carcinoma (SUC) treated with radical cystectomy (RC).
Materials and Methods:
We retrospectively queried our institutional database (2003–18) and Surveillance, Epidemiology, and End Results (SEER)-Medicare (2004–2015) for patients with cT2–4, N0–2, M0 SUC and conventional UC (CUC) treated with RC. Clinicopathologic characteristics were described using descriptive statistics (t test, χ2-test and log-rank-test for group comparison). Overall (OS) and recurrence-free-survival (RFS) after RC were estimated with the Kaplan Meier method and associations with OS were evaluated with Cox proportional hazards models.
Results:
We identified 38 patients with SUC and 287 patients with CUC in our database, and 190 patients with SUC in SEER-Medicare. In the institutional cohort, patients with SUC versus CUC had higher rates of pT3/4 stage (66% vs. 35%, P < 0.001), lower rates of ypT0N0 (6% vs. 35%, P = .02), and worse median OS (17.5 vs. 120 months, P < .001). Further, patients with SUC in the institutional versus SEER-Medicare cohort had similar median OS (17.5 vs. 21 months). In both cohorts, OS was comparable between patients with SUC undergoing NAC + RC vs. RC alone (17.5 vs. 18.4 months, P = .98, institutional cohort; 24 vs. 20 months, P = .56, SEER cohor t). In Cox propor tional hazards models for the institutional RC cohort, SUC was independently associated with worse OS (HR 2.3, CI 1.4–3.8, P = .001).
Conclusion:
SUC demonstrates poor pathologic response to NAC and worse OS compared with CUC, with no OS benefit associated with NAC. A unique pattern of rapid abdominopelvic cystic recurrence was identified.
Keywords: Bladder cancer, Neoadjuvant therapy, Urinary bladder neoplasms, Sarcomatoid histology
Introduction
Bladder cancer (BC) is the 6th most common malignancy in the United States with an estimated 81,180 new cases and 17,100 deaths in 2022.1 Conventional urothelial carcinoma (CUC), which comprises approximately 95% of all cases, represents the most common histology, with the remaining subset including squamous cell carcinoma, adenocarcinoma, small cell carcinoma, and sarcomatoid urothelial carcinoma (SUC) among other rare histologic types.2 Although CUC has been well studied with established treatment guidelines for both localized and metastatic disease, management of variant histologic types remains a clinical challenge due to their rarity and paucity of clinical data.
SUC represents approximately 0.3% of all bladder malignancies and is associated with aggressive tumor behavior and poor prognosis.3 The histopathologic hallmark of SUC is the combination of epithelial (eg, urothelial, squamous) with mesenchymal features (eg, leiomyosarcoma, osteosarcoma).4 Our knowledge regarding this rare histology remains limited, mainly derived from case reports/series,5–7 retrospective cohorts,8–10 and database registries.11, 12 From those, a few common characteristics of SUC are emerging. In general, patients with SUC tend to present at an advanced stage and often carry a devastating prognosis with early recurrence and poor survival, even after definitive treatment modalities are utilized.5–9 Further, the role of neoadjuvant chemotherapy (NAC) remains unclear given conflicting data suggesting both limited and encouraging response rates and effect on survival outcomes.11–13
An improved understanding of oncologic outcomes in patients with SUC is needed to better guide patient selection for definitive therapies and optimize survival. In this study, we present data from our institutional database and the Surveillance, Epidemiology, and End Results (SEER)-Medicare program to delineate survival outcomes, response to NAC, and prognostic characteristics of muscle invasive SUC after radical cystectomy (RC). We hypothesized that SUC would be associated with an inferior response to NAC and poor outcomes after RC compared to CUC.
Patients and Methods
Patient Selection and Study Design
This is a retrospective cohort study examining our institutional database and SEER-Medicare data and was conducted in accordance with the STROBE checklist.14 After obtaining institutional review board approval from the University of Washington (IRB ID: STUDY00005690) and in concordance with the Declaration of Helsinki, our institutional database was retrospectively queried (2003–2018) to identify an institutional cohort of patients with SUC and CUC with the following inclusion criteria: i) adults (≥18 years old), ii) diagnosis of muscle invasive BC (cT2–4, N0–2, M0 stage), iii, a) histologic confirmation of sarcomatoid component (any %) in the transurethral resection of bladder tumor (TURBT) and/or RC specimens (SUC cohort), iii, b) or presence of CUC without variant histology in both the TURBT and RC specimen (CUC cohort). All pathology specimens, including those from referring centers, were analyzed and diagnosis confirmed by a genitourinary pathologist at our institution. We collected demographic and clinicopathologic data such as tumor staging, treatment information, response, and survival outcomes. Our database included data from patients with UC from 2003 to 2018 and was stored in the secure and standardized REDCap capture tool hosted at the Institute of Translational Health Sciences.15, 16 In addition, we included the same clinicopathologic and treatment data extracted from the SEER-Medicare database for a cohort of patients diagnosed with SUC (T2–4 stage) treated with RC between 2004 and 2015, using International Classification of Disease 9 and 10 topography codes for BC (188.0−188.9, C67.0−C67.9), as well as the morphologic code 8122 for SUC, defined by the 2016 World Health Organization classification.2 SEER uses a combination of clinical and pathologic information from imaging, biopsies, TURBT, RC, and autopsies to estimate TNM stage. For that reason, we used the general terms T, N or M for stage breakdown in the SEER cohort, without the defining clinical (c) or pathologic (p) terms (eg, cT, pT), as the origin of the stage-defining specimen (TURBT vs. RC) was not known.
Outcomes of Interest
Primary outcomes of interest were: i) differences in clinical stage of diagnosis, pathologic stage at RC, and recurrence rate (CUC vs. SUC), ii) response to NAC as indicated by ypT0N0 (CUC vs. SUC), and iii) overall survival (OS) from the time of BC diagnosis to death from any cause stratified by: a) histology (CUC vs. SUC), or b) NAC receipt (NAC + RC vs. RC alone) within each histologic subgroup. The secondary outcome of interest was recurrence-free survival (RFS) from the time of RC to initial radiographic evidence of local/distant recurrence or death (CUC vs. SUC). For survival analysis assessing response to NAC, we excluded patients who received carboplatin-based regimens due to concern for inferior outcomes. Further, this analysis was restricted to patients with sarcomatoid histology in the TURBT specimen as treatment decisions regarding NAC are made on this information.
Statistical Analysis
We first performed a descriptive analysis of all patients with SUC treated at our institution, regardless of stage or treatment modality. We then performed a comparative analysis for the prespecified outcomes of interest between patients with SUC and CUC treated with RC. We tested the following covariates of clinical interest: age at diagnosis, ECOG performance status, histologic type, NAC, adjuvant chemotherapy, pT and pN stage, positive surgical margins, and lymphovascular invasion (LVI) at RC. Statistical analysis was performed with IBM SPSS Statistics for Windows, Version 23.0. (Armonk, NY, IBM Corp Released 2015). Normally distributed continuous variables were compared using t tests, and categorical variables were compared using Chi-squared tests (Pearson chi square, Fischer’s exact test for cells with expected values less than 5). Kaplan Meier method was used for estimation of OS and RFS. The Mantel-Cox log-rank test was used for univariate comparison of OS and RFS between groups. Cox proportional hazards model was utilized for identification of prognostic factors related to OS in the entire cohort of patients undergoing RC. Variables significant in univariate Cox regression analysis were included in the final multivariable model. An alpha error of 5% (2-tailed) was set as the cutoff of statistical significance.
Results
Clinicopathologic Characteristics of Institutional SUC Cohort
We identified 45 patients who underwent treatment for bladder cancer with sarcomatoid histology. The primary nonmesenchymal component of these patients was urothelial in 43/45 (96%) cases, and neuroendocrine or squamous in 1/45 (2%) cases each. The latter 2 cases were excluded due to absence of UC component. Baseline patient characteristics for the remaining 43 patients are summarized in Supplemental Table 1. No patients had pure sarcomatoid histology. Sarcomatoid histology was present in 32/43 (74%) cases at the time of TURBT and the remaining were identified only in the RC specimen (n = 11). Squamous features at TURBT were concomitantly present in 11/43 (25%) cases. Histopathology samples of the patients are represented in Supplemental Figure 1A–D.
Treatment Summary of Institutional SUC Cohort
Of the total 43 patients with SUC, 5 patients did not undergo RC (12%). Of those, 3 presented with stage IV disease and were treated with upfront cisplatin-based chemotherapy. All 3 patients progressed within a median 5 months and proceeded to receive second line anti-PD-(L)1 therapy; however, no response was noted with median time to progression of 2.8 months. Of the remaining 2 patients not undergoing RC, these presented with stage I to III disease; 1 patient was treated with primary chemoradiotherapy and remains disease free at 21 months and the other patient progressed on NAC and transitioned to palliative care.
In total, 38 out of 43 patients with SUC underwent RC (88%), with 17/38 (45%) receiving NAC; 16/38 (42%) received gemcitabine-cisplatin (GC) or methotrexate/vinblastine/adriamycin/cyclophosphamide (MVAC), and 1/38 (3%) received gemcitabine-carboplatin. Final pathology at RC was pT3/4 in 25/38 (66%) patients and positive lymph nodes were found in 10/38 (26%). Of patients that received NAC, only 1/17 (6%) patient achieved pathologic complete response (ypCR, ypT0N0), while 2/17 (12%) had < ypT2N0. Clinical T and N staging distribution and NAC regimens for each patient can be found in Supplemental Table 2.
Post-operative recurrence occurred in 17/38 patients (45%). Of these patients, 10/17 (59%) recurred in the pelvis or pelvic lymph nodes while 7/17 (41%) recurred distantly. Median time to recurrence was 4.5 months. Four patients received systemic treatment with anti-PD-(L)1 agents (n = 2) or antibody-drug conjugate as part of a clinical trial (n = 2). No response was noted and the median time to progression was 5.6months. One patient received palliative radiation to bone lesions without systemic treatment.
Among patients with pelvic recurrence, 5/10 (50%) presented with a unique pattern of abdominopelvic malignant cystic collections, with a median time to recurrence of 4.8 months and median post-operative survival of 13.8 months. Chart review revealed that the diagnosis of recurrence in these patients was typically delayed due to the cystic/fluid collection appearance leading to interventions for other common causes of postoperative intrabdominal fluid accumulation (eg, lymphocele, infection, abscess, etc.). These patients were frequently hospitalized and underwent multiple procedures (drains, aspiration, biopsy, etc.) prior to diagnosis of recurrence (made on percutaneous biopsy or fluid cytology). Representative imaging was selected to characterize these recurrences is shown in Supplemental Figures 2A and 2B.
Comparative Analysis of Institutional RC Cohorts
Comparison of Clinicopathologic Data.
We compared the clinicopathologic characteristics (stage of diagnosis, surgical pathology, recurrence rate, NAC) and survival outcomes of 38 patients with cT2–4, N0–2, M0 SUC treated with RC with a cohort of 287 patients with cT2–4, N0–2, M0 pure CUC treated with RC (Table 1). No difference in clinical stage at diagnosis was observed (P > .05). In regard to final pathology at RC, patients with SUC had significantly higher rates of pT3/4 stage (25/38 [66%] vs. 99/287 [35%], P < .001), and lower rates of < pT2 stage (6/38 [16%] vs. 143/287 [50%], P < .001) and pT0N0 stage (3/38 [8%] vs. 71/287 [25%], P = .02) at RC. Additionally, ypCR rate after NAC was significantly lower in patients with SUC vs. CUC (1/17 [6%] vs. 56/162 [35%], P = .02).
Table 1.
Comparison of Clinicopathologic Characteristics (t Test, Pearson Chi-Square, Fisher’s Exact Test) Between Patients With Sarcomatoid and Conventional Urothelial Histology Treated With Radical Cystectomy.
| SUC | CUC | ||
|---|---|---|---|
| Variables | N = 38 | N = 287 | P Value (Pearson χ2, Fischer exact test, t test) |
| Gender | n, % | n, % | |
| Male | 28 (74) | 220 (77) | .686 |
| Female | 10 (26) | 67 (23) | |
| Median age (y, IQR) | 70 (60–76) | 67 (60–76) | .987 |
| Stage at diagnosis | |||
| cT2 | 20 (53) | 162 (56) | .656 |
| cT3/4 | 18 (47) | 125 (44) | |
| Nodal disease (imaging) | 7 (18) | 25 (9) | .078 |
| CIS+ (TURBT) | 11 (29) | 61 (21) | .289 |
| LVI+ (TURBT) | 4 (11) | 36 (13) | .802 |
| Surgical pathology | |||
| pT0N0 | 3 (8) | 71 (25) | .015 |
| pTa/T1/CIS | 3 (8) | 72 (25) | <.001 |
| pT2 | 7 (18) | 45 (16) | .665 |
| pT3/T4 | 25 (66) | 99 (35) | <.001 |
| pTanyN+ | 10 (26) | 58 (20) | .384 |
| Positive margins | 2 (5) | 17 (6) | 1.000 |
| CIS+ | 9 (24) | 61 (21) | .732 |
| LVI+ | 8 (21) | 55 (19) | .782 |
| Recurrence rate | 17 (45) | 81 (28) | .041 |
| Neoadjuvant CT | 17 (45) | 162 (56) | .173 |
| pCR (ypT0N0) | 1/17 (6) | 56/162 (35) | 0.016 |
Abbreviations: CUC = conventional urothelial carcinoma; cT = clinical tumor stage; CT = chemotherapy; CIS = carcinoma in situ; IQR = interquartile range; LVI = lymphovascular invasion; pT = pathologic tumor stage; SUC = sarcomatoid urothelial carcinoma.
Comparison of Survival Outcomes.
Recurrence rates were higher in patients with SUC versus CUC (45% vs. 28%, P = .041, Table 1). Median follow-up time for the entire RC cohort (n = 325) was 79.1 months (95% CI: 67.1–97.1). Median follow-up time for the SUC cohort was 62.8 months (95% CI: 38.4–87.1). Median OS for patients with SUC versus CUC was 17.5 months (95% CI: 8.1–27.0) and 120 months (95% CI: 87.6–152.3), respectively (P < .001, Figure 1) and median RFS for patients with SUC versus CUC was 9.4 months (95% CI: 4.6–14.1) and 109.8 months (95% CI: 81.9–137.6), respectively (P < .001). Receipt of NAC + RC was associated with longer OS in patients with CUC (120 vs. 93.4 months, P = .04) but not in patients with SUC (17.5 vs. 18.4months, P = .98, Figure 2A and 2B). On multivariable Cox regression for the entire RC cohort, presence of sarcomatoid histology was associated with worse OS (HR 2.36, 95% CI: 1.45–3.84, P < .001), adjusting for other potentially prognostic factors (age ≥65 years, ECOG performance status ≥2, pT3/4 stage, pN + disease, LVI, surgical margins, Table 2).
Figure 1.
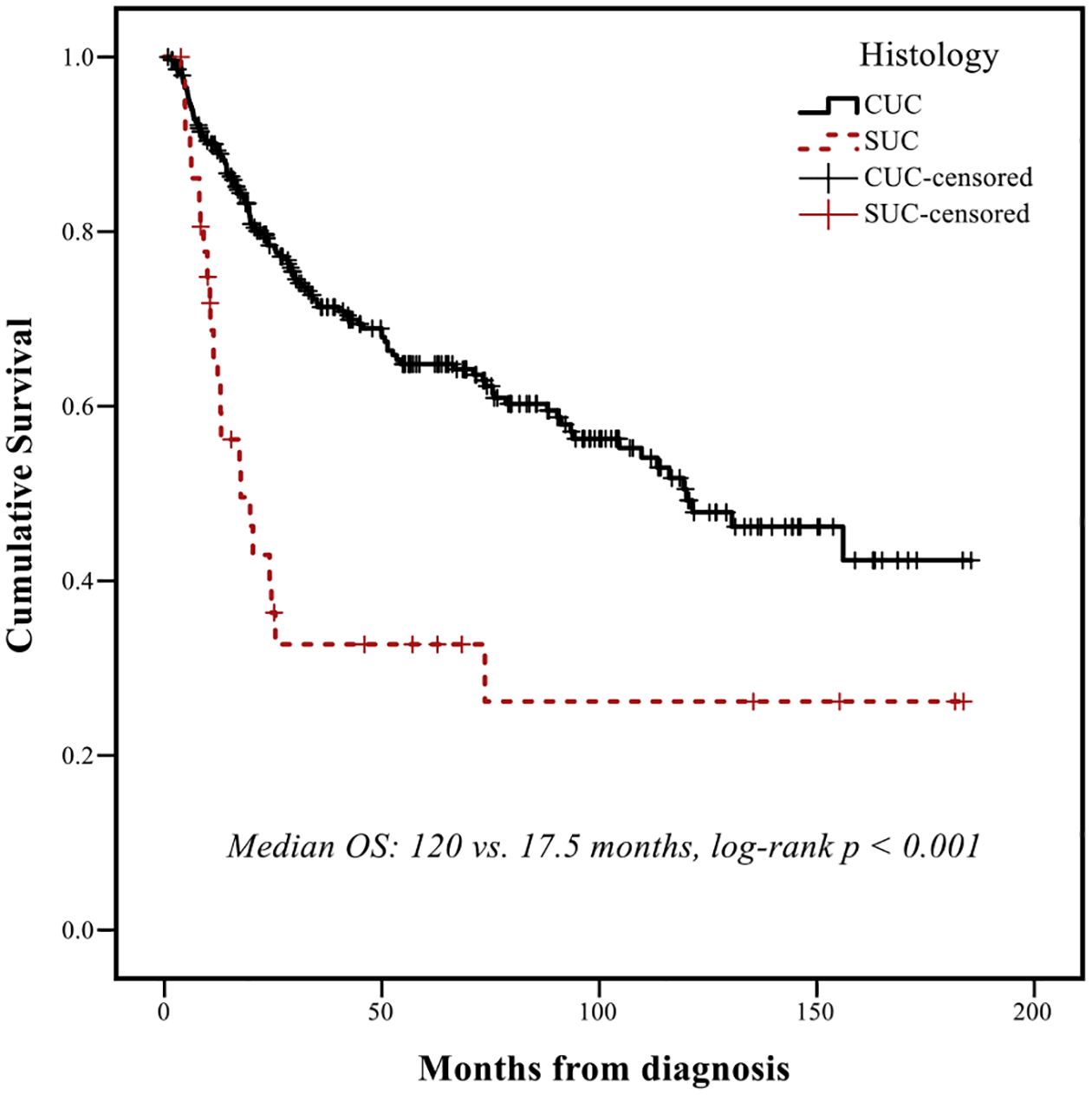
Overall survival (OS) in the institutional cohort of patients treated with radical cystectomy, according to histologic subtype. Abbreviations: CUC = conventional urothelial carcinoma, OS = overall survival, SUC = sarcomatoid urothelial carcinoma.
Figure 2A.
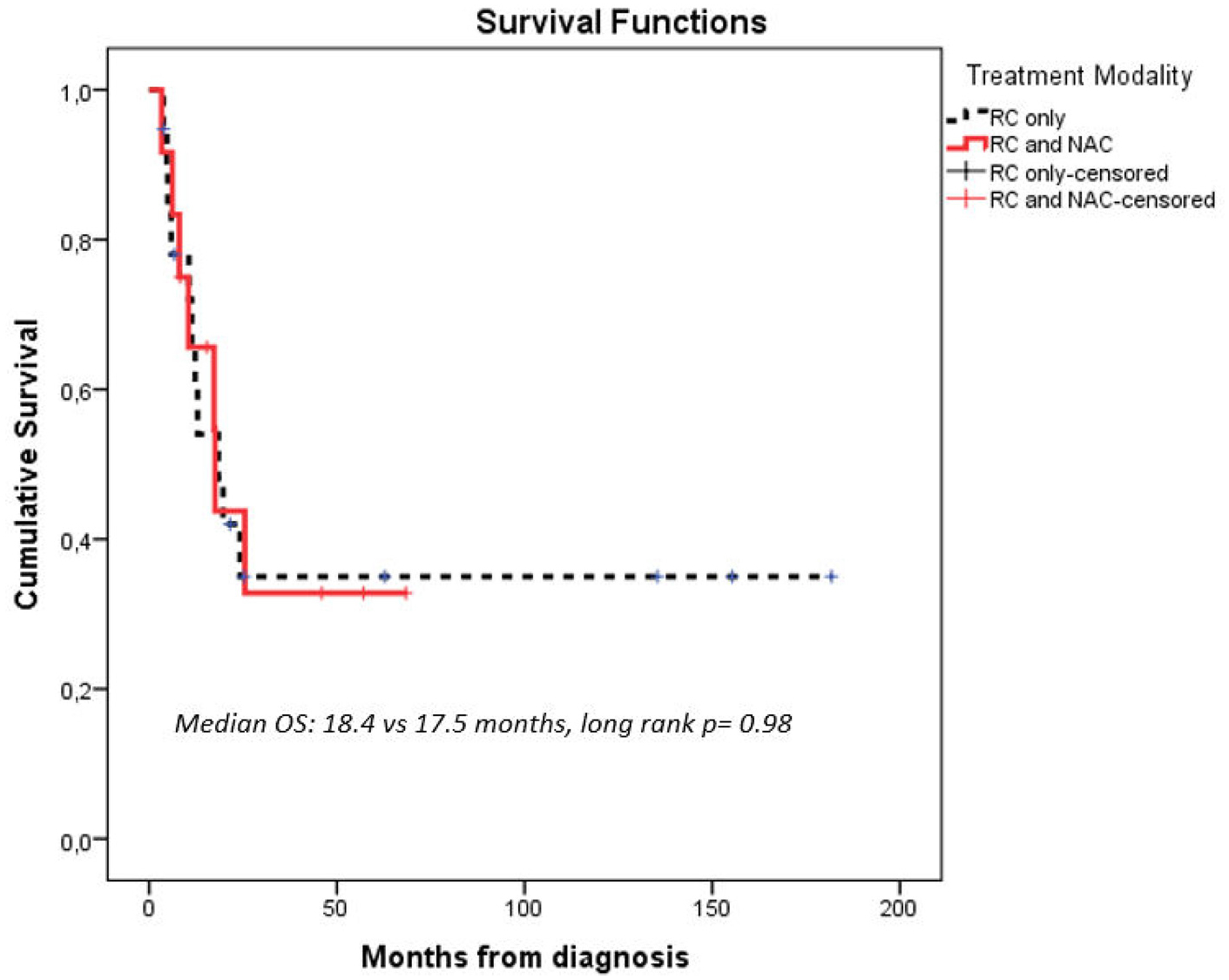
Overall Survival (OS) in institutional patients with sarcomatoid urothelial carcinoma (SUC) treated with radical cystectomy, according to receipt of neoadjuvant chemotherapy (NAC). Abbreviations: NAC = neoadjuvant chemotherapy, OS = overall survival, RC = radical cystectomy.
Figure 2B.
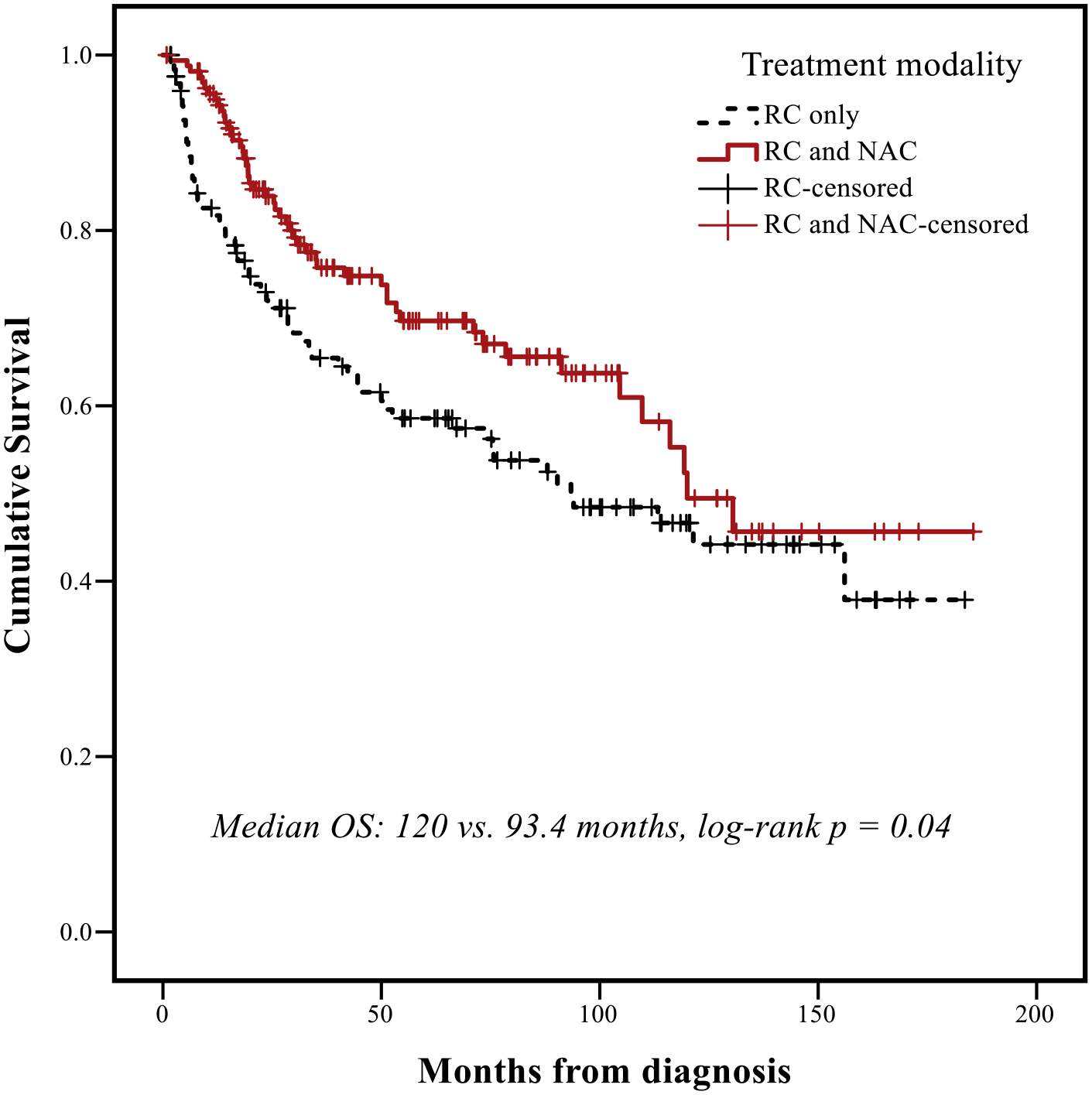
Overall survival (OS) in institutional patients with conventional urothelial histology treated with radical cystectomy, according to receipt of neoadjuvant chemotherapy. Abbreviations: NAC = neoadjuvant chemotherapy, OS = overall survival, RC = radical cystectomy.
Table 2.
Univariate and MULTIVARIATE COX REGREssion for OS in the Institutional Cohort of Patients Treated With Radical Cystectomy (CUC and SUC Combined).
| Overall Survival (From BC Diagnosis) | ||||
|---|---|---|---|---|
| UnivaUnivariate | MultivariaMultivariaMultivariate | |||
| Variables | HR (95% CI) | P-Value | HR (95% CI) | P-Value |
| Age ≥65yo | 1.57 (1.08–2.27) | .018 | 1.68 (1.15–2.44) | .007 |
| Sarcomatoid histology | 2.60 (1.65–4.09) | <.001 | 2.36 (1.45–3.84) | .001 |
| ECOG PS≥2 | 1.93 (1.06–3.50) | .031 | 2.40 (1.30–4.41) | .005 |
| NAC | 0.94 (0.60–1.44) | .76 | 0.96 (0.66–1.39) | .811 |
| pT≥3 | 3.04 (2.15–4.30) | <.001 | 2.23 (1.49–3.36) | <.001 |
| pN≥1 | 2.59 (1.79–3.75) | <.001 | 1.75 (1.14–2.70) | .011 |
| LVI at RC | 2.47 (1.68–3.62) | <.001 | 1.38 (0.87–2.19) | .171 |
| Positive margins | 1.93 (1.04–3.57) | .038 | 1.02 (0.51–2.03) | .958 |
Abbreviations: BC = bladder cancer; CI = confidence interval; CUC = conventional urothelial carcinoma, ECOG PS = eastern cooperative oncology group performance status; HR = hazard ratio; LVI = lymphovascular invasion; NAC = neoadjuvant chemotherapy; pN = pathologic nodal stage; pT = pathologic tumor stage; RC = radical cystectomy; SUC = sarcomatoid urothelial carcinoma.
SEER-Medicare Analysis
A total of 250 patients diagnosed with SUC were identified in the SEER-Medicare database between 2004 and 2015; 190 patients with T2–4 stage were included. Composite stage breakdown was: 108 (57%) T2, 51 (27%) T3, and 31 (16%) T4. Nodal involvement was identified in 29/190 (15%) patients, while 28/190 (15%) patients had metastatic disease. RC was performed in 83/190 (44%) patients and NAC was administered to 26/83 (31%) with 14/26 (54%) receiving a cisplatin-based regimen.
Median follow-up for the SEER SUC cohort (n = 190) was 66 months (95% CI: 59.8–72.1) and median OS was 9 months (95% CI: 6.6–11.4). Patients with SUC treated with RC (± NAC) had median OS of 21 months (95% CI: 8.5–33.5), while the non-RC cohort had median OS of 5 months (95% CI: 3.6–6.4, P < .001). No significant difference in OS was noted between RC + NAC versus RC alone (24 vs. 20 months, P = .56, Figure 2C). In multivariable Cox regression for OS in the SEER-SUC cohort, T3/4 stage and age > 80 years were independently associated with shorter OS (Supplemental Table 3).
Figure 2C.
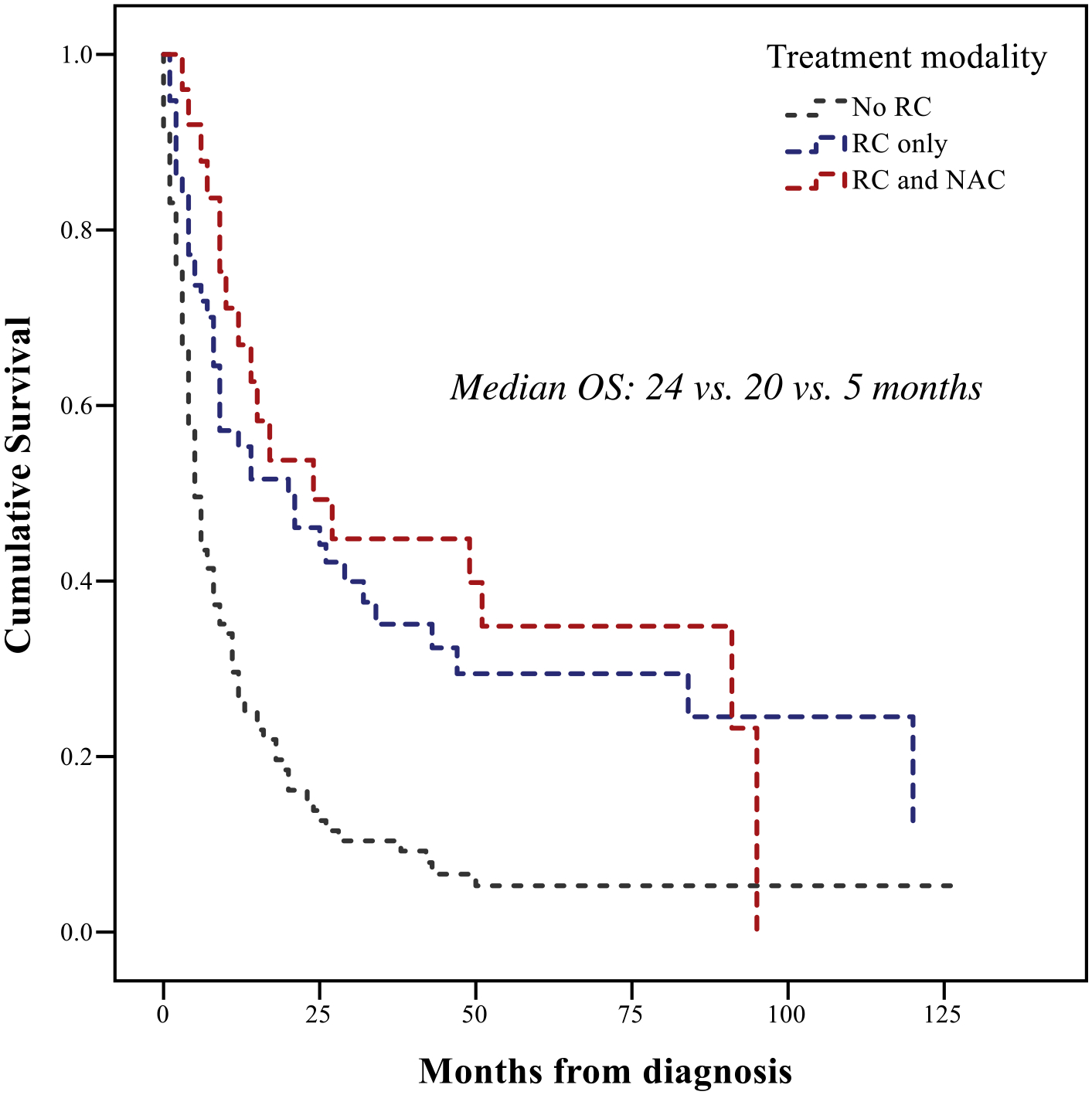
Overall Survival (OS) in SEER-Medicare patients with sarcomatoid urothelial carcinoma (SUC), according to treatment modality. Abbreviations: NAC = neoadjuvant chemotherapy, OS = overall survival, RC = radical cystectomy.
Discussion
This study represents one of the largest institutional series of patients with SUC and demonstrates the increased propensity towards advanced stage presentation, high recurrence rates after RC, and poor survival compared with patients with pure CUC. Additionally, we found poor response to NAC with no associated improvement in OS and only 1 out of 17 patients (6%) achieving a complete pathologic response. These findings were further corroborated by SEER-Medicare data. We have also described a unique pattern of rapid abdominopelvic cystic recurrence associated with delayed diagnosis, which was likely related to the atypical clinical presentation, often mimicking benign postoperative intrabdominal fluid collections. Overall, these findings reflect the need for earlier diagnosis, optimal staging, development of biomarkers, novel treatments for SUC, and vigilant surveillance after RC. Importantly, the benefit of NAC in patients with SUC remains unclear, and consideration of upfront RC in select patients may be a reasonable approach, however further investigation is needed.
Similar clinicopathologic characteristics and survival outcomes have been previously reported (Supplemental Table 4), though these data are limited by small sample sizes, absence of stratification based on treatment modality, and limited number of patients treated with NAC.3, 8, 9, 13, 17–19 Larger cancer database studies have provided additional insight. A SEER-database analysis (2004–2015) compared CUC with SUC outcomes in terms of disease presentation and disease-specific survival, concluding that patients with SUC who underwent RC had superior outcomes indicating that definitive surgical resection remains the cornerstone of management.20 In a National Cancer Database (NCDB) analysis (2004–2014) of 489 patients with SUC, 56% presented at ≥cT2 stage, and the median OS for the entire group was 18.4 months. Survival outcomes were superior in the subgroup that underwent RC (± NAC) and pCR after NAC was 13.6%. Notably, NAC + RC was not associated with survival benefit compared with RC alone.11 Similarly, another NCDB analysis (2003–2012) of patients with rare BC variants demonstrated that NAC was associated with improved OS only in patients with neuroendocrine tumors; the remaining histologic types (including SUC) demonstrated similar OS regardless of NAC receipt, with pCR rate only 8.5% among those who received NAC.12 Our findings derived from our large institutional database and population-based SEER-Medicare data are in concordance with these findings. A recent institutional series led by Almassi et al.13 reported a slightly higher response rate to NAC, with 5 out of 25 patients with SUC who received NAC achieving ypCR (20%).
The histopathologic hallmark of SUC is the combination of malignant epithelial (eg, urothelial, squamous, small cell) with mesenchymal components (eg, leiomyosarcoma, osteosarcoma). For example, Urrea et al.10 reported a series of 28 patients with small cell tumors admixed with sarcomatoid component and describe 3 distinct groups based on the pattern of transition from small cell to sarcomatoid histology: gradual, abrupt, and separate. The “dualistic” nature of this variant has led to the development of 2 pivotal, though contradicting, hypotheses regarding pathogenesis, suggesting that SUC is either composed of 2 distinct malignant clones that occur concomitantly, or the product of deviating differentiation pathways in a single clonal line.4, 21 It has also been shown that SUC carries a basal genomic signature with expression of KRT5, KRT6, KRT14 and CDH3, with downregulation of luminal subtype biomarkers (GATA3, uroplakins, ERBB2, ERBB3, PPAR-γ) and dysregulation of the epithelial-to-mesenchymal transition pathway with increased expression of vimentin, FoxC2, SNAIL, and ZEB1.5, 8, 22 However, ARID1A expression seems to be lower in SUC compared to CUC, but this result was not significantly correlated with survival.22, 23 No studies have assessed a correlation between amount/proportion of sarcomatoid features and clinical outcomes, though presence of even minority component of sarcomatoid features appears to confer aggressive tumor behavior and poor prognosis.
Due to its retrospective nature, our study has several limitations, including small sample size, presence of potential selection and confounding biases, differing or unknown amount/proportions of sarcomatoid component in each case, variability in treatment modalities and monitoring/surveillance schedules, lack of biomarker evaluation, no specification on agents and cycles of NAC and missing data in a number of patients. Further, our SEER-Medicare analysis is subject to missing data, lack of granular clinical and treatment data, and inability to definitively determine pCR due to coding limitations and therefore pathologic outcomes cannot be provided. Moreover, the role of novel agents, for example, antibody drug conjugates, immune checkpoint and FGFR inhibitors need to be further defined in this variant histology.24
Conclusion
We present one of the largest institutional experiences with SUC accompanied by data from SEER-Medicare. This unique and rare BC variant was associated with significantly shorter OS and RFS, and low pathologic response rate to NAC with no associated OS advantage compared to RC alone. Additionally, we characterize a unique pattern of rapid abdominopelvic cystic recurrence. Management of SUC remains a significant challenge, and further investigation of novel therapies and potential predictive biomarkers to improve patient selection is urgently needed.
Supplementary Material
Clinical Practice Points.
NAC prior to radical cystectomy is associated with improved survival in patients with muscle-invasive CUC, however the role of NAC in patients with rare histologic variants such as SUC remains unclear.
In this study, we compared survival outcomes and response to NAC in patients with SUC and CUC treated with radical cystectomy.
We found that SUC is associated with poor response to NAC and worse OS compared to CUC, with no OS benefit associated with NAC.
Management of SUC remains a challenge and further research regarding beneficial interventions and therapies for this rare and aggressive histologic variant of bladder cancer is crucial.
Acknowledgments
Dimitra Rafailia Bakaloudi acknowledges the support of the Kurelt Cancer Research Award. Ali R. Khaki is supported by the National Cancer Institute under training grant (T32CA009515). We extend our gratitude to Seattle Translational Tumor Research program for making this work possible.
Disclosure
Rishi R. Sekar: no conflicts to disclose.
Leonidas N. Diamantopoulos: no conflicts to disclose.
Dimitra Rafailia Bakaloudi: no conflicts to disclose.
Ali R. Khaki: no conflicts to disclose.
Petros Grivas: Consulting: 4D Pharma, Aadi Bioscience, Astellas Pharma, Asieris Pharmaceuticals, AstraZeneca, BostonGene, Bristol Myers Squibb, CG Oncology, Dyania Health, Exelixis, Fresenius Kabi, G1 Therapeutics, Genentech, Gilead Sciences, Guardant Health, ImmunityBio, Infinity Pharmaceuticals, Janssen, Lucence, Mirati Therapeutics, MSD, Pfizer, PureTech, Regeneron, Roche, Seattle Genetics, Silverback Therapeutics, Strata Oncology, QED Therapeutics, Merck KGaA, UroGen Pharma; institutional research funding: Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm Group, G1 Therapeutics, Gilead Sciences, GlaxoSmithKline, Mirati Therapeutics, MSD, Pfizer, QED Therapeutics, Merck KGaA
Brian R. Winters: no conflicts to disclose.
Funda Vakar-Lopez: no conflicts to disclose.
Maria S. Tretiakova: no conflicts to disclose.
Sarah P. Psutka: Honoraria - Prime Education; Travel, Accommodations, Expenses - Prime Education.
Sarah Holt: no conflicts to disclose.
John L. Gore: Research Grant funding from Ferring Pharmaceuticals.
Daniel W. Lin: DSMB for the POTOMAC study with AstraZeneca; Consulting or Advisory Role—Astellas Pharma, Clovis Oncology, Dendreon; Research Funding—GenomeDx; Genomic Health; MDxHealth.
George R. Schade: Patents, Royalties, Other Intellectual Property - Global Cancer Technology.
Andrew C. Hsieh: Honoraria - Hotspot Therapeutics; Research Funding - eFFECTOR Inc; Patents, Royalties, Other Intellectual Property - MTOR modulators and uses thereof Patent number: 9629843; Use of Translational Profiling To Identify Target Molecules For Therapeutic Treatment, Publication number: 20140288097.
John K. Lee: no conflicts to disclose.
Todd Yezefski: no conflicts to disclose.
Michael T. Schweizer: Paid consultant/advisor to Janssen; Research Grant funding to University of Washington from Janssen, AstraZeneca, Zenith, Pfizer and Hoffmann-La Roche.
Heather H. Cheng: Research Funding - Astellas Medivation, Clovis Oncology, Color Foundation, Inovio Pharmaceuticals, Janssen, Sano.
Evan Y. Yu: consulting for Amgen, AstraZeneca, Bayer, Churchill, Dendreon, EMD Serono, Incyte, Janssen, Merck, Pharmacyclics, QED, Seattle Genetics, and Tolmar; institutional research support from Bayer, Daiichi-Sankyo, Dendreon, Merck, Taiho, and Seattle Genetics; and personal fees from Clovis (last 3 years).
Lawrence D. True: Stock and Other Ownership Interests—Lightspeed Micro Research Funding, Ventana Medical Systems; Patents, Royalties, Other Intellectual Property—Lens on an open-top lightsheet microscope.
Robert B. Montgomery: Research Funding—AstraZeneca, ESSA, Ferring, Janssen Oncology.
Jonathan L. Wright : Royalties—UpToDate; Clinical Trials - Merck, Nucleix, Altor Biosciences; BMS, Janssen, Veracyte;Consulting—ImmunityBio.
Abbreviations:
- BC
Bladder cancer
- CUC
Conventional urothelial carcinoma
- ECOG
Eastern Cooperative Oncology Group
- KM
Kaplan-Meier
- LVI
Lymphovascular invasion
- NAC
Neoadjuvant chemotherapy
- OS
Overall survival
- pCR
Pathologic complete response
- RC
Radical cystectomy
- RFS
Recurrence free survival
- SEER
Surveillance, epidemiology, and end results
- SUC
Sarcomatoid urothelial carcinoma
- TURBT
Transurethral resection of bladder tumor.
References
- 1.American Cancer Society. Cancer Facts and Figures 2022. Atlanta, Ga: American Cancer Society; 2022. [Google Scholar]
- 2.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. European urology. 2016;70(1):106–119. [DOI] [PubMed] [Google Scholar]
- 3.Baseskioglu B, Duman BB, Kara IO, Can C, Yildirim M, Acikalin M. Early detection and gemcitabine/cisplatin combination positively effect survival in sarcomatoid carcinoma of the urinary bladder. Asian Pacific journal of cancer prevention. APJCP. 2012;13(11):5729–5733. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong AB, Wang M, Eble JN, et al. TP53 mutational analysis supports monoclonal origin of biphasic sarcomatoid urothelial carcinoma (carcinosarcoma) of the urinary bladder. Mod Pathol. 2009;22(1):113–118. [DOI] [PubMed] [Google Scholar]
- 5.Narayan VM, Gupta S, Davicioni E, Murugan P, Gibb EA, Konety B. Genomic analysis and treatment response of a bladder urothelial carcinoma with sarcomatoid variant histology. Clin Genitourin Cancer. 2019;17:e888–e892. [DOI] [PubMed] [Google Scholar]
- 6.Stamatiou K, Galariotis N, Michailidis I, Petrakopoulou N, Moustou H, Zizi-Sermpetzoglou A. Sarcomatoid carcinoma of the urinary bladder: a clinicopathological study of 4 cases and a review of the literature. Korean J Urol. 2010;51(10):724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertz A, Lerma L, Ahn J, et al. Two cases of Aggressive Sarcomatoid Urothelial Carcinoma reveal potential molecular targets. Eur J Med Case Rep. 2021;5(8):230–237. [Google Scholar]
- 8.Sanfrancesco J, McKenney JK, Leivo MZ, Gupta S, Elson P, Hansel DE. Sarcomatoid urothelial carcinoma of the bladder: analysis of 28 Cases with emphasis on clinicopathologic features and markers of epithelial-to-mesenchymal transition. Arch Pathol Lab Med. 2016;140(6):543–551. [DOI] [PubMed] [Google Scholar]
- 9.Fatima N, Canter DJ, Carthon BC, et al. Sarcomatoid urothelial carcinoma of the bladder: a contemporary clinicopathologic analysis of 37 cases. The Can J Urol. 2015;22(3):7783–7787. [PubMed] [Google Scholar]
- 10.Urrea YR, Epstein JI. Sarcomatoid carcinoma associated with small cell carcinoma of the urinary bladder: a series of 28 cases. Hum Pathol. 2017;67:169–175. [DOI] [PubMed] [Google Scholar]
- 11.Sui W, Matulay JT, Onyeji IC, et al. Contemporary treatment patterns and outcomes of sarcomatoid bladder cancer. World J Urol. 2017;35(7):1055–1061. [DOI] [PubMed] [Google Scholar]
- 12.Vetterlein MW, Wankowicz SAM, Seisen T, et al. Neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer with variant histology. Cancer. 2017;123(22):4346–4355. [DOI] [PubMed] [Google Scholar]
- 13.Almassi N, Vertosick EA, Sjoberg DD, et al. Pathological and oncological outcomes in patients with sarcomatoid differentiation undergoing cystectomy. BJU Int. 2022;129(4):463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson SP, Farooq A, Laniado M, Motiwala H. The demographic features, clinical outcomes, prognosis and treatment options for patients with sarcomatoid carcinoma of the urinary bladder: a single centre experience. Int Braz J Urol. 2018;44(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabate Arroyo XA, Rodrigo Lara H, Carrillo Garcia P, Brugarolas Rossello J, Piza Reus P. Sarcomatoid urothelial bladder carcinoma in adults: histology, symptomatology, treatments and survival. Actas Urol Esp. 2019;43(2):106–110. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Gillaspie C, Kunadharaju R, Talmon GA. Sarcomatoid urothelial carcinoma: a single cancer center experience. World J Oncol. 2011;2(4):175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamantopoulos LN, Korentzelos D, Alevizakos M, Wright JL, Grivas P, Appleman LJ. Sarcomatoid urothelial carcinoma: a population-based study of clinicopathologic characteristics and survival outcomes. Clin Genitourin Cancer. 2022;20(2):139–147. [DOI] [PubMed] [Google Scholar]
- 21.Cheng L, Zhang S, Alexander R, et al. Sarcomatoid carcinoma of the urinary bladder: the final common pathway of urothelial carcinoma dedifferentiation. Am J Surg Pathol. 2011;35(5):e34–e46. [DOI] [PubMed] [Google Scholar]
- 22.Guo CC, Majewski T, Zhang L, et al. Dysregulation of EMT drives the progression to clinically aggressive sarcomatoid bladder cancer. Cell Rep. 2019;27(6) 1781–93.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Lu S, Lombardo K, Monahan R, Amin A. ARID1A alteration in aggressive urothelial carcinoma and variants of urothelial carcinoma. Human Pathol. 2016;55:17–23. [DOI] [PubMed] [Google Scholar]
- 24.Miller NJ, Khaki AR, Diamantopoulos LN, et al. Histological subtypes and response to PD-1/PD-L1 blockade in advanced urothelial cancer: a retrospective study. J Urol. 2020;204(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


