Abstract
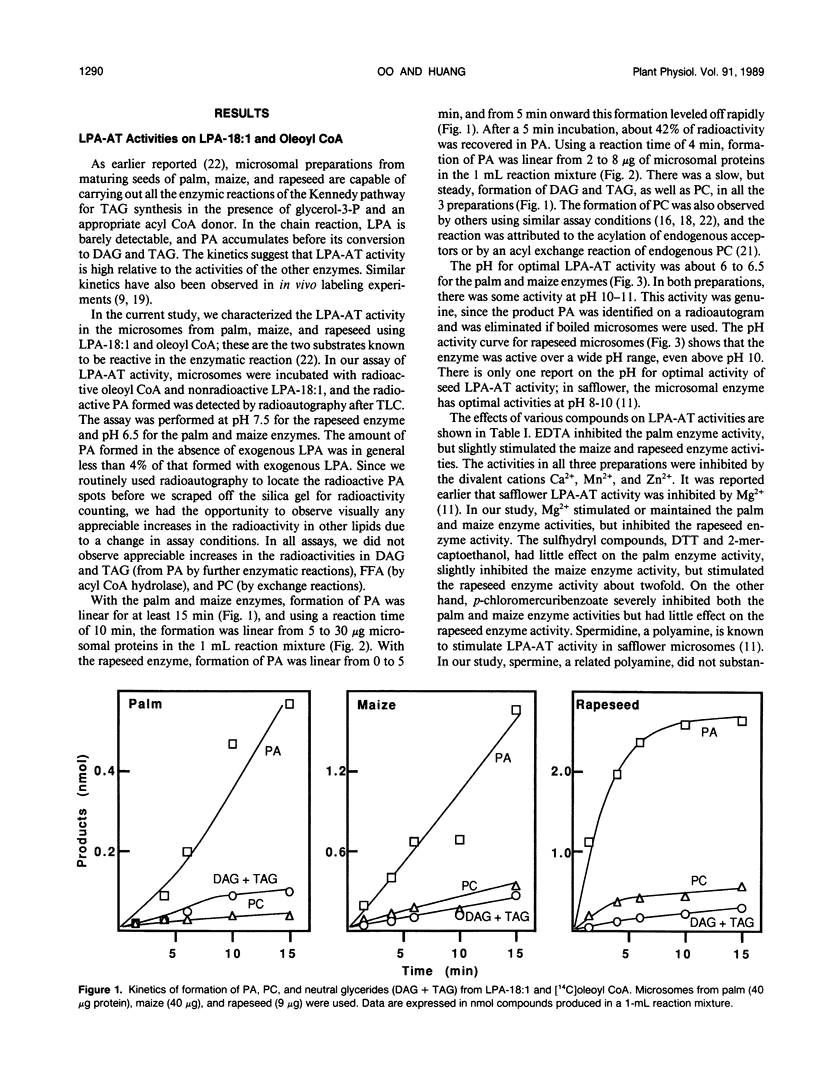
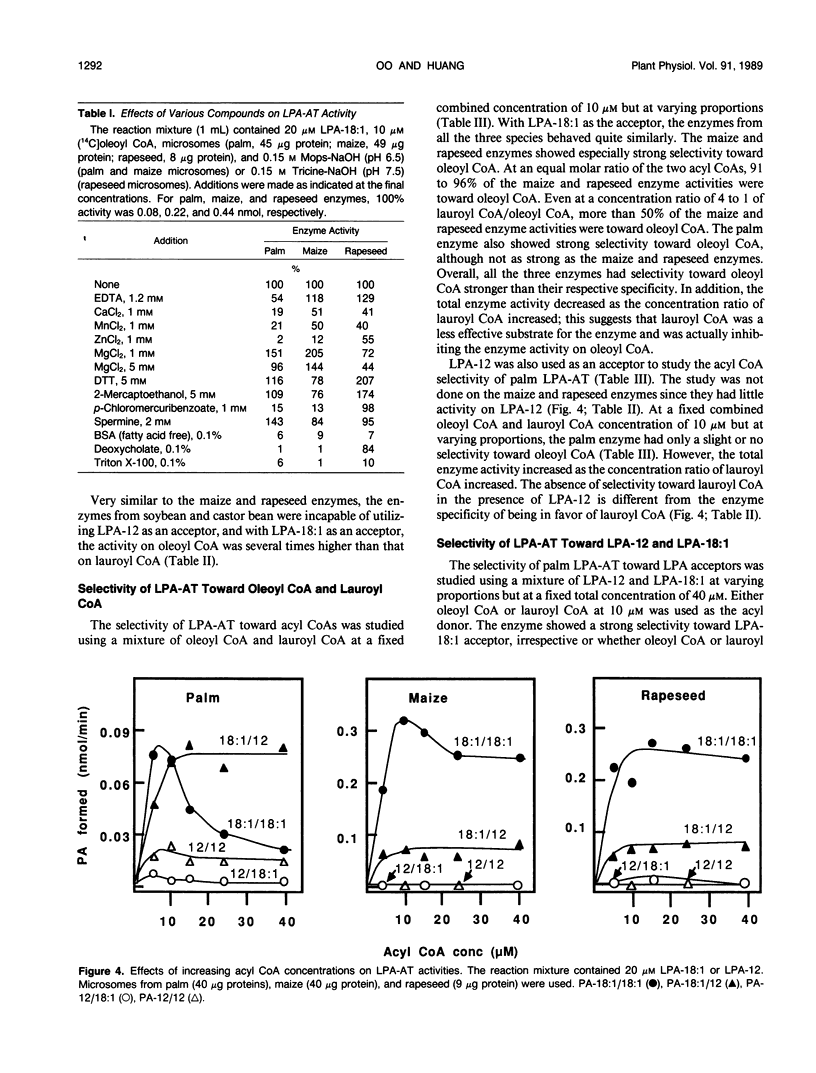
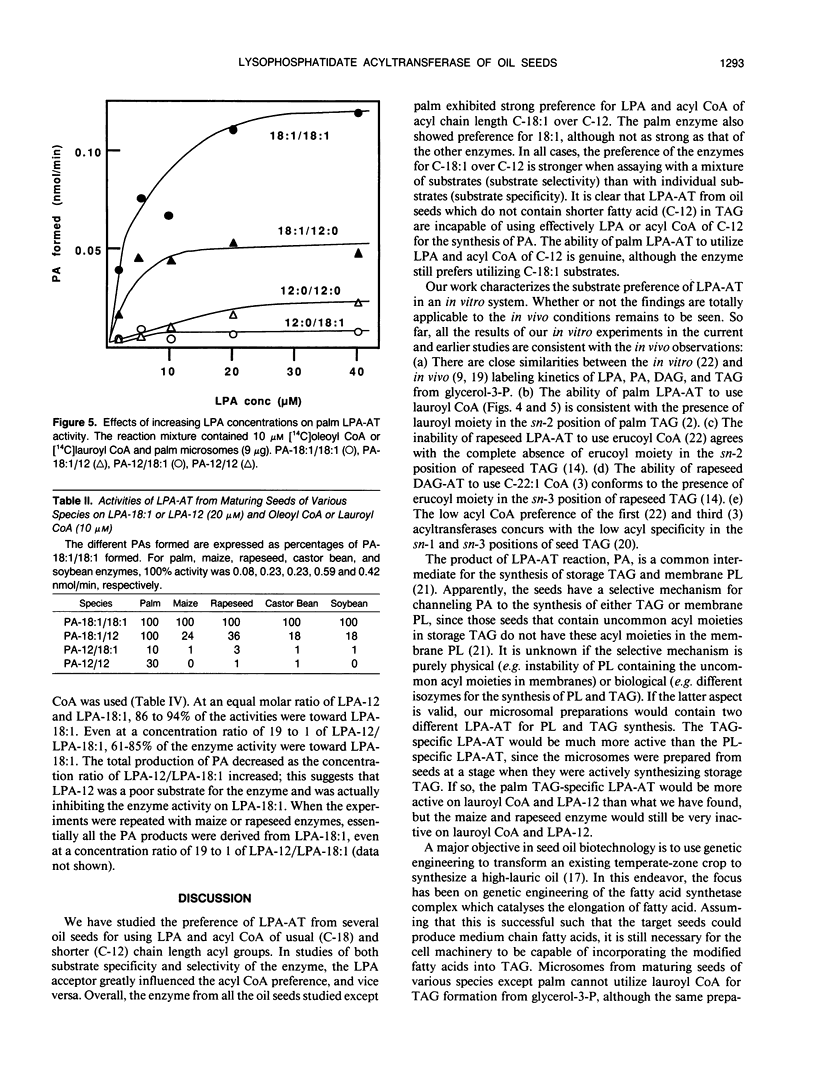
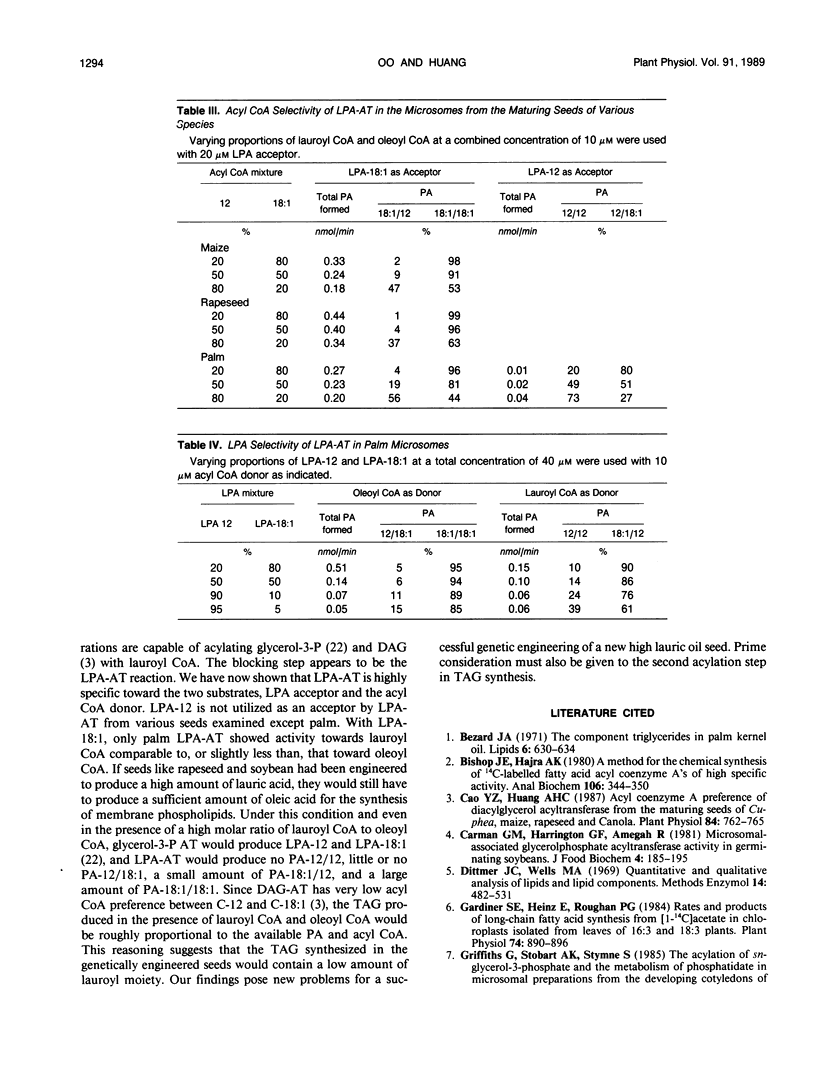
Lysophosphatidate (LPA) acyltransferase (EC 2.3.1.51) in the microsomes from palm endosperm (Syagrus cocoides Martius), maize scutellum (Zea mays L.), and rapeseed cotyledon (Brassica napus L.) of maturing seeds were studied for their specificities toward the acyl moiety of the substrates lysophosphatidate and acyl coenzyme A (CoA). The LPA acceptor greatly influenced the acyl CoA specificity of the enzyme and vice versa. With 1-oleoyl-lysophosphatidate (LPA-18:1), the palm enzyme was equally active on oleoyl CoA and lauroyl CoA, whereas the maize and rapeseed enzymes were more active on oleoyl CoA than on lauroyl CoA. With 1-lauroyl-lysophosphatidate (LPA-12), which generated less activity than LPA-18:1, the palm enzyme was three times more active on lauroyl CoA than on oleoyl CoA. LPA-12 was an inactive substrate for the maize and rapeseed enzymes. The selectivity of the enzymes was also studied using a mixture of LPA-18:1 and LPA-12, as well as lauroyl CoA and oleoyl CoA. Under this selectivity condition and compared to the specificity condition, the enzymes from all the three seeds exerted stronger preference for oleoyl moiety in either the LPA or acyl CoA, and again, only the palm enzyme could act on LPA-12. Similar studies, although in lesser detail, showed that the enzymes from soybean and castor bean were similar to the maize and rapeseed enzymes in having little activity on substrates containing lauroyl moiety. The results demonstrate the importance of the acyl group in the sn-1 position of LPA in determining the acyl preference in the sn-2 position in phosphatidate synthesis. The palm enzyme appears to be the only one capable of synthesizing phosphatidates containing high amounts of lauric moieties.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. E., Hajra A. K. A method for the chemical synthesis of 14C-labeled fatty acyl coenzyme A's of high specific activity. Anal Biochem. 1980 Aug;106(2):344–350. doi: 10.1016/0003-2697(80)90531-x. [DOI] [PubMed] [Google Scholar]
- Cao Y. Z., Huang A. H. Acyl coenzyme a preference of diacylglycerol acyltransferase from the maturing seeds of cuphea, maize, rapeseed, and canola. Plant Physiol. 1987 Jul;84(3):762–765. doi: 10.1104/pp.84.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. E., Heinz E., Roughan P. G. Rates and products of long-chain Fatty Acid synthesis from [1-C]acetate in chloroplasts isolated from leaves of 16:3 and 18:3 plants. Plant Physiol. 1984 Apr;74(4):890–896. doi: 10.1104/pp.74.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Stobart A. K., Stymne S. The acylation of sn-glycerol 3-phosphate and the metabolism of phosphatidate in microsomal preparations from the developing cotyledons of safflower (Carthamus tinctorius L.) seed. Biochem J. 1985 Sep 1;230(2):379–388. doi: 10.1042/bj2300379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurr M. I., Blades J., Appleby R. S., Smith C. G., Robinson M. P., Nichols B. W. Studies on seed-oil triglycerides. Triglyceride biosynthesis and storage in whole seeds and oil bodies of Crambe abyssinica. Eur J Biochem. 1974 Apr 1;43(2):281–290. doi: 10.1111/j.1432-1033.1974.tb03411.x. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Asahi T., Fujii S. 1-Acyl-sn-glycerol-3-phosphate acyltransferase in maturing safflower seeds and its contribution to the non-random fatty acid distribution of triacylglycerol. Eur J Biochem. 1987 Sep 1;167(2):339–347. doi: 10.1111/j.1432-1033.1987.tb13342.x. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Takahashi T., Fujii S. Diacylglycerol acyltransferase in maturing safflower seeds: its influences on the fatty acid composition of triacylglycerol and on the rate of triacylglycerol synthesis. Biochim Biophys Acta. 1988 Jan 19;958(1):125–129. doi: 10.1016/0005-2760(88)90253-6. [DOI] [PubMed] [Google Scholar]
- Ichihara K. sn-Glycerol-3-phosphate acyltransferase in a particulate fraction from maturing safflower seeds. Arch Biochem Biophys. 1984 Aug 1;232(2):685–698. doi: 10.1016/0003-9861(84)90589-7. [DOI] [PubMed] [Google Scholar]
- MATTSON F. H., VOLPENHEIN R. A. The specific distribution of fatty acids in the glycerides of vegetable fats. J Biol Chem. 1961 Jul;236:1891–1894. [PubMed] [Google Scholar]
- Rochester C. P., Bishop D. G. The role of lysophosphatidylcholine in lipid synthesis by developing sunflower (Helianthus annuus L.) seed microsomes. Arch Biochem Biophys. 1984 Jul;232(1):249–258. doi: 10.1016/0003-9861(84)90541-1. [DOI] [PubMed] [Google Scholar]
- Singh H., Privett O. S. Incorporation of 33P in soybean phosphatides. Biochim Biophys Acta. 1970 Feb 10;202(1):200–202. doi: 10.1016/0005-2760(70)90236-5. [DOI] [PubMed] [Google Scholar]
- Stymne S., Stobart A. K., Glad G. The role of the acyl-CoA pool in the synthesis of polyunsaturated 18-carbon fatty acids and triacylglycerol production in the microsomes of developing safflower seeds. Biochim Biophys Acta. 1983 Jul 12;752(2):198–208. doi: 10.1016/0005-2760(83)90113-3. [DOI] [PubMed] [Google Scholar]
- Sun C., Cao Y. Z., Huang A. H. Acyl coenzyme a preference of the glycerol phosphate pathway in the microsomes from the maturing seeds of palm, maize, and rapeseed. Plant Physiol. 1988 Sep;88(1):56–60. doi: 10.1104/pp.88.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]


