Abstract
Aim:
To understand how attitudes toward pharmacogenomic (PGx) testing among healthcare providers varies by specialty.
Methods:
Providers reported comfort ordering PGx testing and its perceived utility on web-based surveys before and after genetics education. Primary quantitative analyses compared primary care providers (PCPs) to specialty providers at both timepoints.
Results:
PCPs were more likely than specialty care providers to rate PGx testing as useful at both timepoints. Education increased comfort ordering PGx tests, with larger improvements among PCPs than specialty providers. Over 90% of cardiology and internal medicine providers rated PGx testing as useful at pre- and post-education.
Conclusion:
PCPs overwhelmingly perceive PGx to be useful, and provider education is particularly effective for improving PCPs' confidence. Education for all specialties will be essential to ensure appropriate integration into routine practice.
Keywords: attitude, clinical, decision support systems, family practice, genetics, health education, health personnel, medicine, pharmacogenomic testing, physicians, testing
Allelic variation in genes associated with alterations in pharmacokinetics and pharmacodynamics can affect medication efficacy and risks of adverse drug responses [1]. Pharmacogenomic (PGx) testing detects these variations, and has the potential to revolutionize patient care by allowing healthcare providers to anticipate patient responses to a drug and guide medication choices and doses accordingly [2–6]. Evidence-based guidelines for over 100 drug–gene interactions currently exist [7], with advances gradually emerging for an expanding number of medication classes with relevance to an increasing number of specialties. PGx testing as a tool to improve patient care in all areas of medicine is limited when providers are lacking in both educational resources and enthusiasm toward its uses.
Two factors that influence the willingness of providers to use PGx testing include attitudes toward and confidence about using its results [8,9]. In particular, perceived usefulness and comfort with incorporating results into clinical practice have both strong theoretical support and empirical evidence as predictors of genetic test utilization [10–13]. A study of cancer specialists, for example, showed that the likelihood they would discuss somatic mutation testing findings depended on the assessed actionability of findings [14]. The same study also found that cancer specialists were far more likely to order testing if they felt confident interpreting results [14]. It is likely that perceived usefulness and comfort using genetics services will also influence whether healthcare providers adopt PGx testing in many specialty settings.
The adoption of PGx testing by primary care providers (PCPs) is of particular importance [15]. PCPs are well positioned to discuss, order and respond to PGx testing accordingly, as medication management falls within their scope of clinical practice. Evidence suggests PCPs understand the clinical benefits of PGx tests and see themselves as having primary responsibility for presenting them to patients and responding to findings [16]. However, they often desire additional support to confidently communicate, respond to and utilize these results [5,6,17]. Educational gaps remain a persistent barrier to PGx implementation, particularly among PCPs [9,18–21]. Medical schools are increasingly incorporating PGx into their curricula [22], and provider genomics educational efforts have been broadly successful at improving attitudes, confidence and knowledge [15,23,24]. A better understanding of the impact of provider education on particular specialties may help improve curriculum development even further [24].
To address this gap in the literature, we performed a secondary analysis of data collected on the impact of our system-wide genetics education program at Sanford Health [25]. In prior work, we showed that Sanford Health's 2-year genetic education initiative improved providers' perceived preparedness toward genomic testing and perceived utility of PGx testing [26]. Here, we extended that work to examine how attitudes toward PGx testing vary by specialty, as well as the impact of required education on those outcomes.
Materials & methods
Overview
Data were analyzed from evaluations of a 2-year genomics education program. Details about Sanford Health's PGx program and the genomics education program have been published previously [26–29]. Briefly, PGx testing was launched with robust clinical decision support (CDS) in 2014 as part of the Sanford Imagenetics (i.e., internal medicine and genetics) initiative to reinforce responsible implementation of genetic testing into all aspects of clinical care. Healthcare providers also had direct access to a team of PGx clinical pharmacists for consultation as desired. Clinical pharmacists completed a comprehensive review outlining any guideline-based recommendations within a note housed in the electronic medical record for any patient who completed PGx testing. To augment a vision of greater use of genetics in all aspects of medicine, all Sanford Health physicians and advanced practice providers (APPs) participated in a mandatory educational program from 2017 to 2019. The content of this program did not vary for providers in different specialties or with different roles. Providers completed eight web-based modules that included one titled, ‘The genetics of drug response’, and another titled, ‘Pharmacogenomics and patient care’. Annual raises for physicians and APPs were contingent upon completion of modules, and providers were given 3 months to complete each successive module [26]. Internal medicine providers also completed an additional 20 hour web-based education program titled, ‘Essentials of genomic medicine’ starting in 2014 [25]. Between 2102 and 2822 individuals completed each educational module [26].
Educational modules were developed to support the launch of the Sanford Chip program, an elective preemptive screen available to adult primary care patients where all patients received PGx panel testing for eight genes (Supplementary Table 1; later expanded [27]) and could opt into a medically actionable predisposition disease screening portion [25]. All PGx test results were compared against existing medication lists within a patient's medical record to identify drug–gene interactions which may have warranted medication changes. In addition, results were stored in patients' medical records and activated automated CDS to alert healthcare providers about potential drug–gene or drug–gene–disease interactions at the time of a medication order [27–29] .
Survey data about provider characteristics, attitudes & confidence
Prior to the start of education and again after the educational program had concluded, providers completed surveys. Survey completion was optional and not incentivized. Provider characteristics were self-reported and included age in 10-year increments, gender, clinical specialty, clinical role, years out of residency, medical school training (US based or not) and the setting of their residency (university based, hospital based, other).
Herein, we focus on provider comfort in PGx test ordering, assessed by asking providers to respond to the statement, “I feel comfortable ordering a pharmacogenetic test to predict risk of adverse events or likelihood of response (e.g., CYP2C9/VKORC1 and warfarin therapy)”. Response options to this item were ‘strongly disagree’, ‘disagree’, ‘agree’ and ‘strongly agree’. Perceived utility of PGx testing was also assessed by asking providers to respond to the statement, “and how useful do you think pharmacogenetic results would be for managing your patient's health?”. Response options included ‘not at all useful’, ‘not very useful’, ‘somewhat useful’ and ‘very useful’.
Categorization of provider groups
At pre-education, respondents could endorse multiple primary specialties (although no physician or APP did so) and write in others. At post-education, respondents could choose a single response option and write in others. The study team categorized providers into two groups according to whether they classified themselves as a PCP (internal medicine or family medicine) or a specialty care provider (any other specialty). Providers in multiple specialties per their structured and unstructured responses were categorized in all reported specialties.
Data analysis
Available case analyses on de-identified data were limited to physicians and APPs (physician's assistants and nurse practitioners), given that the education program was mandatory for these provider types. Chi-square and Wilcoxon rank sum tests were used to compare the characteristics of the respondents in each provider group separately for the pre- and post-education assessments. Analyses that examined how comfort and perceived utility differed between provider groups used logistic regression models rather than ordinal logistic regression to avoid assuming proportional odds where the impact of independent variables is consistent for each increase in level (e.g., assuming that the odds ratio [OR] that a PCP will respond ‘agree’ rather than ‘disagree’ compared with a non-PCP is the same as the OR that a PCP will respond ‘strongly agree’ rather than ‘agree’ compared with a non-PCP). To simplify these models, responses to the comfort ordering PGx test item were dichotomized into ‘agree’ or ‘disagree’, and responses for the perceived utility of PGx testing item were dichotomized to compare responses of ‘somewhat useful’ and ‘very useful’ against ‘not at all useful’ and ‘not very useful’. The statistical model to compare PCPs and specialty care providers included pre- and post-education data. Models were adjusted for provider characteristics to minimize risks for confounding, with covariates that were based on the prior published approach [26] and included age, gender, provider type (physician or APP), survey timepoint (pre- or post-education) and an interaction term for provider type and survey timepoint. Interaction terms were included based on prior analyses that showed statistical significance [26] and model comparisons that showed that they improved model precision in analyses of comfort ordering PGx tests (p < 0.001 per likelihood ratio tests). We included interaction terms in analyses of perceived usefulness to maintain consistency in our analytic approach, although their inclusion did not improve the precision of the model (p = 0.163). Analyses that examined whether the impact of education differed between provider care groups were conducted using logistic regression models that included an interaction term for provider groups and survey timepoints. Secondary analyses used the same approach to examine particular specialties with at least 25 survey respondents at pre- and post-education. These models also included terms age, gender, role, provider type (physician or APP), survey timepoint (pre- or post-education), an interaction term for provider type and survey timepoint, and interaction terms for specialties and survey timepoint. Given trends in primary analyses, we also conducted exploratory analyses where age was changed to a dichotomous variable to compare respondents over and under the age of 50 years.
Complete case analyses were conducted using R version 4.2.1 (R Foundation for Statistical Computing). Responses from 28 providers at pre-education and 182 providers at post-education were omitted because they did not report their specialty. Statistical significance was set at p = 0.05. This research project was deemed exempt from human subjects research by the Sanford Health Research Institutional Review Board.
Results
Data from 1002 physicians and 638 APPs who completed the pre-education assessment and 578 physicians and 395 APPs who completed the post-education assessment were analyzed. Characteristics of healthcare providers included in analyses are summarized in Table 1. Survey completion rates were 81.8% at pre-education and 50.9% at post-education, as noted previously [26]. 510 providers at pre-education and 344 providers at post-education were classified as practicing in primary care (31.1% and 35.4%, respectively) (Supplementary Table 2).
Table 1. . Characteristics of healthcare providers included in analyses†.
| Characteristics | Pre-education (n = 1640) | Post-education (n = 973) |
|---|---|---|
| Specialty | ||
| Primary care | 510 (31.1%) | 344 (35.4%) |
| Specialist | 1130 (68.9%) | 629 (64.6%) |
| Role | ||
| Physician | 1002 (61.1%) | 578 (59.4%) |
| Advanced practice provider | 638 (38.9%) | 395 (40.6%) |
| Age, years | ||
| <30 | 96 (5.9%) | 44 (4.5%) |
| 30–39 | 599 (36.5%) | 292 (30.0%) |
| 40–49 | 409 (24.9%) | 247 (25.4%) |
| 50–59 | 291 (17.7%) | 205 (21.1%) |
| 60–69 | 210 (12.8%) | 165 (17.0%) |
| 70+ | 35 (2.1%) | 20 (2.1%) |
| Gender | ||
| Female | 856 (52.2%) | 530 (54.5%) |
| Male | 784 (47.8%) | 443 (45.5%) |
| Years out of residency‡ | ||
| <5 years | 280 (27.9%) | 95 (16.4%) |
| 5–9 years | 201 (20.1%) | 107 (18.5%) |
| 10–14 years | 134 (13.4%) | 79 (13.7%) |
| 15–19 years | 127 (12.7%) | 68 (11.8%) |
| 20+ years | 260 (25.9%) | 229 (39.6%) |
| Specialty | ||
| Anesthesiology | 105 (6.4%) | 28 (2.9%) |
| Cardiology | 40 (2.4%) | 36 (3.7%) |
| Emergency medicine | 56 (3.4%) | 32 (3.3%) |
| Family medicine | 377 (23.0%) | 258 (26.5%) |
| Internal medicine | 133 (8.1%) | 86 (8.8%) |
| Obstetrics/gynecology | 75 (4.6%) | 41 (4.2%) |
| Oncology | 56 (3.4%) | 29 (3.0%) |
| Orthopedics | 85 (5.2%) | 45 (4.6%) |
| Pediatrics | 156 (9.5%) | 96 (9.9%) |
| Psychiatry/behavioral health | 36 (2.2%) | 28 (2.9%) |
| Radiology | 46 (2.8%) | 45 (4.6%) |
| Surgery | 113 (6.9%) | 69 (7.1%) |
Only specialties with more than 25 providers at both the pre- or post-education timepoints are summarized here. Specialties with less than 25 providers at the pre- or post-education timepoints are summarized in supplemental materials (Supplementary Table 2).
Specialties with less than 25 providers at the pre- or post-education timepoints are summarized in supplemental materials (Supplementary Table 2).
Restricted to providers who identified as physicians.
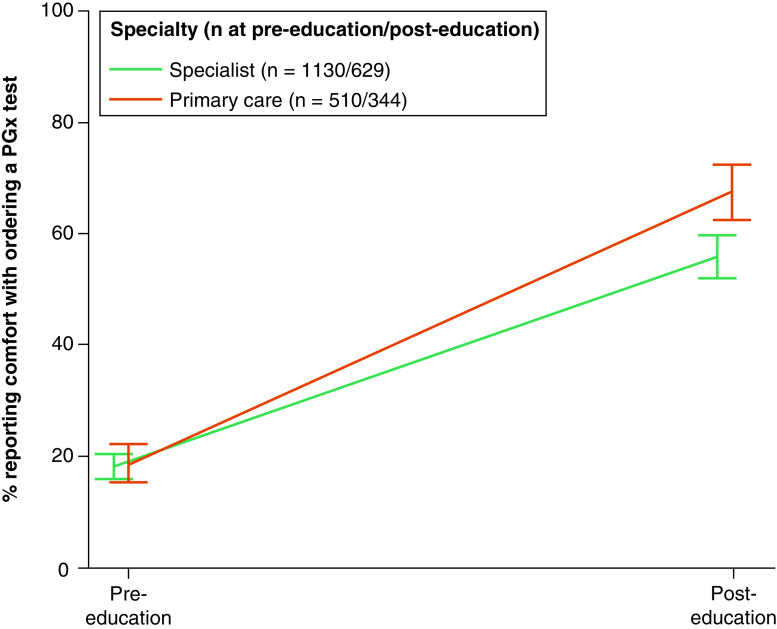
No differences were observed in the percentage of providers who reported feeling comfortable ordering PGx tests when comparing PCPs and specialists at pre-education (18.5 and 18.1%, respectively; p = 0.82). A greater percentage of both PCPs and specialists reported feeling comfortable ordering PGx tests at post-education (67.6 and 55.8%, respectively; p < 0.001), with the increase being larger for PCPs (interaction between specialty [PCP vs specialist] and timepoint; p = 0.017) (Figure 1 & Supplementary Table 3). Analyses also showed that male providers were more likely than female providers to report comfort with ordering PGx tests across timepoints (OR: 1.27; p = 0.029). We observed notable differences between specific specialties (Figure 2). At pre-education, oncology, cardiology and internal medicine providers were most likely to report comfort ordering PGx tests (39.7, 38.5 and 33.8%, respectively), while emergency medicine and anesthesiology providers were least likely to report comfort (7.3 and 8.1%, respectively). The largest increases in the likelihood of reporting comfort after education were observed among providers specializing in family medicine (from 12.3 to 67.4%; p < 0.001), anesthesiology (from 8.1 to 60.2%; p = 0.013) and emergency medicine (from 7.3 to 56.3%; p = 0.032). Exploratory analyses that compared providers over and under the age of 50 years showed no differences (p = 0.58) (Supplementary Figure 1).
Figure 1. . Comfort ordering a pharmacogenomic test, primary care and specialists.
Providers responded to the statement, “I feel comfortable ordering a pharmacogenetic test to predict risk of adverse events or likelihood of response (e.g., CYP2C9/VKORC1 and warfarin therapy)”. Percentages responding ‘agree’ or ‘strongly agree’ were adjusted for respondents' role, age and gender. Error bars represent 95% CIs.
CI: Confidence interval; PGx: Pharmacogenomic.
Figure 2. . Comfort ordering a pharmacogenomic test, by specialty and timepoint.
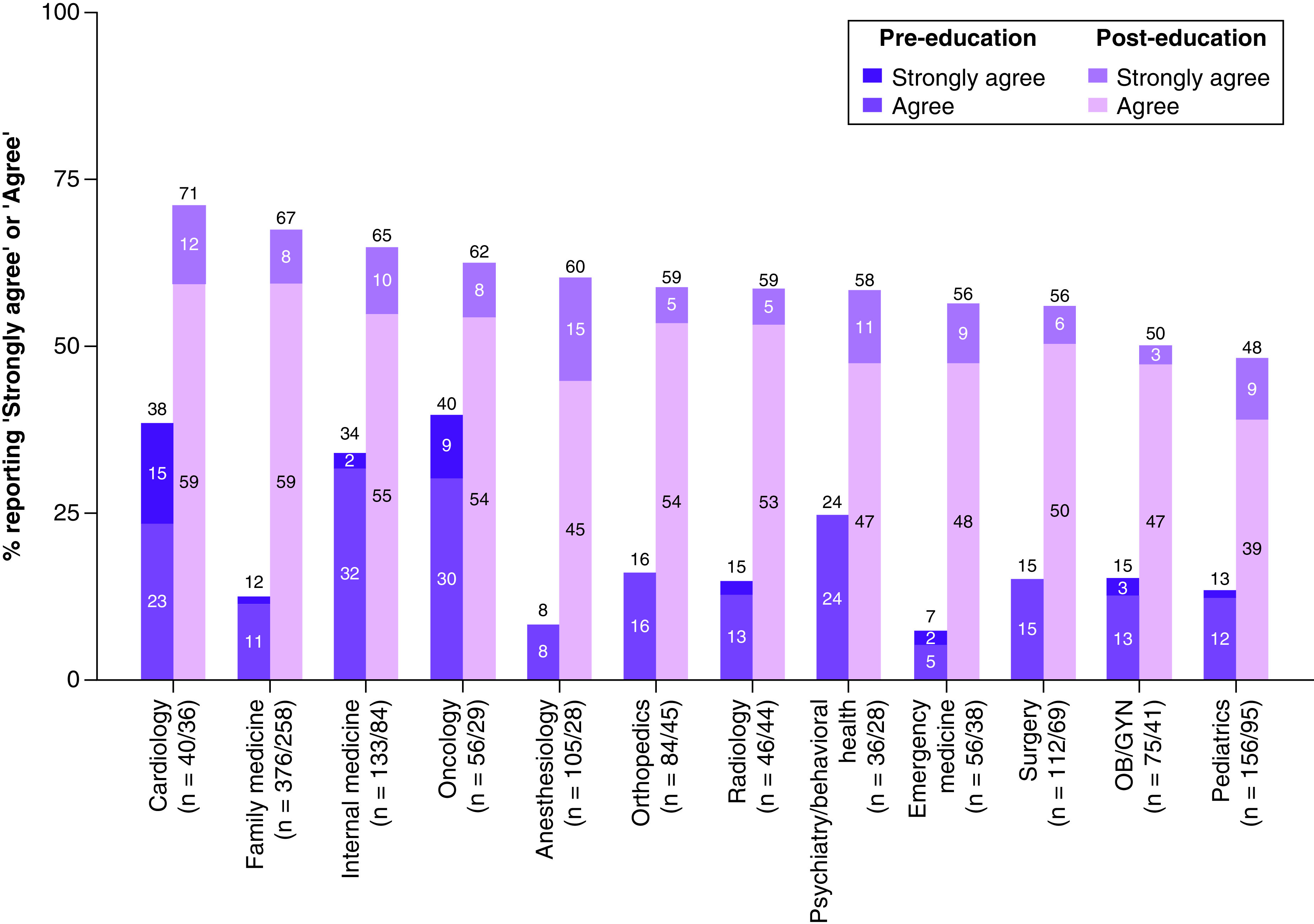
Providers responded to the statement, “I feel comfortable ordering a pharmacogenetic test to predict risk of adverse events or likelihood of response (e.g., CYP2C9/VKORC1 and warfarin therapy)”. Percentages responding ‘agree’ or ‘strongly agree’ were adjusted for respondents' role, age and gender.
OB/GYN: Obstetrics/gynecology; PGx: Pharmacogenomic.
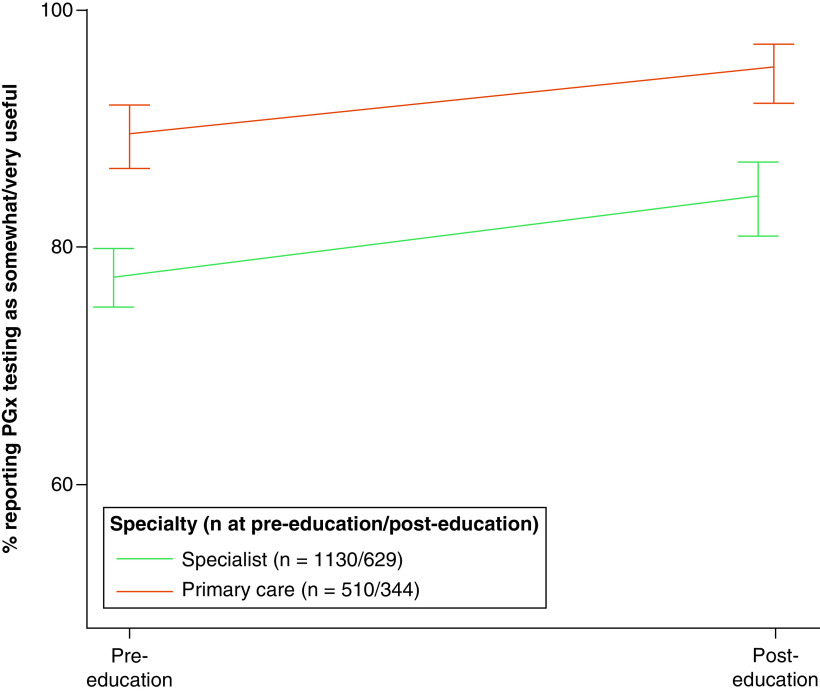
Analyses of the perceived utility of PGx tests showed that providers in primary care were more likely to rate PGx testing as somewhat or very useful more often than providers in other specialties (89.6 vs 77.5% at pre-education, respectively; 95.1 vs 84.3% at post-education, respectively; both p < 0.001) (Figure 3 & Supplementary Table 4). Changes from pre- to post-education did not vary between primary care and specialty providers (p for differences by timepoint by specialty = 0.25). Analyses also showed that male providers were less likely than female providers to rate PGx testing as somewhat or very useful across timepoints (OR: 0.69; p = 0.003). We observed notable differences between specific specialties (Figure 4). Providers in cardiology were most likely to rate PGx testing as somewhat or very useful at both the pre- and post-education timepoints. In fact, all cardiology providers at pre-education and all but one cardiology provider at post-education rated PGx testing as somewhat or very useful. Emergency medicine providers were least likely to rate PGx testing as somewhat or very useful at both timepoints. At post-education, over 70% of respondents from all specialties except emergency medicine rated PGx testing as somewhat or very useful, and over half of cardiology, internal medicine and family medicine providers rated PGx testing as very useful.
Figure 3. . Perceived usefulness of pharmacogenomic testing, primary care versus specialists.
Providers responded to the question, “How useful do you think pharmacogenetic results would be for managing your patient's health?”. Percentages responding ‘somewhat useful’ or ‘very useful’ were adjusted for respondents' role, age and gender. Error bars represent 95% CIs.
CI: Confidence interval; PGx: Pharmacogenomic.
Figure 4. . Perceived usefulness of pharmacogenomic testing, by specialty and timepoint.
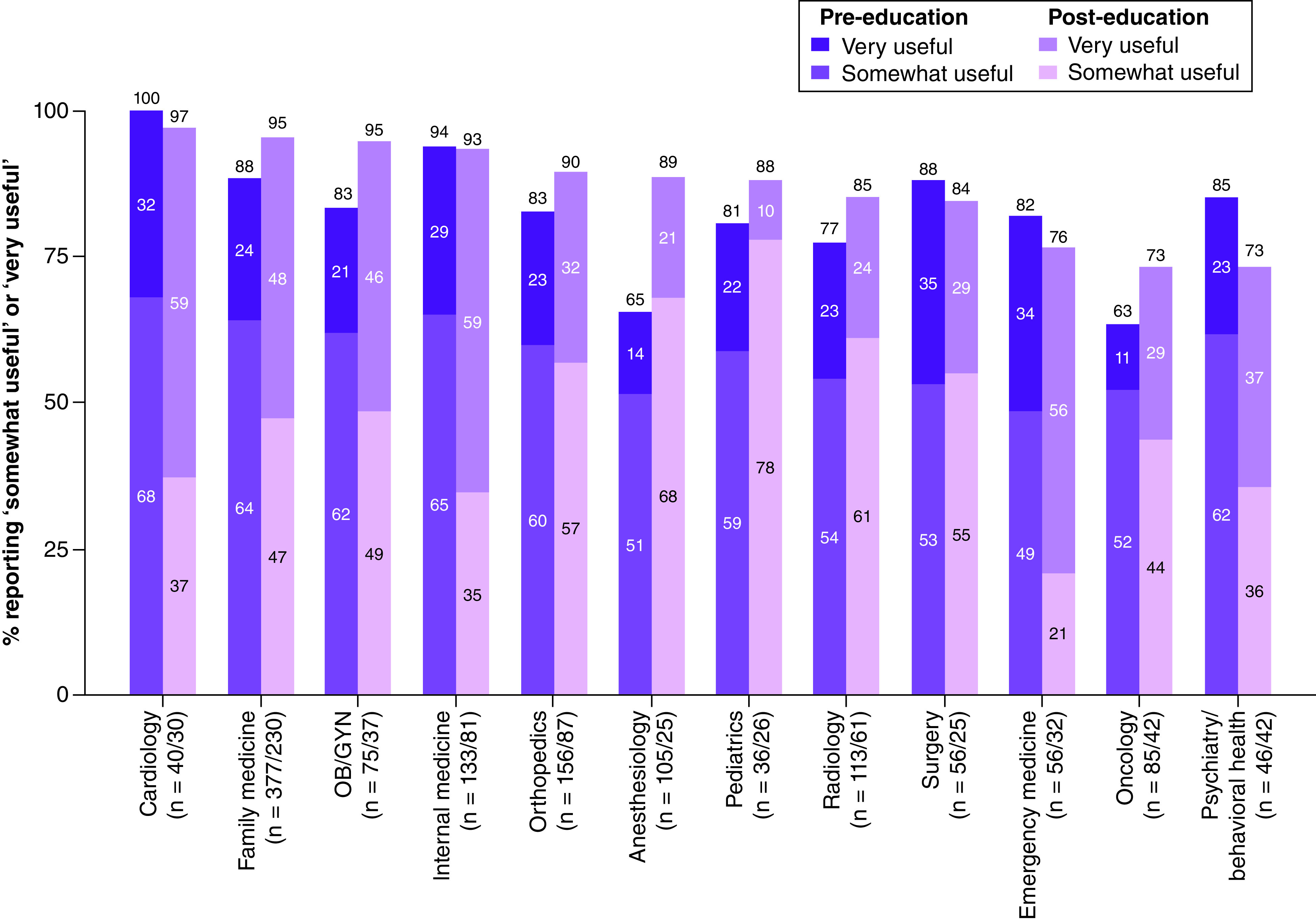
Providers responded to the question, “How useful do you think pharmacogenetic results would be for managing your patient's health?”. Percentages responding ‘very useful’ or ‘somewhat useful’ were adjusted for respondents’, role, age and gender.
OB/GYN: Obstetrics/gynecology; PGx: Pharmacogenomic.
Discussion
With the utility and scope of PGx testing rapidly increasing, it is critical for healthcare providers of all specialties to be receptive and prepared to order and respond to test results. In this report, we summarize how healthcare providers' comfort toward PGx testing and beliefs about its utility vary by specialty. Prior to education, perceptions about the utility of PGx testing were high across specialties, particularly in primary care, but comfort with ordering PGx testing was low across specialties. Education greatly improved comfort ordering PGx testing across specialties, with proportionally larger improvements among primary family medicine specialists. Findings complement our prior-reported results by highlighting how a provider education program developed with a goal of moving genetic medicine into primary care was particularly influential for PCPs.
As PGx guidelines continue to expand and improve, it is becoming increasingly clear that PGx testing will grow ever more pertinent to PCPs. A high percentage of medications impacted by Sanford Health's PGx program are prescribed by PCPs. It is possible that the incremental impact of education on PCPs' comfort after education reflects greater relevance of the curriculum to their practices. The Sanford PGx panel has expanded substantially since its introduction of a four gene panel in 2014, expanding its clinical applications across specialties. It is possible that when assessed by clinical subspecialties there would be an insignificant increase in comfort or usefulness because these providers were utilizing PGx testing in clinic well before the educational rollout. This relevance is only likely to grow, as Clinical Pharmacogenetics Implementation Consortium guidelines in development address drug–gene interactions for beta blockers and antipsychotic medications, both of which are likely to be managed by PCPs [30]. Additionally, providers in most specialties are likely to need to respond to PGx test results for medications prescribed by other providers [31,32]. Moreover, PCPs are likely to need to manage PGx results for testing that patients received from outside of clinical settings, including large research studies such as the All of Us Research Program [33]. Given how PGx applications in primary care will only continue to increase, educational efforts like ours at Sanford Health will be increasingly critical.
More granular analyses by specialty presented additional findings of note. Prior to education, cardiology providers expressed greater comfort with ordering PGx testing than other specialty groups. Findings likely reflect how initial PGx options at Sanford Health, including PGx panel tests, had strong early implications for cardiology care. PGx CDS to help guide antiplatelet therapy were implemented in some clinics as early as 2014 and expanded in 2015 to address drug–gene interactions associated with statin orders and anticoagulation management [27]. In addition, growing evidence from clinical trials, including work in which Sanford Health participated, demonstrated clinical benefits of PGx testing in patients with acute coronary syndromes [34–36]. For these reasons, it is likely that cardiology providers were early adopters of PGx testing at Sanford Health and often familiar with how to order and manage PGx testing prior to the mandatory provider education program.
Internal medicine providers also had stronger perceptions than specialty providers about the utility of PGx testing prior to the provider education program. Multiple internal medicine providers were engaged in the development of the Imagenetics initiative in 2014 and subsequent planning of the PGx program [25]. They also completed a lengthy web-based lecture series as early as 2014 [25], and internal medicine physicians served as physician champions for the Imagenetics initiative within their clinics prior to the required education rollout. It is likely that this engagement contributed to the positive perceptions about the utility of PGx testing and higher comfort ordering PGx testing at pre-education. This conjecture is supported by evidence from providers in anesthesiology, where the likelihood of reporting comfort with ordering PGx testing increased from 8.1% pre-education to 60.2% post-education. Specialists in anesthesiology collaborated with the PGx team for curation of CDS for drug–gene interactions associated with malignant hyperthermia in 2018 [29], after the pre-education assessment but prior to the post-education assessment. Our findings highlight possible benefits of engaging providers in the development of genetics education programs and ensuring educational content and CDS are tailored to the needs of specific specialties.
We also note that specialists in cardiology, psychiatry, surgery and emergency medicine were less likely to rate PGx testing as very useful at post-education than at pre-education. Although differences between timepoints for these two specialties were not statistically significant, it is notable that some of the most common PGx and high-profile applications exist in these clinics. It is possible that, with experience, providers found ordering PGx testing to be more challenging or that results had less of an impact on patient management than providers originally anticipated. It is also possible that the increase in PGx testing has increased the number of CDS alerts that providers encounter, leading to ‘alert fatigue’ [37–39]. With this last concern in mind, Sanford has begun to implement ‘non-interruptive alerts’ that warn ordering providers about drug–gene interactions but do not require providers to acknowledge them when consequences for patients are not severe [40].
Lastly, our analyses also showed interesting demographic differences. Male providers felt more comfortable ordering PGx tests, but were less likely to rate them as useful. These differences were not observed in prior analyses that examined comfort of perceived preparedness [26]. It is possible that these patterns reflect general patterns in healthcare and genetics, where male providers tend to be more assertive than women [41] while women tend to be more interested in genetic testing [42]. Observations about older providers being more likely to report utility to PGx testing could reflect observations of experienced providers who may have more examples about how genetic testing has changed the way they manage their patients.
Limitations
These secondary analyses utilized anonymous data and did not allow for change assessment from pre- to post-education for specific individuals. It is possible that providers with more favorable attitudes overall toward PGx testing were more likely to complete the post-education assessment. All APPs within the Sanford Health System were required to complete the 2-year genomics education course as part of continuing education, which may have had an influence on participants' responses to be more favorable or biased toward PGx testing. As such, a smaller percentage of providers completed the post-education survey than completed the pre-education survey, possibly because many thought the pre-education survey was mandatory and learned that the post-education survey was not. Survey items about comfort and perceived usefulness were not validated prior to use, and respondents may not have interpreted questions as intended. Changes from pre- to post-education in the format of the question about providers' primary specialty may have affected how providers responded. Furthermore, our approach to analyses omitted potential interaction effects between provider specialties and other demographic and practice characteristics. Additionally, these modules were administered in one healthcare system that provided CDS and clinical pharmacy consultations, and therefore may not be generalizable to other hospital systems, particularly those that are lacking the infrastructure to support providers as they work to implement genomics into clinical care. The CDS provided by Sanford Imagenetics was not considered during analysis. The use of logistic regression to analyze outcomes increased the possibility of false negative findings.
Conclusion
With the applications of PGx testing rapidly evolving, it is likely that PGx education will become increasingly relevant to providers of all clinical specialties. As testing panels expand and new drug–gene relationships emerge, the vast majority of providers are likely to prescribe medications with available PGx guidance. Whether providers take advantage of these opportunities is likely to depend on their comfort with ordering PGx tests and their perceptions of its utility for their patients. Overall, our findings demonstrate high perceptions of utility across specialty, and show how education can have a particularly significant impact on PCPs' comfort ordering PGx testing. These results are encouraging, and support the notion that with institutional support, providers of all clinical specialties can implement PGx testing into their clinical practice.
Summary points.
Regardless of specialty, provider confidence to order pharmacogenomic (PGx) testing prior to education was low, but increased after education, with larger improvements among primary care providers (from 18.5 to 67.6%) than specialty care providers (from 18.1 to 55.8%).
Primary care providers were more likely to rate PGx testing as useful compared with specialty providers (89.6 vs 77.5% at pre-education; 95.1 vs 84.3% at post-education; both p < 0.001).
Over 95% of cardiology and internal medicine providers rated PGx testing as useful at both timepoints, with an increase in reported comfort from 35% (pre-education) to over 60% (post-education).
For PGx testing to expand across specialties, provider education is essential to its appropriate integration into clinical care.
Supplementary Material
Acknowledgments
Work was completed on behalf of the Imagenetics Medical/Economic Impact and Behavioral Responses to Integrating the Sanford Chips (METRICS) team. Members of Imagenetics METRICS are included in the Supplementary Information.
The following people contributed to the development of the educational modules: A Aifaoui, J Baye, M Bell, L Davis-Keppen, K DeBerg, C Hajek, A Hutchinson, P Crotwell Leiferman, A Massmann, L Mullineaux, N Petry, D Platt, A Schultz and D Isum Ward.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/pgs-2023-0039
Author contributions
Conceptualization: KD Christensen, A Massmann, CL Preys, J Van Heukelom, CL Blout Zawatsky. Data curation: KD Christensen, C Hajek, MR Hickingbotham, A Massmann, CL Preys, J Van Heukelom, ES Zoltick. Formal analysis: KD Christensen, CL Preys. Funding acquisition: KD Christensen, RC Green, C Hajek. Methodology: KD Christensen, MR Hickingbotham, A Massmann, CL Preys, J Van Heukelom, ES Zoltick, A Schultz. Project administration: C Hajek, A Schultz. Supervision: KD Christensen, A Schultz. Validation: A Massmann, J Van Heukelom. Writing (original draft): KD Christensen, CL Preys, CL Blout Zawatsky. Writing (review and editing): KD Christensen, RC Green, C Hajek, A Massmann, CL Preys, J Van Heukelom, CL Blout Zawatsky, A Schultz, ES Zoltick.
Financial & competing interests disclosure
The Imagenetics Initiative and Sanford Chip Program and this work were supported by Sanford Health. CL Blout Zawatsky, RC Green, MR Hickingbotham, ES Zoltick and KD Christensen were supported by a research grant from Sanford Health for the submitted work. KD Christensen was supported by grant no. K01-HG009173 from the NIH. CL Blout Zawatsky, RC Green, MR Hickingbotham, ES Zoltick and KD Christensen were also supported by a research grant from Sanford Health for the submitted work. Employees of the funding organization (A Massmann, J Van Heukelom, C Hajek, A Schultz) contributed to the management, analysis and interpretation of the data, preparation, review or approval of the manuscript, and decision to submit the manuscript for publication. RC Green has received compensation for advising AIA, Embryome, Genome Web, Genomic Life, Grail, Humanity, OptumLabs, Plumcare and Verily, and is co-founder of Genome Medical, Inc. C Hajek is an employee of Helix OpCo, LLC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The study was deemed exempt from human subjects research by the Sanford Health Research Institutional Review Board.
Data sharing statement
Analytic code will be made available at request. Inquiries can be directed to the corresponding author.
References
Papers of special note have been highlighted as: • of interest
- 1.Roden DM, McLeod HL, Relling MV et al. Pharmacogenomics. Lancet 394(10197), 521–532 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature 526(7573), 343–350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinshillboum R, Wang L. Pharmacogenomics: precision medicine and drug response. Mayo Clin. Proc. 92(11), 1711–1722 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moyer AM, Caraballo PJ. The challenges of implementing pharmacogenomic testing in the clinic. Expert Rev. Pharmacoecon. Outcomes Res. 17(6), 567–577 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Albassam A, Alshammari S, Ouda G, Koshy S, Awad A. Knowledge, perceptions and confidence of physicians and pharmacists towards pharmacogenetics practice in Kuwait. PLOS ONE 13(9), e0203033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemke AA, Selkirk CGH, Glaser NS et al. Primary care physician experiences with integrated pharmacogenomic testing in a community health system. Per. Med. 14(5), 389–400 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Clinical Guideline Annotations. PharmGKB, CA, USA. www.pharmgkb.org/guidelineAnnotations (Accessed 5 February 2023). [Google Scholar]
- 8.Horowitz CR, Orlando LA, Slavotinek AM et al. The Genomic Medicine Integrative Research Framework: a conceptual framework for conducting genomic medicine research. Am. J. Hum. Genet. 104(6), 1088–1096 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crellin E, McClaren B, Nisselle A, Best S, Gaff C, Metcalfe S. Preparing medical specialists to practice genomic medicine: education an essential part of a broader strategy. Front. Genet. 10(789), eCollection, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Physicians request additional educational supports that more broadly address genetics. Exploration into speciality specific educational needs is required to best support physicians.
- 10.Champion VL, Skinner CS. The health belief model. In: Health Behavior and Health Education Theory, Research and Practice (4th Edition). Glanz K, Rimer BK, Viswanath K (Eds). Jossey-Bass, CA, USA, 45–65 (2008). [Google Scholar]
- 11.Fishbein M. Developing effective behavior change interventions: some lessons learned from behavioral research. In: NIH Pub No. 95-4035 Reviewing the Behavioral Science Knowledge Base on Technology Transfer. Backer TE, David SL, Soucy GP (Eds). (NIDA Research Monograph).National Institute on Drug Abuse. MD, USA, 246–261 (1995). [PubMed] [Google Scholar]
- 12.Sweeny K, Ghane A, Legg AM, Huynh HP, Andrews SE. Predictors of genetic testing decisions: a systematic review and critique of the literature. J. Genet. Couns. 23(3), 263–288 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Carroll JC, Wilson BJ, Allanson J et al. GenetiKit: a randomized controlled trial to enhance delivery of genetics services by family physicians. Fam. Pract. 28(6), 615–623 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Demeshko A, Pennisi DJ, Narayan S, Gray SW, Brown MA, McInerney-Leo AM. Factors influencing cancer genetic somatic mutation test ordering by cancer physician. J. Transl. Med. 18(1), 431 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campion M, Goldgar C, Hopkin RJ, Prows CA, Dasgupta S. Genomic education for the next generation of health-care providers. Genet. Med. 21(11), 2422–2430 (2019). [DOI] [PubMed] [Google Scholar]; • The current genomics workforce is not adequate to address the growing need for genomics specialists. To promote the expansion of genomics in medicine, meaningful genomics education is required in all medical training programs.
- 16.Haga SB, Burke W, Ginsburg GS, Mills R, Agans R. Primary care physicians' knowledge of and experience with pharmacogenetic testing. Clin. Genet. 82(4), 388–394 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Primary care physicians report that pharmacogenomic (PGx) testing would be useful in their clinics, but are lacking the knowledge and skillset to implement this.
- 17.Haga SB, Tindall G, O'Daniel JM. Professional perspectives about pharmacogenetic testing and managing ancillary findings. Genet. Test. Mol. Biomarkers 16(1), 21–24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Healthcare providers across disciplines are interested in utilizing PGx testing in their clinics, but not confident in their ability to interpret and implement such testing. Providers report that access to resources and educational materials would improve confidence.
- 18.Li J, Xu T, Yashar BM. Genetics educational needs in China: physicians' experience and knowledge of genetic testing. Genet. Med. 17(9), 757–760 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Salm M, Abbate K, Appelbaum P et al. Use of genetic tests among neurologists and psychiatrists: knowledge, attitudes, behaviors, and needs for training. J. Gene. Couns. 23(2), 156–163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klitzman R, Chung W, Marder K et al. Attitudes and practices among internists concerning genetic testing. J. Genet. Couns. 22(1), 90–100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein ME, Parvez MM, Shin JG. Clinical implementation of pharmacogenomics for personalized precision medicine: barriers and solutions. J. Pharm. Sci. 106(9), 2368–2379 (2017). [DOI] [PubMed] [Google Scholar]; • There are similar barriers and limitations across the globe to implementing PGx testing into clinical care. A key solution would be increased education, and institutional supports for clinical application.
- 22.Karas Kuželički N, Prodan Žitnik I, Gurwitz D et al. Pharmacogenomics education in medical and pharmacy schools: conclusions of a global survey. Pharmacogenomics 20(9), 643–657 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Luzum JA, Luzum MJ. Physicians' attitudes toward pharmacogenetic testing before and after pharmacogenetic education. Pers. Med. 13(2), 119–127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talwar D, Tseng TS, Foster M, Xu L, Chen LS. Genetics/genomics education for nongenetic health professionals: a systematic literature review. Genet. Med. 19(7), 725–732 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Christensen KD, Bell M, Zawatsky CLB et al. Precision population medicine in primary care: the Sanford Chip experience. Front. Genet. 12(274), eCollection, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajek C, Hutchinson AM, Galbraith LN et al. Improved provider preparedness through an 8-part genetics and genomic education program. Genet. Med. 24(1), 214–224 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petry N, Baye J, Aifaoui A et al. Implementation of wide-scale pharmacogenetic testing in primary care. Pharmacogenomics 20(12), 903–913 (2019). [DOI] [PubMed] [Google Scholar]; • Comprehensive overview of the work we did within the Sanford PGx program: successful implementation of PGx testing in primary care requires careful consideration, implementation of clinical tools, access to genetics professionals and a robust clinical education program.
- 28.Baye J, Massmann A, Petry N et al. Development and early evaluation of clinical decision support for long QT syndrome population screening. J. Transl. Genet. Genom. 6(3), 375–387 (2022). [Google Scholar]
- 29.Baye JF, Petry NJ, Jacobson SL et al. Malignant hyperthermia susceptibility: utilization of genetic results in an electronic medical record to increase safety. Pharmacogenomics 21(17), 1207–1215 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Clinical Pharmacogenetics Implementation Consortium. Prioritization of CPIC Guidelines (2022) https://cpicpgx.org/prioritization-of-cpic-guidelines/ (Accessed 5 February 2023).
- 31.Pet DB, Holm IA, Williams JL et al. Physicians' perspectives on receiving unsolicited genomic results. Genet. Med. 21(2), 311–318 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vears DF, Senecal K, Borry P. Reporting practices for unsolicited and secondary findings from next generation sequencing technologies: perspectives of laboratory personnel. Hum. Mutat. 38(8), 905–911 (2017). [DOI] [PubMed] [Google Scholar]
- 33.All of Us Research Program Investigators. The ‘All of Us’ Research Program. N. Engl. J. Med. 381(7), 668–676 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galli M, Benenati S, Franchi F et al. Comparative effects of guided vs potent P2Y12 inhibitor therapy in acute coronary syndrome: a network meta-analysis of 61 898 patients from 15 randomized trials. Eur. Heart J. 43(10), 959–967 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik AH, Gupta R, Chakraborty S et al. Effect of genotype-guided oral P2Y12 inhibitor selection after percutaneous coronary intervention: a systematic review and meta-analysis of randomized clinical trials. Cardiovasc. Revasc. Med. 41, 115–121 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Beitelshees AL, Thomas CD, Empey PE et al. CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention in diverse clinical settings. J. Am. Heart Assoc. 11(4), e024159 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolley CL, Slight SP, Husband AK, Watson N, Bates DW. Improving medication-related clinical decision support. Am. J. Health Syst. Pharm. 75(4), 239–246 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Hinderer M, Boeker M, Wagner SA et al. Integrating clinical decision support systems for pharmacogenomic testing into clinical routine – a scoping review of designs of user–system interactions in recent system development. BMC Med. Inform. Decis. Mak. 17(1), 81–81 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman JM, Flynn AJ, Juskewitch JE, Freimuth RR. Biomedical data science and informatics challenges to implementing pharmacogenomics with electronic health records. Annu. Rev. Biomed. Data Sci. 3, 289–314 (2020). [Google Scholar]
- 40.Massmann A, Petry NJ. Impact of transitioning to an active, noninterruptive CYP2C19/proton pump inhibitor alert on prescribing patterns. Am. J. Health Syst. Pharm. (2023) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 41.Bertakis KD. The influence of gender on the doctor–patient interaction. Patient Educ. Couns. 76(3), 356–360 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Peterson EB, Chou WS, Gaysynsky A et al. Communication of cancer-related genetic and genomic information: a landscape analysis of reviews. Transl. Behav. Med. 8(1), 59–70 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.