Abstract
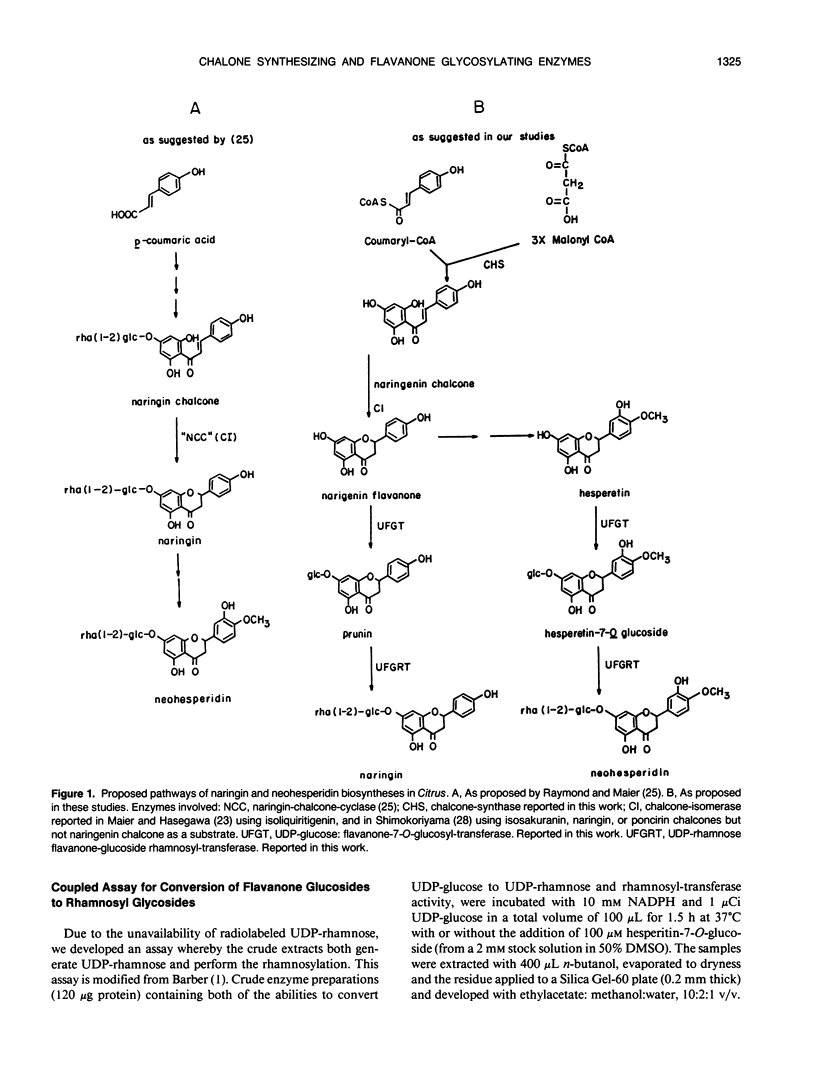
Previous indirect evidence suggested that the biosynthesis of flavonoids in Citrus may not proceed via the usual chalcone synthase reaction and that glycosylation occurs during chalcone formation and not afterward, as has been reported in other species. We detected chalcone-synthase and UDP-glucose:flavanone-7-O-glucosyl-transferase activities in cell-free extracts of Citrus. The glucosylated flavanone was further rhamnosylated when exogenous UDP-glucose and NADPH were added to the extract. Chalcone-synthase activity was detected in cell-free extracts derived from young leaves and fruits. Young fruits (2 millimeter diameter) had the highest chalcone synthase activity. UDP-glucose:flavanone-7-O-glucosyl-transferase activity was measured in cell-free extracts derived from young leaves and fruits of Citrus mitis and Citrus maxima. The highest UDP-glucose:flavanone-7-O-glucosyl-transferase activity was found in young C. maxima leaves. These data indicate that Citrus contains a flavonoid pathway similar to that studied in other species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER G. A. The enzymic synthesis of uridine diphosphate L-rhamnose. Biochem Biophys Res Commun. 1962 Jul 3;8:204–208. doi: 10.1016/0006-291x(62)90264-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- De Luca V., Ibrahim R. K. Enzymatic synthesis of polymethylated flavonols in Chrysosplenium americanum. I. Partial purification and some properties of S-adenosyl-L-methionine:flavonol 3-, 6-, 7-, and 4'-O-methyltransferases. Arch Biochem Biophys. 1985 May 1;238(2):596–605. doi: 10.1016/0003-9861(85)90205-x. [DOI] [PubMed] [Google Scholar]
- Ebel J., Hahlbrock K., Grisebach H. Purification and properties of an o-dihydricphenol meta-O-methyltransferase from cell suspension cultures of parsley and its relation to flavonoid biosynthesis. Biochim Biophys Acta. 1972 May 12;268(2):313–326. doi: 10.1016/0005-2744(72)90326-9. [DOI] [PubMed] [Google Scholar]
- Goldstein S. M., Kaempfer C. E., Kealey J. T., Wintroub B. U. Human mast cell carboxypeptidase. Purification and characterization. J Clin Invest. 1989 May;83(5):1630–1636. doi: 10.1172/JCI114061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann M. L., Heller W., Grisebach H. Induction and characterization of a microsomal flavonoid 3'-hydroxylase from parsley cell cultures. Eur J Biochem. 1983 Aug 15;134(3):547–554. doi: 10.1111/j.1432-1033.1983.tb07601.x. [DOI] [PubMed] [Google Scholar]
- Jourdan P. S., McIntosh C. A., Mansell R. L. Naringin Levels in Citrus Tissues : II. Quantitative Distribution of Naringin in Citrus paradisi MacFad. Plant Physiol. 1985 Apr;77(4):903–908. doi: 10.1104/pp.77.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzaler F., Hahlbrock K. Enzymatic synthesis of aromatic compounds in higher plants: formation of naringenin (5,7,4'-trihydroxyflavanone) from p-coumaroyl coenzyme A and malonyl coenzyme A. FEBS Lett. 1972 Nov 15;28(1):69–72. doi: 10.1016/0014-5793(72)80679-3. [DOI] [PubMed] [Google Scholar]
- Poulton J. E., Hahlbrock K., Grisebach H. O-Methylation of flavonoid substrates by a partially purified enzyme from soybean cell suspension cultures. Arch Biochem Biophys. 1977 Apr 30;180(2):543–549. doi: 10.1016/0003-9861(77)90071-6. [DOI] [PubMed] [Google Scholar]
- Ragni M. V., Tegtmeier G. E., Levy J. A., Kaminsky L. S., Lewis J. H., Spero J. A., Bontempo F. A., Handwerk-Leber C., Bayer W. L., Zimmerman D. H. AIDS retrovirus antibodies in hemophiliacs treated with factor VIII or factor IX concentrates, cryoprecipitate, or fresh frozen plasma: prevalence, seroconversion rate, and clinical correlations. Blood. 1986 Mar;67(3):592–595. [PubMed] [Google Scholar]
- Riov J., Monselise S. P., Kahan R. S. Ethylene-controlled Induction of Phenylalanine Ammonia-lyase in Citrus Fruit Peel. Plant Physiol. 1969 May;44(5):631–635. doi: 10.1104/pp.44.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter A., Ortmann R., Grisebach H. Purification and properties of an enzyme from cell suspension cultures of parsley catalyzing the transfer of D-glucose from UDP-D-glucose to flavonoids. Biochim Biophys Acta. 1972 Jan 20;258(1):71–87. doi: 10.1016/0005-2744(72)90967-9. [DOI] [PubMed] [Google Scholar]