Abstract
A greatly improved most-probable-number (MPN) method for selective enumeration of sulfate-reducing bacteria (SRB) is described. The method is based on the use of natural media and radiolabeled sulfate (35SO42−). The natural media used consisted of anaerobically prepared sterilized sludge or sediment slurries obtained from sampling sites. The densities of SRB in sediment samples from Kysing Fjord (Denmark) and activated sludge were determined by using a normal MPN (N-MPN) method with synthetic cultivation media and a tracer MPN (T-MPN) method with natural media. The T-MPN method with natural media always yielded significantly higher (100- to 1,000-fold-higher) MPN values than the N-MPN method with synthetic media. The recovery of SRB from environmental samples was investigated by simultaneously measuring sulfate reduction rates (by a 35S-radiotracer method) and bacterial counts by using the T-MPN and N-MPN methods, respectively. When bacterial numbers estimated by the T-MPN method with natural media were used, specific sulfate reduction rates (qSO42−) of 10−14 to 10−13 mol of SO42− cell−1 day−1 were calculated, which is within the range of qSO42− values previously reported for pure cultures of SRB (10−15 to 10−14 mol of SO42− cell−1 day−1). qSO42− values calculated from N-MPN values obtained with synthetic media were several orders of magnitude higher (2 × 10−10 to 7 × 10−10 mol of SO42− cell−1 day−1), showing that viable counts of SRB were seriously underestimated when standard enumeration media were used. Our results demonstrate that the use of natural media results in significant improvements in estimates of the true numbers of SRB in environmental samples.
Sulfate-reducing bacteria (SRB) are of great ecological importance in the mineralization of organic matter in anaerobic environments. For example, in marine sediments up to 50% of the organic matter may be oxidized by sulfate reduction (25). In some low-sulfate environments, such as freshwater lakes, bacterial sulfate reduction may still be important in the mineralization process (5, 14, 16, 20). SRB also have great economic importance in the oil industry, where they cause severe problems, including souring of oil and gas deposits and corrosion of production facilities (13, 21, 33).
Considerable efforts have been directed toward the development of rapid and dependable methods for detection and enumeration of SRB in natural and industrial environments. In general, the methods used to enumerate SRB can be divided into the following two categories: (i) direct detection methods and (ii) culture methods. The direct detection methods developed recently include the use of antibodies raised against SRB (7, 28), an immunoassay for the enzyme adenosine-5′-phosphosulfate (APS) reductase (34), and the use of 16S rRNA probes (2, 37). Although promising, these techniques are still in the developmental phase, and several problems are encountered when they are used in situ (1, 41). For example, rRNA fluorescent probes are difficult to use in sediments due to the high background autofluorescence of inorganic particles (32). Furthermore, not all known types of dissimilatory sulfate reducers in environmental samples can be unequivocally identified with RNA probes described previously (37, 42).
Culture methods for enumeration of SRB based on the most-probable-number (MPN) technique (3) have been used extensively for several decades. A variety of MPN media have been developed for specific environments, including activated sludge, marine sediments, and samples from the oil drilling industry (10, 22, 35, 36, 40). Most of these enumeration media contain lactate as the main carbon and energy source. In all cases, the presence of SRB in MPN tubes is evaluated by the formation of a black precipitate of ferrous sulfide (FeS).
Several studies have demonstrated that the numbers of viable SRB in marine sediments are underestimated by a factor of at least 1,000 when standard MPN techniques are used with synthetic growth media (12, 24, 37). In this paper, we describe the development and evaluation of a greatly improved MPN technique for enumeration of SRB in environmental samples.
MATERIALS AND METHODS
Sampling procedures.
Sediment samples were collected by obtaining cores (23) from shallow permanently water-covered sediments in Kysing Fjord on the east coast of Jutland, Denmark. Material used to prepare sediment medium was collected from the top sediment layer (upper 2 cm), which had an organic content of 2 to 10% (dry weight) (18, 43). Activated sludge samples were obtained from an aeration basin at a municipal wastewater treatment plant (Marselisborg, Aarhus, Denmark). Sludge and sediment samples were stored in 100-ml serum flasks that were filled to capacity and sealed with gas-tight rubber stoppers while they were transported to the laboratory.
Strain.
Desulfobulbus propionicus DSM 2032 was obtained from the Deutsche Sammlung von Microorganismen und Zellkulturen, Braunschweig, Germany.
Preparation of natural MPN media.
Sediment medium for tracer MPN (T-MPN) enumerations performed with Kysing Fjord sediment was prepared as follows. Sediment was diluted 1:1 (vol/vol) with water from the sampling site and homogenized in a blender. The sediment suspension was successively passed through 1-, 0.5-, and 0.25-mm-mesh sieves (Endecotts Ltd., London, United Kingdom) and finally autoclaved for 20 min at 121°C. After autoclaving, the sediment suspension was cooled during vigorous magnetic stirring while it was purged with oxygen-free N2. Aliquots (8.9 ml) of the sediment suspension were anaerobically dispensed into culture tubes (type 2047; Bellco), and the tubes were sealed under an N2 atmosphere with butyl rubber stoppers. The tubes were incubated for 24 h at room temperature, autoclaved a second time, and stored at 5°C. The sediment medium was reduced immediately before inoculation by aseptically adding 0.1 ml of a freshly prepared sodium dithionite (Na2S2O4) solution to a final concentration of 200 μM.
Sludge medium for T-MPN enumerations was prepared from undiluted activated sludge as described above for the sediment medium except that the concentration of sulfate was adjusted to 6 to 7 mM with a concentrated solution of Na2SO4. This was done to ensure that the levels of sulfate during incubation were high enough (mean in situ concentration, 1.5 mM) (see below). The sludge medium was reduced as described above for sediment medium. Below, both the sediment medium and the sludge medium are referred to as natural media. The final pH values of the natural media were 7.0 ± 0.2, which were similar to the in situ pH values.
Preparation of synthetic MPN media.
API-RST medium, an improved version of the API RP-38 medium used for enumeration of SRB, was prepared as described by Tanner (40), except that sodium dithionite (final concentration, 200 μM) was substituted for ascorbic acid and cysteine-HCl as the reducing agent. Baar’s medium (culture medium 1249) was prepared as described previously (4), with the following modifications: the NaCl concentration was adjusted to 1% (wt/vol) (as in API-RST medium), and sodium dithionite was used as the reducing agent at a final concentration of 200 μM. Postgate’s B medium was prepared as described by Postgate (36) by using autoclaved seawater from the sampling site. DSM medium 194, a bicarbonate-buffered, sulfide-reduced, defined medium having a low iron content (9), was used for pure-culture experiments performed with Desulfobulbus propionicus. All MPN media (except DSM medium 194) contained yeast extract and lactate as the main electron donor. Below, these media are referred to as synthetic media.
General anaerobic techniques.
Strictly anaerobic and aseptic conditions were maintained throughout the experiments. The anaerobic techniques used were essentially the syringe methods of Macy et al. (30) performed with anoxic gases, N2, or N2-CO2 (90:10).
Measurement of sulfate reduction rates in sediment slurries, activated-sludge enrichment cultures, and pure cultures. (i) Sediment slurries.
Sulfate reduction rates and MPN values were determined as follows. Sediment slurries were prepared under constant N2 gassing by diluting anaerobic surface sediment 1:1 with anoxic water from the sampling site in serum flasks containing glass beads. Each slurry was mixed vigorously by shaking and preincubated under an N2 atmosphere for 2 h at 22°C. After preincubation, 20 ml of the sediment slurry was removed with a syringe, and 10-ml portions were placed into two sterile Bellco culture tubes containing an N2-CO2 (90:10) gas phase. One tube was immediately used to inoculate MPN tubes containing natural and synthetic media (see below). The other tube was used to measure the sulfate reduction rate with radiolabeled sulfate as follows. A 1-ml sample was removed for sulfate analysis before 0.1 ml of a sterile isotope solution (200 kBq of carrier-free 35SO42−; Isotope Laboratory, Risø, Denmark) was injected. The tube was incubated in the dark at 22°C, and 0.5-ml samples were removed at appropriate times and injected into 2 ml of a 20% (wt/vol) zinc acetate solution in order to stop the biological activity and preserve the 35S-sulfides produced.
(ii) Activated-sludge enrichment cultures.
Samples (100 ml) of activated sludge were amended with sulfate (1 ml of a 0.3 M Na2SO4 solution) and preincubated under an N2 atmosphere for 6 days at 22°C to increase the indigenous population of SRB. Enrichment was used only during evaluation of the method in order to facilitate the statistical analysis because low numbers of SRB were sometimes observed in sludge samples. After preincubation, 10.0-ml aliquots of the enriched sludge were transferred with a 50-ml sterile syringe and an 18-gauge needle into three sterile Bellco culture tubes containing an N2-CO2 (90:10) gas phase. The same syringe was filled only once in order to reduce experimental error. One tube (tube 1) was immediately used to inoculate MPN tubes containing natural and synthetic media (see below). Tubes 2 and 3 were used for radiotracer measurements of the sulfate reduction rate as described above for sediment slurries. To one of these tubes (tube 2) chloramphenicol and streptomycin were added at final concentrations of 20 and 100 mg/liter, respectively.
(iii) Pure cultures.
Sulfate reduction rates in pure cultures of Desulfobulbus propionicus were determined in the presence and absence of antibiotics. The experiments were carried out in culture tubes (type 2047; Bellco) containing 9 ml of defined growth medium (DSM medium 194). Three tubes were each inoculated with 1 ml of an exponential-phase culture, and the experiment was conducted as described above for the sludge sample experiment. The initial cell density in tube 1, which was used for T-MPN determination with DSM medium 194, was determined immediately after inoculation with a Bürker-Türk counting chamber. The tubes were incubated at 30°C for 25 h, and samples were removed at intervals as described above.
Enumeration of SRB by the T-MPN and N-MPN methods.
T-MPN enumeration was performed as follows. Each 10-ml sample examined was transferred into a sterile Bellco culture tube containing glass beads and mixed vigorously by vortexing. The suspended sample was immediately used to prepare 10-fold MPN dilutions (in triplicate) in synthetic and natural media; each tube contained 200 kBq of 35SO42−. In each T-MPN experiment, three uninoculated tubes were included as controls for medium sterility and isotope carryover during the distillation procedure. The tubes used for the MPN analysis and to determine sulfate reduction rates were incubated at the same temperature (22 or 30°C). During incubation, 1-ml subsamples were removed with a syringe from the T-MPN tubes and immediately injected into test tubes containing 2 ml of 20% (wt/vol) zinc acetate. Unless stated otherwise, the presence of SRB in MPN dilution tubes containing synthetic media was evaluated by using the normal MPN (N-MPN) method (formation of black FeS precipitate). In MPN dilution tubes containing natural media, the numbers of SRB were estimated only by the T-MPN method.
Recovery of reduced 35S by distillation.
The amounts of reduced 35S-sulfur in subsamples from T-MPN tubes were determined by using the single-step chromium reduction method described by Fossing and Jørgensen (11), which allows simultaneous measurement of acid-volatile sulfur and chromium-reducible sulfur, yielding total reduced inorganic sulfur (TRIS). Sulfate reduction rates (SRR) were calculated with the following equation: SRR = [a × 1.06 × (SO42−)]/[(a + A) × T], where (SO42−) is the sulfate concentration, a is the total radioactivity of ZnS, A is the total radioactivity of sulfate after incubation, T is the incubation time, and 1.06 is the 32S/35S correction factor for the expected isotope fractionation (23). Sulfate reduction rates were expressed as number of moles per liter per day.
The amount of reduced 35S-sulfur in T-MPN tubes, expressed as the percentage of TRIS (TRIS%), was calculated with the following equation: TRIS% = [a/(a + A)] × 100. The detection limit for sulfate reduction (presence of SRB in T-MPN tubes) was 0.1% TRIS; samples with values above this limit were considered positive for SRB.
Sulfate analysis.
The sulfate concentrations in activated sludge, sediment slurries, and DSM medium 194 were determined by suppressed ion chromatography as previously described (6).
MPN calculations and statistical analysis.
A computer program (15) was used to calculate MPN values and the standard errors of the MPN estimates. The statistical method of Cochran (8), a Student t test, was used to determine whether MPN values obtained with different media were significantly different. Differences were considered significant at the 95% confidence level.
RESULTS
MPN enumeration of SRB in sediment slurries with natural and synthetic media.
The MPN of SRB in a sediment slurry from Kysing Fjord determined by the T-MPN method with sediment medium (natural medium) and by the N-MPN method with API-RST medium (synthetic medium) differed by a factor of ∼3,000 after 34 days of incubation (Table 1). Estimates obtained by the N-MPN method (API-RST medium) yielded significantly higher bacterial counts only on day 2. No significant differences in the MPN values between the two methods were observed from day 5 to day 11. However, from day 14 on, the bacterial numbers determined by the T-MPN method with sediment medium were significantly higher than the bacterial numbers determined by the N-MPN method with API-RST medium. A constant sulfate reduction rate of 0.98 mM SO42− day−1 (0 to 9 h; r2 = 0.996) was obtained for the Kysing Fjord slurry used for the enumeration experiments shown in Table 1. Based on this sulfate reduction rate, specific sulfate reduction rates (qSO42−) of 75 × 10−15 and 228 × 10−12 mol cell−1 day−1 were calculated by using the viable counts obtained by the T-MPN method with natural medium and the N-MPN method with synthetic medium, respectively (Table 1, day 34).
TABLE 1.
Enumeration of SRB in a sediment slurry from Kysing Fjord with synthetic medium (API-RST medium) and sediment medium
| Medium | Detection method | MPN · ml−1 on:
|
|||||
|---|---|---|---|---|---|---|---|
| Day 2 | Day 5 | Day 8 | Day 11 | Day 14 | Day 34 | ||
| Sediment medium | Tracera | 2.3 × 101 (a)c | 9.3 × 102 (a) | 2.1 × 104 (a) | 2.1 × 104 (a) | 1.4 × 106 (a) | 1.3 × 107 (a) |
| API-RST medium | Blackeningb | 4.3 × 103 (b) | 4.3 × 103 (a) | 4.3 × 103 (a) | 4.3 × 103 (a) | 4.3 × 103 (b) | 4.3 × 103 (b) |
Positive tubes were detected by the 35SO42− T-MPN method, and the detection limit was 0.1% TRIS.
Positive tubes were visually detected by the N-MPN method, i.e., by detection of a black precipitate (FeS).
MPN values in the same column followed by the same letter are not significantly different as determined by the Student t test (P > 0.05).
In order to investigate whether the low MPN values obtained with the synthetic medium (Table 1) were due to differences in the scoring procedure (blackening of the culture medium versus TRIS production), an additional experiment was performed with Kysing Fjord sediment. In this experiment, data obtained by the T-MPN method with sediment medium were compared with data obtained with three different synthetic media (Postgate’s B medium, Baar’s medium, API-RST medium) which are often used for enumeration of SRB. With the synthetic media, MPN values were estimated by using both T-MPN and N-MPN scores (Table 2). As shown in Table 2, the MPN values obtained by the T-MPN method with sediment medium were significantly higher (at least 1,600-fold higher) than the MPN values obtained with any of the synthetic media on day 28. This was true irrespective of the scoring method used for evaluation of growth in synthetic media. Although growth of SRB in Postgate’s B medium exhibited a long lag phase, the final MPN estimates for SRB obtained by the N-MPN and T-MPN methods with the three synthetic media on day 28 were not significantly different. A constant sulfate reduction rate of 1.63 mM SO42− day−1 (0 to 9 h; r2 = 0.997) was determined for the Kysing Fjord slurry used for the MPN enumeration experiments (Table 2). Based on this sulfate reduction rate, a qSO42− value of 109 × 10−15 mol of SO42− cell−1 day−1 was calculated by using the T-MPN value obtained with sediment medium (Table 2, day 28). The qSO42− values calculated by using MPN estimates determined with synthetic media were several orders of magnitude higher (177 × 10−12 to 709 × 10−12) (Table 3).
TABLE 2.
Enumeration of SRB in a sediment slurry from Kysing Fjord by using different enumeration media and different scoring methods
| Medium | Detection method | MPN · ml−1 on:
|
||
|---|---|---|---|---|
| Day 7 | Day 14 | Day 28 | ||
| Sediment medium | Tracera | 4.2 × 105 (a)c | 4.2 × 106 (a) | 1.5 × 107 (a) |
| Postgate’s B medium | Blackeningb | 2 (b) | 2 (b) | 2.3 × 103 (b) |
| Tracer | 2 (x) | 7 (x) | 2.8 × 102 (x) | |
| Baar’s medium | Blackening | 9.2 × 103 (a) | 9.2 × 103 (c) | 9.2 × 103 (b) |
| Tracer | 7 (x) | 4.0 × 101 (x) | 3.5 × 102 (x) | |
| API-RST medium | Blackening | 9.2 × 103 (a) | 9.2 × 103 (c) | 9.2 × 103 (b) |
| Tracer | 4.2 × 102 (y) | 4.2 × 102 (y) | 3.5 × 103 (x) | |
Positive tubes were detected by the 35SO42− T-MPN method, and the detection limit was 0.1% TRIS.
Positive tubes were visually detected by the N-MPN method, i.e., by detection of a black precipitate (FeS).
MPN values in the same column followed by the same letter are not significantly different as determined by the Student t test (P > 0.05). For comparisons of the T-MPN method with sediment medium and the N-MPN method with synthetic media the letters a, b, and c are used. For comparisons of the T-MPN method with sediment medium and the T-MPN method with synthetic media the letters x and y are used.
TABLE 3.
Comparison of qSO42− values determined by different experimental methods
| Expt | qSO42− (mol of SO42− cell−1 day−1)
|
|||||
|---|---|---|---|---|---|---|
| T-MPN method with natural media | N-MPN method with API-RST medium | N-MPN method with Baar’s medium | N-MPN method with Postgate’s B medium | T-MPN method with DSM medium 194 | Direct count method with DSM medium 194a | |
| Sediment slurry | 75 × 10−15 | 228 × 10−12 | ||||
| Sediment slurry | 109 × 10−15 | 177 × 10−12 | 177 × 10−12 | 709 × 10−12 | ||
| Activated sludge | 657 × 10−15 | |||||
| Desulfobulbus propionicus | 243 × 10−15 | 41 × 10−15 | ||||
Cell numbers were determined with a Bürker-Türk counting chamber.
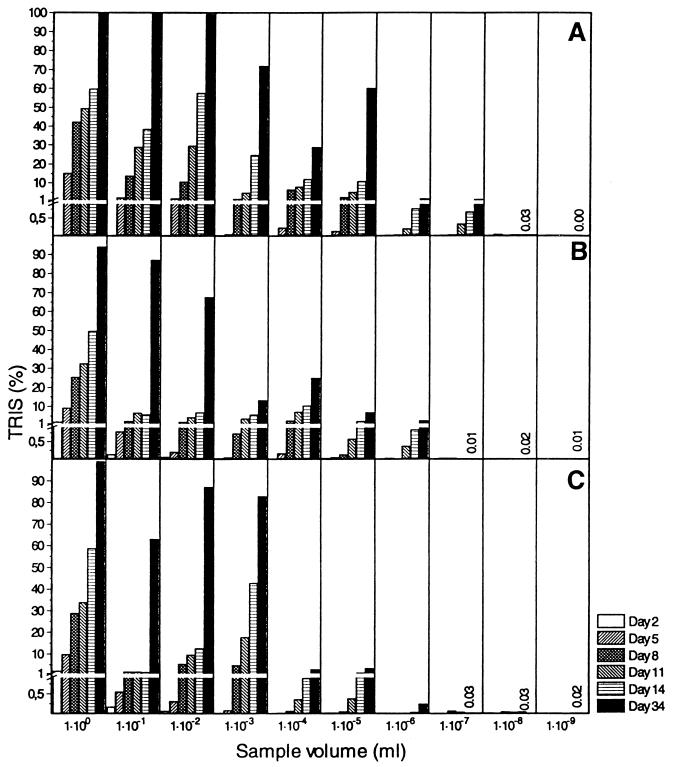
Figure 1 shows a typical time course of TRIS production for three independent MPN dilution series (Table 1) containing sediment medium. TRIS production increased with time in all positive tubes, and even though bacterial sulfate reduction was evaluated by a sensitive radiotracer technique, long incubation times were necessary in order to obtain positive scores in the tubes containing the highest dilutions. None of the three control tubes containing uninoculated sediment medium were positive (TRIS concentration, <0.05%) even after 34 days of incubation (data not shown).
FIG. 1.
Time course of TRIS production during T-MPN enumeration of SRB in a sediment slurry from Kysing Fjord (sediment medium; initial sulfate concentration, 17 mM; 20 kBq of 35SO42−/ml). TRIS values are the fractions of added 35SO42− converted to TRIS. Panels A, B, and C show the results obtained with three independent dilution series (series A, B, and C, respectively) prepared with the same sample. The detection limit for the presence of SRB in MPN tubes was 0.1% TRIS. After 34 days of incubation, the dilution series A, B, and C were positive with 10−7 ml of sample (1.30% TRIS), 10−6 ml of sample (2.56% TRIS), and 10−6 ml of sample (0.25% TRIS), respectively, yielding a viable count of 1.3 × 107 cells/ml. TRIS values for tubes considered negative for SRB on day 34 are shown by numbers. Note the enlarged scale for TRIS values of ≤ 1%.
Enumeration of SRB in activated-sludge enrichment cultures by T-MPN and N-MPN methods.
SRB in an anaerobic sludge enrichment culture were enumerated by the T-MPN method (sludge medium and API-RST medium) and the N-MPN method (API-RST medium) at 30°C (Table 4). As shown in Table 4, the viable counts of SRB determined by the T-MPN method with sludge medium were significantly higher than the viable counts determined by both the N-MPN method and the T-MPN method with API-RST medium from day 8 on. After 34 days of incubation, the SRB counts determined by the T-MPN method (sludge medium) and the N-MPN method (API-RST medium) were very different (1.5 × 107 and 4.3 × 104 cells · ml−1, respectively). There was a general tendency for T-MPN values to be lower than N-MPN values with the same synthetic medium, which indicates that blackening of the medium may not always be due to dissimilatory sulfate reduction (Tables 2 and 4).
TABLE 4.
Enumeration of SRB in an activated-sludge enrichment culture by using synthetic medium (API-RST medium) and sludge medium
| Medium | Detection method | MPN · ml−1 on:
|
||||
|---|---|---|---|---|---|---|
| Day 1 | Day 4 | Day 8 | Day 14 | Day 34 | ||
| Sludge medium | Tracera | 23.1 (a,x)c | 4.1 × 105 (a) | 1.3 × 107 (a) | 1.5 × 107 (a) | 1.5 × 107 (a) |
| API-RST medium | Blackeningb | 2.3 × 104 (b) | 4.3 × 104 (a) | 4.3 × 104 (b) | 4.3 × 104 (b) | 4.3 × 104 (b) |
| Tracer | 2 (x) | 9.2 × 103 (x) | 2.3 × 104 (x) | 2.3 × 104 (x) | 2.3 × 104 (x) | |
Positive tubes were detected by the T-MPN method, and the detection limit was 0.1% TRIS.
Positive tubes were visually detected by the N-MPN method, i.e., formation of FeS.
MPN values in the same column followed by the same letter are not significantly different as determined by the Student t test (P > 0.05). For comparisons of the T-MPN method with sludge medium and the N-MPN method with API-RST medium the letters a and b are used. For comparisons of the T-MPN method with sludge medium and the T-MPN method with API-RST medium the letter x is used.
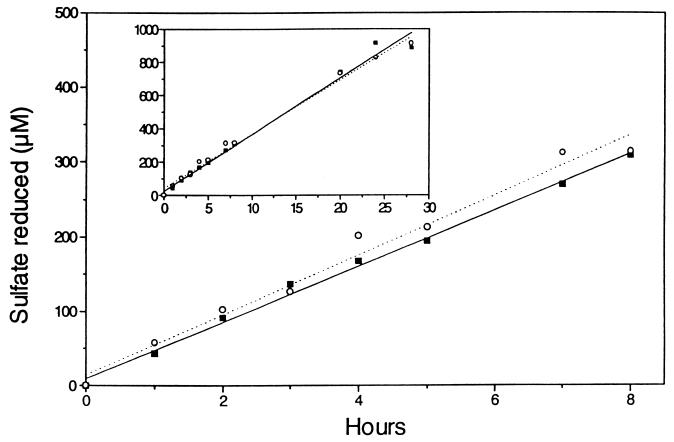
Experimentally determined qSO42− values obtained for SRB in the sludge experiment are shown in Table 3. These values were calculated from the MPN values in Table 4, and a constant sulfate reduction rate of 0.94 mM day−1 (0 to 8 h) obtained with a parallel sample (Fig. 2). Sulfate reduction was linear for up to 28 h, which indicates that the sulfate-reducing population did not change significantly throughout this period (Fig. 2). As shown in Fig. 2, sulfate reduction was not affected by the presence of the antibiotics chloramphenicol and streptomycin (Fig. 3).
FIG. 2.
Time course of sulfate reduction in an activated sludge enrichment culture used for MPN determination (Table 4). Sulfate reduction was measured in the presence (▪) and in the absence (○) of antibiotics (chloramphenicol and streptomycin). The initial sulfate concentration was 4.2 mM. The isotope concentration was 20 kBq of 35SO42−/ml. The inset shows a 28-h time course for the same experiment.
FIG. 3.
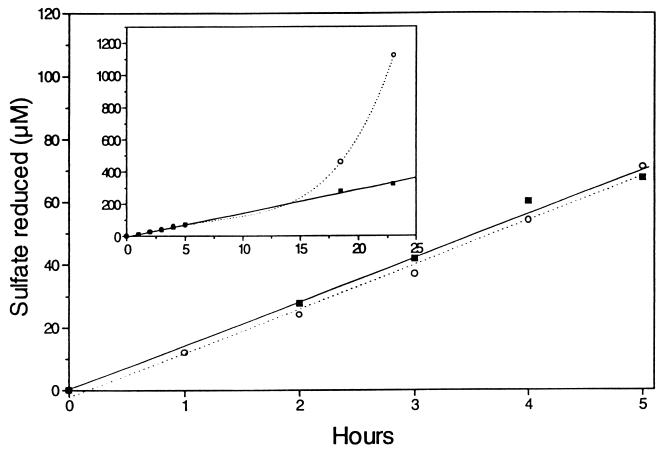
Sulfate reduction by Desulfobulbus propionicus after inoculation into defined growth medium (DSM 194 medium) in the presence (▪) and in the absence (○) of antibiotics (chloramphenicol and streptomycin). The initial sulfate concentration was 23.1 mM. The isotope concentration was 20 kBq of 35SO42−/ml. The inset shows a 24-h time course for the same experiment.
T-MPN enumeration of a pure culture of Desulfobulbus propionicus.
The average cell density in the experimental culture was 8.3 × 106 cells · ml−1 as determined by direct counting, whereas the T-MPN method yielded a slightly lower value, 1.4 × 106 cells · ml−1, after 18 days of incubation (95% confidence limits, 0.4 × 106 to 5.1 × 106 cells · ml−1). A sulfate reduction rate of 0.34 mM day−1 (r2 = 0.996) was obtained for the Desulfobulbus propionicus culture used for MPN enumeration (Fig. 3). From these data a qSO42− value of 41 × 10−15 mol of SO42− cell−1 day−1 was calculated by using direct count cell numbers, and a qSO42− value of 243 × 10−15 mol of SO42− cell−1 day−1 was calculated when the cell number estimated by the T-MPN method was used (Table 3).
The Desulfobulbus culture containing antibiotics exhibited a constant sulfate reduction rate for 24 h, indicating that cell growth was completely inhibited (Fig. 3). In the culture without antibiotics, sulfate reduction started to increase exponentially after about 15 h of incubation, hence indicating the start of exponential growth (Fig. 3).
DISCUSSION
The viable counts of SRB determined by the T-MPN method with natural media (slurries of marine sediment and anaerobic sludge) were typically 100- to 1,000-fold higher than the N-MPN and T-MPN estimates obtained when different synthetic media were used (Tables 1, 2, and 4) and yielded qSO42− values similar to those reported in pure-culture studies of SRB. This indicates that viable counts of SRB were not seriously underestimated by the T-MPN method with natural media described here. The high counting efficiency of the T-MPN method with natural media was primarily due to the use of natural media prepared from sterilized sample material, since the MPN values obtained with the T-MPN and N-MPN methods with the same synthetic media were much lower (Tables 2 and 4).
Source material is often included in isolation and enumeration media. Thus, clarified rumen fluid has been used extensively as a constituent of media used for the isolation of rumen microorganisms (29, 31). Other examples include the use of soil extract (47) and bacterial extracts (44) in isolation media. Furthermore, many media contain extracts from blood, serum, animal tissues, fecal material, or digestor or sewage sludge supernatant mainly in order to promote growth of microorganisms with fastidious and/or unknown requirements. However, unsupplemented natural media have to our knowledge not been used previously for MPN enumerations of SRB.
In a comprehensive study of dissimilatory sulfate reduction in marine sediments, Jørgensen (24) concluded that sulfate reduction rates in coastal sediments were roughly proportional to colony counts of SRB, even though the actual numbers of bacteria appeared to be grossly underestimated. Jørgensen reported qSO42− values for SRB in different marine sediments on the order of 10−12 to 10−11 mol of SO42− cell−1 day−1, values which are much higher than the values determined in pure-culture studies (10−15 to 10−14 mol of SO42− cell−1 day−1). This difference in metabolic rates (qSO42−) led Jørgensen to the conclusion that viable counts of SRB in marine sediment obtained by the N-MPN method with synthetic media underestimate the true numbers by at least 2 orders of magnitude. He suggested that the low recovery of SRB from sediments could be due to clumping of cells and the inability of many naturally occurring SRB to grow in the enumeration media used. Many media used for enumeration of SRB contain lactate as the sole electron donor, and this compound is a substrate which cannot be utilized by several species of cultured SRB, including most Desulfobacter species, Desulfotomaculum acetoxidans, Desulfovibrio baarsii, some Desulfobacterium species, Desulfococcus niacini, and Desulfonema magnum (45). In order to improve the recovery of SRB, Gibson et al. (12) used MPN media containing surfactants and different growth substrates (acetate, lactate, propionate, butyrate, and H2-CO2) known to be important for sulfate reduction in situ. Although these modifications did increase the viable counts, the numbers of SRB were still underestimated, as shown by the resulting high qSO42− values (mean qSO42−, 4.42 × 10−12 mol of SO42− · cell−1 · day−1). Gibson et al. suggested that a large part of the natural population of SRB is physiologically different from laboratory isolates and is not able to grow in enumeration media containing high levels of organic substrates. Bak and Pfennig (5) also proposed that substrate inhibition was the cause of the low bacterial counts obtained during enrichment of SRB at butyrate concentrations of >7 mM. It is well-known that many bacteria from oligotrophic environments can be isolated only in media containing low levels of substrate (38). The concentration of organic substrates in enumeration media is typically ≥20 mM, which could be inhibitory to ecologically important types of SRB not yet isolated.
Addition of particles to growth media has been reported to stimulate bacterial metabolism and growth (26, 39, 46). The high particle content (approximately 20 to 30%, vol/vol) of the natural media could have contributed to the high MPN numbers observed in the present investigation. The natural media used for enumeration of SRB in the present study were not amended with electron donors and therefore contained only naturally occurring substrates at in situ concentrations. As shown in Fig. 1, natural media prepared from Kysing Fjord sediment contained sufficient electron donors to support complete reduction of approximately 17 mM sulfate in the tubes containing the lowest dilutions (natural media prepared from activated sludge supported reduction of at least 9 mM sulfate). Part of the electron donors used for sulfate reduction were probably produced during incubation by hydrolytic and fermentative bacteria coinoculated with the SRB. It should be noted that MPN enumerations carried out with sediment media having lower organic contents did not result in complete reduction of sulfate in the media; instead, TRIS values between 15 and 60% were typically obtained after 4 weeks of incubation.
The detection limit (scoring limit) for the presence of SRB in T-MPN enumeration tubes was set at 0.1% TRIS. This value was chosen on the basis of numerous distillations of sterile media (control tubes injected with 20 kBq of radioactive sulfate ml−1). Usually, TRIS values obtained with sterile media were much lower than 0.05% and not significantly higher than the radioactive background values, showing that the carryover of 35SO42−-containing aerosols into the zinc acetate traps was negligible.
Theoretical calculations (assuming worst-case scenarios), growth experiments performed with Escherichia coli, and MPN enumerations with sulfate-depleted freshwater sediments showed that interference from assimilatory sulfate reduction (i.e., false positives) could be neglected at sulfate concentrations greater than approximately 6 mM (data not shown).
Consequently, when the T-MPN method with natural media is used for enumeration of SRB in low-sulfate environments, such as freshwater sediments, false positives in the highest-dilution tubes can be avoided by adding nonradioactive sulfate at a final concentration of ≥6 mM to the natural media. Alternatively, the detection limit can be increased to 0.5% or a higher percentage of TRIS.
As shown in Fig. 1, a prolonged incubation period (34 days) was required before a TRIS value of ≥0.1% (scoring limit) was observed in the series C tube containing 10−6 ml of sample. Hence, our method does not decrease the long incubation periods often used in MPN studies. However, compared to synthetic media, significantly higher estimated T-MPN values were obtained after 14 days of incubation in sediment medium (Tables 1 and 2) and after only 8 days of incubation in sludge medium (Table 4).
Table 3 summarizes qSO42− values calculated from data obtained in this study. Cell densities obtained by the T-MPN method with natural media yielded qSO42− values on the order of 10−14 to 10−13 mol of SO42− cell−1 day−1 for both sediment slurries and activated sludge. Our qSO42− values are thus at least 2 orders of magnitude lower than previously reported values obtained with marine sediments (10−12 to 10−11 mol of SO42− cell−1 day−1) (24), as well as activated sludge and anaerobic sludge (10−11 and 10−7 mol of SO42− cell−1 day−1 (27), and similar to the values obtained in pure-culture studies (10−15 to 10−14 mol of SO42− cell−1 day−1) (17, 19, 24).
All MPN techniques inherently tend to underestimate the actual numbers of SRB in environmental samples due to clumping of cells and attachment of cells to particulate matter. Gibson and coworkers (12) demonstrated that viable counts of SRB in marine sediment could be increased by 0 to 200% (average, 53%) by adding surfactants to enumeration media. The T-MPN method with natural media described in the present study always yielded 100- to 1,000-fold-higher viable counts of SRB than the T-MPN and N-MPN methods with synthetic media without any use of surfactants. The high MPN counting efficiencies obtained in this study thus suggest that cell clumping and attachment of SRB to particulate matter are not the main causes of the low levels of recovery of SRB observed with synthetic media.
The T-MPN method with natural media has several advantages over previously described methods for enumerating SRB: (i) it uses the natural environment of the SRB as the growth medium; (ii) it can easily be adapted to different environments; (iii) it yields significantly higher MPN counts than synthetic enumeration media; (iv) it selectively counts SRB performing dissimilatory sulfate reduction; and (v) it is insensitive to false positives due to assimilatory sulfate reduction by coexisting anaerobic bacteria and other H2S-producing reactions.
The natural media used in the present study were prepared from sample material which had been sterilized twice by autoclaving and diluted to a suitable consistency. It is noteworthy that even though this treatment must have resulted in drastic changes in in situ conditions (chemically and physically), it produced growth media which were far superior to any of the synthetic enumeration media tested. Since the ecological niches of uncultured SRB in nature are not known, it may be very difficult to develop suitable cultivation techniques for these bacteria based on synthetic media. Attempts should be made to identify and isolate those SRB present in the highest MPN dilution tubes containing natural media since these bacteria are likely to be the bacteria which are predominant in the environment investigated.
The principle of using natural media should be widely applicable to MPN enumeration of microorganisms in diverse ecosystems, especially those physiotypes which can be detected by radiotracers or sensitive chemical methods (e.g., methanogens, methanotrophs, and denitrifiers).
ACKNOWLEDGMENTS
We thank T. H. Blackburn for critically reading the manuscript and valuable comments. We also thank Dorthe T. Ganzhorn, Tove Wiegers, and Annette N. Jensen for skillful technical assistance.
REFERENCES
- 1.Amann R I, Ludwig W, Schleifer K H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann R I, Stromley J, Devereux R, Key R, Stahl D A. Molecular and microscopic identification of sulfate-reducing bacteria in multispecies biofilms. Appl Environ Microbiol. 1992;58:614–623. doi: 10.1128/aem.58.2.614-623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Public Health Association. Standard methods for the examination of water and wastewater. 17th ed. Washington, D.C: American Public Health Association; 1989. Estimation of bacterial density; pp. 977–980. [Google Scholar]
- 4.American Type Culture Collection. Bacteria and bacteriophages. 19th ed. Rockville, Md: American Type Culture Collection; 1996. [Google Scholar]
- 5.Bak F, Pfennig N. Sulfate-reducing bacteria in littoral sediment of Lake Constance. FEMS Microbiol Ecol. 1991;85:43–52. [Google Scholar]
- 6.Brandt K K, Ingvorsen K. Desulfobacter halotolerans sp. nov., a halotolerant acetate-oxidizing sulfate-reducing bacterium isolated from sediments of Great Salt Lake, Utah. Syst Appl Microbiol. 1997;20:366–373. [Google Scholar]
- 7.Christensen B, Torsvik T, Lien T. Immunomagnetically captured thermophilic sulfate-reducing bacteria from North Sea oil field waters. Appl Environ Microbiol. 1992;58:1244–1248. doi: 10.1128/aem.58.4.1244-1248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cochran W G. Estimation of bacterial densities by means of the “most probable number.”. Biometrics. 1950;6:105–116. [PubMed] [Google Scholar]
- 9.Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH. Catalogue of strains 1993. 5th ed. Braunschweig, Germany: Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH; 1993. [Google Scholar]
- 10.Fedorak P M, Semple K M, Westlake D W S. A statistical comparison of two culturing methods for enumerating sulfate-reducing bacteria. J Microbiol Methods. 1987;7:19–27. [Google Scholar]
- 11.Fossing H, Jørgensen B B. Measurement of bacterial sulfate reduction in sediments: evaluation of a single-step chromium reduction method. Biogeochemistry. 1989;8:205–222. [Google Scholar]
- 12.Gibson G R, Parkers R J, Herbert R A. Evaluation of viable counting procedures for the enumeration of sulfate-reducing bacteria in estuarine sediments. J Microbiol Methods. 1987;7:201–210. [Google Scholar]
- 13.Hamilton W A. Sulphate-reducing bacteria and anaerobic corrosion. Annu Rev Microbiol. 1985;39:195–217. doi: 10.1146/annurev.mi.39.100185.001211. [DOI] [PubMed] [Google Scholar]
- 14.Hordijk K A, Hagenaars C P M M, Cappenberg T E. Kinetic studies of bacterial sulfate reduction in freshwater sediments by high-pressure liquid chromatography and microdistillation. Appl Environ Microbiol. 1985;49:434–440. doi: 10.1128/aem.49.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurley M A, Roscoe M E. Automated statistical analysis of microbial enumeration by dilution series. J Appl Bacteriol. 1983;55:159–164. [Google Scholar]
- 16.Ingvorsen K, Brock T D. Electron flow via sulfate reduction and methanogenesis in the hypolimnion of Lake Mendota. Limnol Oceanogr. 1982;27:559–564. [Google Scholar]
- 17.Ingvorsen K, Jørgensen B B. Kinetics of sulfate uptake by freshwater and marine species of Desulfovibrio. Arch Microbiol. 1984;139:61–66. [Google Scholar]
- 18.Ingvorsen K, Jørgensen B B. Seasonal variation in H2S emission to the atmosphere from intertidal sediments in Denmark. Atmos Environ. 1982;16:855–865. [Google Scholar]
- 19.Ingvorsen K, Zehnder A J B, Jørgensen B B. Kinetics of sulfate and acetate uptake by Desulfobacter postgatei. Appl Environ Microbiol. 1984;47:403–408. doi: 10.1128/aem.47.2.403-408.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingvorsen K, Zeikus J G, Brock T D. Dynamics of bacterial sulfate reduction in a eutrophic lake. Appl Environ Microbiol. 1981;42:1029–1036. doi: 10.1128/aem.42.6.1029-1036.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iverson W P. Microbial corrosion of metals. Adv Appl Microbiol. 1987;32:1–36. [Google Scholar]
- 22.Jain D K. Evaluation of the semisolid Postgate’s B medium for enumerating sulfate-reducing bacteria. J Microbiol Methods. 1995;22:27–38. [Google Scholar]
- 23.Jørgensen B B. A comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments. I. Measurement with radiotracer techniques. Geomicrobiology. 1978;1:11–27. [Google Scholar]
- 24.Jørgensen B B. A comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments. III. Estimation from chemical and bacteriological field data. Geomicrobiology. 1978;1:49–64. [Google Scholar]
- 25.Jørgensen B B. Mineralization of organic matter in the sea bed—the role of sulphate reduction. Nature. 1982;296:643–645. [Google Scholar]
- 26.Laanbroek H J, Geerligs H J. Influence of clay particles (Illite) on substrate utilization by sulfate-reducing bacteria. Arch Microbiol. 1983;134:161–163. [Google Scholar]
- 27.Lens P N, De Poorter M-P, Cronenberg C C, Verstraete W H. Sulfate reducing and methane producing bacteria in aerobic wastewater treatment systems. Water Res. 1995;29:871–880. [Google Scholar]
- 28.Lillebæk R. Application of antisera raised against sulfate-reducing bacteria for indirect immunofluorescent detection of immunoreactive bacteria in sediment from the German Baltic Sea. Appl Environ Microbiol. 1995;61:3436–3442. doi: 10.1128/aem.61.9.3436-3442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macy J M. Nonpathogenic members of the genus Bacteroides. In: Starr M P, Stolp H, Trüper H G, Balows A, Schlegel H G, editors. The prokaryotes. New York, N.Y: Springer-Verlag; 1981. pp. 1450–1463. [Google Scholar]
- 30.Macy J M, Snellen J E, Hungate R E. Use of syringe methods for anaerobiosis. Am J Clin Nutr. 1972;25:1318–1323. doi: 10.1093/ajcn/25.12.1318. [DOI] [PubMed] [Google Scholar]
- 31.Mah R A, Smith M R. The methanogenic bacteria. In: Starr M P, Stolp H, Trüper H G, Balows A, Schlegel H G, editors. The prokaryotes. New York, N.Y: Springer-Verlag; 1981. pp. 948–977. [Google Scholar]
- 32.Muyzer G, Ramsing N B. Molecular methods to study the organization of microbial communities. Water Sci Technol. 1995;32:1–9. [Google Scholar]
- 33.Odom J M. Industrial and environmental concerns with sulfate-reducing bacteria. ASM News. 1990;56:473–476. [Google Scholar]
- 34.Odom J M, Jessie K, Knodel E, Emptage M. Immunological cross-reactivities of adenosine-5′-phosphosulfate reductases from sulfate-reducing and sulfide-oxidizing bacteria. Appl Environ Microbiol. 1991;57:727–733. doi: 10.1128/aem.57.3.727-733.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pankhurst E S. The isolation and enumeration of sulphate-reducing bacteria. In: Shepton D A, Board G G, editors. Isolation of anaerobes. Vol. 5. New York, N.Y: Academic Press; 1971. pp. 223–240. [Google Scholar]
- 36.Postgate J R. The sulphate reducing bacteria. Cambridge, Great Britain: Cambridge University Press; 1984. [Google Scholar]
- 37.Ramsing N B, Fossing H, Ferdelman T G, Andersen F, Thamdrup B. Distribution of bacterial populations in a stratified fjord (Mariager Fjord, Denmark) quantified by in situ hybridization and related to chemical gradients in the water column. Appl Environ Microbiol. 1996;62:1391–1404. doi: 10.1128/aem.62.4.1391-1404.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schut F, Prins R A, Gottschal J C. Oligotrophy and pelagic marine bacteria: facts and fiction. Aquat Microb Ecol. 1997;12:177–202. [Google Scholar]
- 39.Szewzyk U, Schink B. Attachment to amorphous iron sulfide increases the activity of strictly anaerobic, gallic acid-degrading bacteria. FEMS Microbiol Lett. 1991;78:115–120. [Google Scholar]
- 40.Tanner R S. Monitoring sulfate-reducing bacteria: comparison of enumeration media. J Microbiol Methods. 1989;10:83–90. [Google Scholar]
- 41.Tatnall R E, Stanton K M, Ebersole R C. Testing for the presence of sulfate-reducing bacteria. Mater Perform. 1988;27:71–80. [Google Scholar]
- 42.Teske A, Wawer C, Muyzer G, Ramsing N B. Distribution of sulfate-reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most-probable-number counts and denaturing gradient gel electrophoresis of PCR-amplified ribosomal DNA fragments. Appl Environ Microbiol. 1996;62:1405–1415. doi: 10.1128/aem.62.4.1405-1415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thode-Andersen S, Jørgensen B B. Sulfate reduction and the formation of 35S-labeled FeS, FeS2, and So in coastal marine sediments. Limnol Oceanogr. 1989;34:793–806. [Google Scholar]
- 44.Wais A C. Recovery of halophilic archaebacteria from natural environments. FEMS Microbiol Ecol. 1988;53:211–216. [Google Scholar]
- 45.Widdel F. Microbiology and ecology of sulfate- and sulfur-reducing bacteria. In: Zehnder A J B, editor. Biology of anaerobic microorganisms. New York, N.Y: Wiley; 1988. pp. 469–585. [Google Scholar]
- 46.Widdel F, Kohring G-W, Mayer F. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limicola gen. nov. sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol. 1983;134:286–294. [Google Scholar]
- 47.Wirth S J, Wolf G A. Dye-labelled substrates for the assay and detection of chitinase and lysozyme activity. J Microbiol Methods. 1990;12:197–205. [Google Scholar]