Abstract
Background and Objectives
Elevated intracranial pressure (ICP) in myelin oligodendrocyte glycoprotein (MOG) antibody–associated disease (MOGAD) has been largely unexplored. The objectives of this study were to determine the frequency of increased ICP in MOGAD and its association with disease course and outcomes and to highlight cases requiring medical and/or surgical management of increased ICP.
Methods
In this retrospective, single-center cohort study, we examined the clinical and paraclinical data from the initial presentation and follow-up data of children diagnosed with MOGAD. In those with opening pressure (OP) measurements, univariate analyses were used to evaluate factors associated with increased ICP, which was defined as OP > 28 cm H2O. We also present a case series of patients with or without OP measurement who required medical and/or surgical management of increased ICP.
Results
Of 86 children with MOGAD, 43 (50.0%) had an OP recorded and 7 (8.1%) required ICP management. In those with OP recorded, the median (interquartile range) OP for the different MOGAD phenotypes were: 30.0 (22.8–41.6) (acute disseminated encephalomyelitis, ADEM), 20.5 (16.1–23.6) (optic neuritis), 17.0 (17.0–22.5) (myelitis), and 19.5 (16.5–29.3) (other) cm H20. Overall, 20.9% had increased ICP based on an OP > 28 cm H2O, of whom 77.8% presented with ADEM. In a subgroup analysis of those presenting with ADEM, those with an elevated ICP had longer hospital stay (p = 0.007) and neurologic disability (defined as modified Rankin Scale >1) (p = 0.049). In those with or without OP recorded, 7 (6 with ADEM, one with cerebral cortical encephalitis) required ICP-directed therapies. Findings on brain MRI in these 7 children revealed extensive disease burden with bilateral cerebral involvement and evidence of restricted diffusion. While neuropsychological data in this small subset revealed significant variability, all sustained identifiable deficits after discharge, including attention-deficit hyperactivity disorders and language and learning disorders.
Discussion
In pediatric MOGAD, increased OP and ADEM at initial presentation were associated with longer hospital stays and greater long-term morbidity. Although invasive ICP monitoring has not been specifically advocated in the management of MOGAD, it is important to recognize signs and symptoms of increased ICP in these patients and consider ICP monitoring and management strategies based on clinical and radiologic findings, especially in those presenting with ADEM and with OP > 28 cm H2O.
Introduction
Myelin oligodendrocyte glycoprotein (MOG) antibody–associated disease (MOGAD) is a CNS demyelination disease distinct from multiple sclerosis (MS) and anti-aquaporin-4 (AQP4)-IgG–positive neuromyelitis optic spectrum disorder. There have been limited previous reports of increased intracranial pressure (ICP) in patients with MOGAD.1-11 The clinical course and outcomes of hospitalized children with MOGAD and increased ICP remains largely unexplored because most published studies have been case reports or small case series. In this single-center cohort study of pediatric MOGAD, we describe the frequency of increased ICP using lumbar puncture (LP) opening pressure (OP) measurements and evaluate its association with disease course and outcome. We also highlight a series of patients with MOGAD who required medical and/or surgical management of increased ICP.
Methods
Patients
We retrospectively analyzed the medical records of 86 children seen in the Demyelinating Disease Clinic in Dallas, Texas, from 2009 to 2022 who have been diagnosed with MOGAD. Diagnosis required fulfillment of the criteria recently proposed by an international panel of experts.12 This includes 3 main criteria: (1) the presence of one of 6 defined core clinical demyelinating events, (2) a positive anti-MOG-IgG test, and (3) the exclusion of alternative causes.12 For those who did not experience one of the 6 events at onset or at relapse, the patient was included if he/she had clear positive titers (≥1:100) and there was no better diagnostic explanation. All patients had positive serum anti-MOG-IgG cell-based assays per the individual assay cutoffs specified by the recently proposed international guidelines.12 We included patients who had OP measurement on the first attack and/or required medical and/or surgical management of increased ICP (with or without OP measurement). Only those with OP measurement were included in the group analyses while those who required medical and/or surgical management were highlighted individually.
Data Collection
MOGAD-demyelinating events were categorized into one of the 6 defined core clinical demyelinating events: acute disseminated encephalomyelitis (ADEM), optic neuritis, myelitis, cerebral monofocal/polyfocal deficits, brainstem/cerebellar deficits, and cerebral cortical encephalitis often with seizures.12 If they presented with symptoms and imaging that did not fit into one of the 6 demyelination categories, they were categorized as “other clinical event.” For the group analysis, those with cerebral monofocal or polyfocal deficits, brainstem or cerebellar deficits, cerebral cortical encephalitis, and other clinical event were included together under “other.” A relapse was defined as a new clinical episode accompanied by radiologic evidence, appearing ≥1 month subsequent to the onset of the past acute attack for non-ADEM presentations and ≥3 months subsequent to the past acute attack for ADEM presentations.13 An OP ≤ 28 cm H2O is generally regarded as normal in children.14 Hence, we defined >28 cm H2O as elevated ICP in our analysis.
Demographic, investigative, and disease course data were collected. Results from standard neuropsychological tests were also collected. If a patient had multiple OP measurements during the first attack, then the highest value was recorded. If a patient required acute measures to lower ICP such as hypertonic saline boluses or pentobarbital infusions that were specifically given for ICP control, then they were included in the group requiring ICP management. Glasgow Coma Scale (GCS) was determined based on the history and physical examination documented on the first hospital day (HD). A GCS ≤12, indicative of at least a moderate-degree brain injury based on traumatic brain injury (TBI) literature, was used for further analysis. Disease severity and neurologic outcomes at the last follow-up were assessed retrospectively with the pediatric modified Rankin Scale (mRS).15 A mRS score >1, indicative of at least some disability, was considered a poor outcome.
Statistical Analysis
Data were summarized with counts (percentages) for categorical variables and as median with interquartile range (IQR) for continuous variables. Clinical features at the first MOGAD attack and long-term outcome were compared in patients with an OP ≤ 28 and OP > 28 cm H2O in the entire cohort and the subgroup with ADEM presentation, respectively. Group differences were examined using 2-proportion Z-test for categorical data or Mann-Whitney U test for continuous and ordinal data. p < 0.05 (2-sided) was considered significant. Given the retrospective nature of the study, no correction for multiple analyses was performed.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. Written informed consent for publication was obtained from all patients' parents or legal guardians of the selected cases included.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Cohort With OP Measured on LP
Among 86 children diagnosed with MOGAD, 82 (95.3%) had an LP and 43 (50.0%) had an OP measured and documented during their initial attack. We found no significant difference between those who did and did not have OP measurements when examining age, sex, presence of headache on admission, GCS ≤12 on admission, ADEM presentation or intensive care unit (ICU) stay (eTable 1, links.lww.com/NXI/A925). 20.9% (9/43) had OP > 28 cm H2O (increased ICP), and 79.1% (34/43) had an OP ≤ 28 cm H2O (normal ICP). 7.0% (3/43) had positive anti-MOG-IgG by fixed cell-based assays, and 93.0% (40/43) had positive anti-MOG-IgG by live cell-based assays. By MOGAD phenotypes, increased ICP was present in 50.0% (7/14) patients with ADEM, 4.5% (1/22) patients with optic neuritis, 0.0% (0/3) patients with myelitis, and 25.0% (1/4) patients with the other phenotype. The median (IQR) OP for the different MOGAD phenotypes were 30.0 (22.8–41.6) (ADEM), 20.5 (16.1–23.6) (optic neuritis), 17.0 (17.0–22.5) (myelitis), and 19.5 (16.5–29.3) cm H2O (other; 2 with cerebral monofocal/polyfocal deficits, one with cerebral cortical encephalitis, and one with concurrent myelitis and optic neuritis). Overall, 7 of 9 patients (77.8%) who presented with increased ICP had an ADEM phenotype. Those who presented with ADEM were more likely to have elevated ICP than normal ICP (p = 0.001), whereas those with optic neuritis were more likely to have normal ICP than elevated ICP (p = 0.007) (Table 1). Patients with an elevated ICP were more likely to have depressed mental status (GCS ≤12) (p = 0.005), require ICU admission (p = 0.010), and have received ICP-directed therapies (p < 0.001). They also had longer ICU (p < 0.001) and hospitalization (p < 0.001) stays and longer follow-up duration (p = 0.002). At follow-up, those with increased ICP also had a higher proportion with disability (mRS >1) (p = 0.004). Examining the ADEM group alone, the associations between elevated ICP and longer hospitalization and increased likelihood of long-term neurologic disability remained statistically significant (p = 0.011 and p = 0.049, respectively) (Table 2).
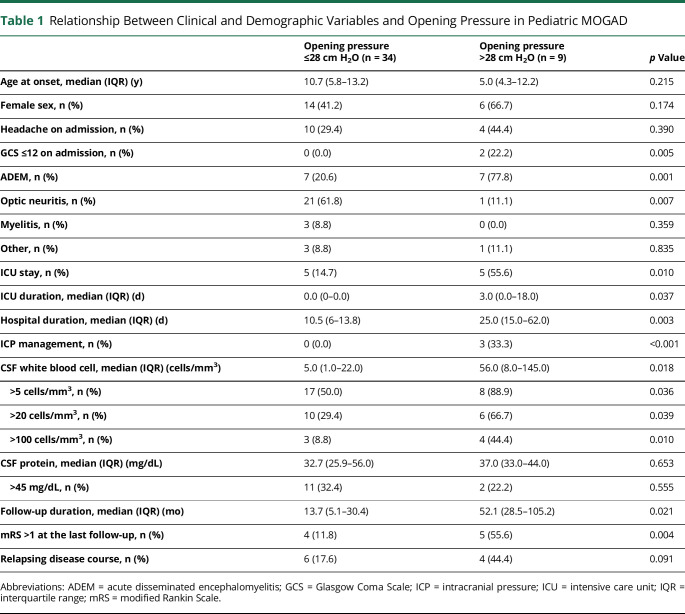
Table 1.
Relationship Between Clinical and Demographic Variables and Opening Pressure in Pediatric MOGAD
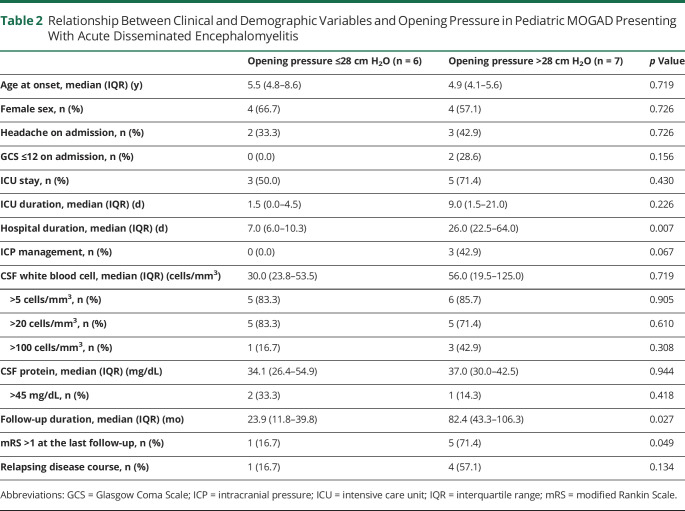
Table 2.
Relationship Between Clinical and Demographic Variables and Opening Pressure in Pediatric MOGAD Presenting With Acute Disseminated Encephalomyelitis
Cases Requiring ICP Management (With or Without OP Measured)
Seven patients had acute medical therapies and 3 had additional neurosurgical procedures for the management of increased ICP during their initial presentation. Their clinical and paraclinical data, as well as outcomes, are presented in eAppendix 1 and in Table 3 and eTable 2 (links.lww.com/NXI/A925). Four patients did not have an OP documented on LP, but they had clinical and radiologic findings consistent with increased ICP and received ICP-directed care. The 3 patients who did have OP measurements all had elevated OP (42.5, 52, and >55 cm H2O). Six patients presented with ADEM and one presented with cerebral cortical encephalitis.
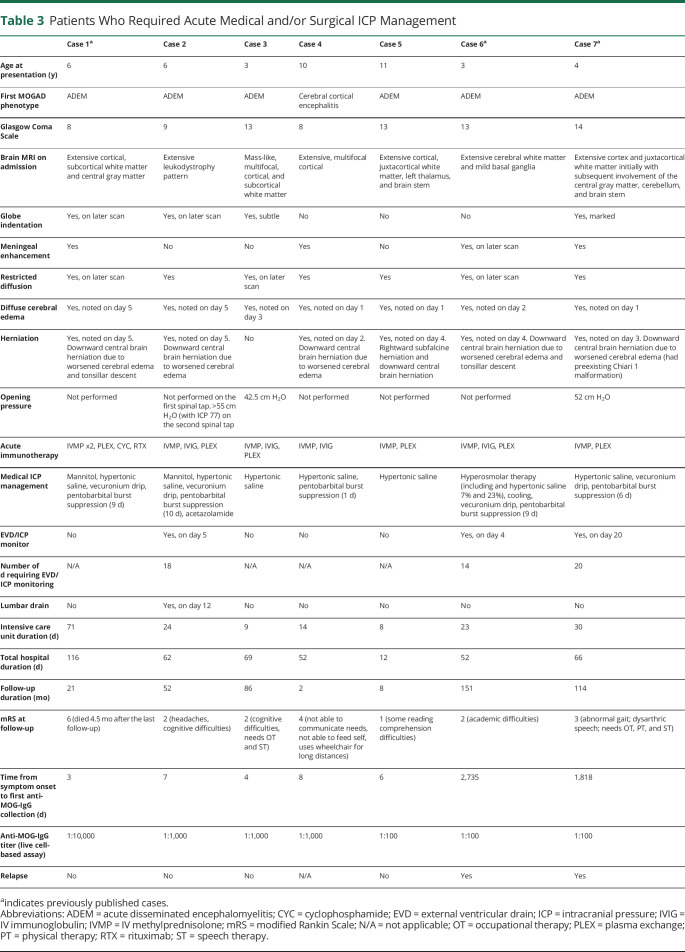
Table 3.
Patients Who Required Acute Medical and/or Surgical ICP Management
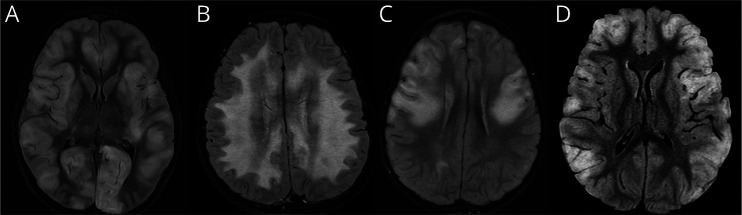
All patients presented with head CT scan findings consistent with mild cerebral edema on HD 1. Six patients had downward central brain herniation on repeat head CT scan by HD 2 to 5. In addition, 2 patients had mild tonsillar descent and one patient had rightward subfalcine herniation. Restricted diffusion on brain MRI was seen in all 7 patients on either the initial scan (4/7) or on follow-up imaging performed 7 to 11 days later. Follow-up MRI during admission usually demonstrated that lesions became more confluent involving additional areas of the brain. There was posterior globe indentation by the optic nerve head, an imaging feature suggestive of raised ICP, in 4 of 7 patients. There was leptomeningeal enhancement in 4 of 7 patients (3 on the initial imaging and one on follow-up). The predominant location of brain imaging abnormalities was cortical, juxtacortical, and peripheral white matter, with most lesions in the frontal and parietal lobes. Less commonly, lesions were located in the central gray matter, brain stem, and cerebellum. One patient had a diffuse leukodystrophy pattern of white mater involvement. Representative MRI scans with globe indentation, restricted diffusion, and leptomeningeal enhancement are shown in Figure 1. The imaging finding with peripheral and central restricted diffusion is similar to that of acute leukoencephalopathy with restricted diffusion (ALERD)16 (Figure 1, B and C). Representative MRI FLAIR images of the differing patterns of brain involvement are shown in Figure 2.
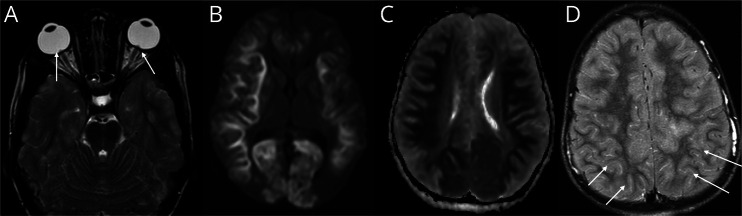
Figure 1. Representative MRI Scans of Posterior Globe Indentation, Restricted Diffusion, and Meningeal Enhancement From Patients Who Required ICP Management.
(A) Axial T2-weighted image from case 7 shows indentation of the posterior globe by optic nerve heads (arrows) and increased perioptic nerve CSF; (B) axial diffusion weighted imaging from case 1 shows restricted diffusion in the cortex and juxtacortical white matter in both cerebral hemispheres; (C) axial apparent diffusion coefficient image from case 1 shows true restricted diffusion in the cortex and juxtacortical white matter in both cerebral hemispheres; (D) postcontrast axial FLAIR image from case 4 shows scattered leptomeningeal enhancement (arrows). ICP = intracranial pressure.
Figure 2. Different Patterns of Brain Involvement on Axial FLAIR Images From Patients Who Required ICP Management.
(A) Image from case 1 shows extensive cortical, subcortical white matter and central gray matter abnormalities; (B) image from case 2 shows diffuse white matter increased signal in a leukodystrophy pattern; (C) image from case 3 shows mass-like, multifocal, cortical, and peripheral white matter frontoparietal abnormalities; (D) image from case 4 shows peripheral, multifocal, cortical, and juxtacortical signal changes in bilateral frontal and parietal lobes. ICP = intracranial pressure.
We did not include 4 patients who were given only acetazolamide for ICP management because this medication generally requires a period of days to weeks to take effect with oral dosing17 and variable response with IV dosing.18 In acetazolamide-treated patients, one patient presented with optic neuritis with an OP of 40 cm H2O, did not require an ICU stay, and had mRS 0 at follow-up. Another presented with cerebral monofocal/polyfocal deficits (headaches, cranial nerve VI palsy, and blurry vision) with an OP of 54 cm H2O and was managed as aseptic meningitis. She did not require an ICU stay and had mRS 0 at follow-up. Two patients presented with ADEM and had an OP of 28 and 32 cm H2O; both required an ICU stay for close observation and had mRS 0 and 2 at follow-up, respectively.
Discussion
Overall, in pediatric patients with MOGAD with OP measurements on LP at initial presentation, 20.9% had an OP > 28 cm H2O consistent with definitions for increased ICP. Patients with elevated ICP had longer hospital stays, an increased likelihood of ICU admission, and a longer stay in the ICU. They also had longer outpatient follow-up and greater likelihood of neurologic disability. Seven patients (3 from the cohort with OP measured and 4 additional patients without OP measured) required ICP-directed therapies; 4 had only medical management and 3 required additional surgical management (placement of an ICP monitor or lumbar drain) because of signs of cerebral edema and increased ICP. These data suggest that increased OP (and, by extension, increased ICP) in patients with MOGAD is associated with greater utilization of health care resources and worse outcomes. In addition, patients with MOGAD who present with ADEM phenotypes are at the highest risk of developing elevated ICP and of clinical deterioration requiring ICP-directed care in the ICU.
In the 7 patients who required medical/surgical management of increased ICP, we observed that all had restricted diffusion on brain MRI during admission. Cortical and peripheral T2 or FLAIR lesions are frequently seen in pediatric patients with MOGAD, but extensive restricted diffusion is not common.19,20 Diffusion restriction indicates cytotoxic edema with potential irreversible tissue injury. The pattern of restriction diffusion has been described with unilateral cortical FLAIR-hyperintense lesions in anti-MOG–associated encephalitis with seizures.21-23 All 7 patients in our series started with bilateral cerebral involvement and frequently progressed on subsequent scans, either becoming more confluent or with new areas of involvement. Long-term follow-up imaging revealed areas of gliosis, cortical laminar necrosis, and volume loss. Although this has been reported before,11 this imaging finding is atypical for MOGAD, in which lesions are more likely to resolve with no or minimal scarring.22 These findings in our selected cases may represent either an aggressive necrotizing form of MOGAD or secondary tissue damage from elevated ICP, low cerebral perfusion pressure, and subsequent ischemic injury.
Examining the neuropsychological outcome in the 7 cases, we found significant variability across this small subset although all had identified deficits. Most met clinical criteria for a diagnosis such as attention-deficit hyperactivity disorder, language disorder, and/or learning disorder. Notably, 3 were described as having a developmental delay, and one had a preexisting learning disorder in reading. For those with longitudinal evaluation data, reductions in performance were noted in some areas over time, illustrating the notion of “growing into deficits” when a child fails to maintain skill development at the expected rate for their age. There are numerous factors that may influence cognitive outcomes in the context of MOGAD including age at onset, time since onset, disease location and severity, brain and cognitive reserve, and sociodemographic factors, among others. Such factors should be carefully considered and integrated into future studies.
The standard treatment of acute MOGAD attacks is high-dose steroid alone or in combination with IV immunoglobulin and/or plasma exchange to control the underlying inflammation. However, in cases with elevated ICP, the addition of ICP monitoring and management strategies may be needed to mitigate secondary brain injury until immunomodulatory agents can effectively reduce the neuroinflammatory responses.
While invasive ICP monitoring has not been specifically advocated in the management of increased ICP in ADEM or other encephalitis disorders, we suggest that a pathophysiologic rationale can be made for monitoring and treatment of increased ICP in a selected subset of patients with CNS autoimmune disorders. There are 2 potential mechanisms of preventable brain injury: (1) ischemia due to low cerebral perfusion states caused by elevated ICP and (2) direct compressive caused by mass effect, such as uncal or tonsillar herniation. If undetected and/or not emergently treated before brain herniation, the brain is likely to sustain significant injury with lasting deleterious effects. In our case series, the 2 children (cases 2 and 6) who had invasive ICP monitoring early in their course and received aggressive ICP-directed care had good neurologic outcomes with mild disability. By contrast, 3 children (cases 1, 4, and 7), whose ICP monitoring was not performed or was performed late in their course after medical management (including barbiturate-induced burst suppression), demonstrated poor neurologic outcomes and significant disability (including one child ultimately dying of complications of severe brain injury). Notably, however, not all patients who have clinical or radiologic findings of increased ICP require invasive ICP monitoring. This is illustrated in cases 3 and 5, who received only hypertonic saline for management and did not have ICP monitors placed with good neurologic outcomes. In these cases, it is critical to perform frequent neurologic examinations to evaluate whether the patient is responding to therapy or declining and would benefit from more aggressive management. Whether ICP monitoring is associated with an improved outcome in patients with MOGAD with elevated ICP remains to be further studied and cannot be concluded from our case series. It could be argued that the inflammatory disease process itself was the cause of poor outcomes. However, in those with extensive lesions on MRI who required aggressive medical therapy (including burst suppression) to control ICP, neurologic morbidity was minimal in cases in which an ICP monitor was initiated early in the course compared with those without or late ICP monitor placement.
Standard therapies for ICP prevention such as head-of-bed elevation, targeting higher serum sodium levels, prevention of hypercarbia, hypoxia, and hypotension are important in preventing secondary brain injury. These may be sufficient in milder cases of increased ICP in patients with a reassuring neurologic examination. We recommend that neurosurgical services be involved early in the care of these patients, especially for those who present with ADEM with depressed mental status, OP > 28 cm H2O, or signs of diffuse cerebral edema on initial imaging. Surgical interventions can be diagnostic, such as the placement of an ICP monitor to guide medical management, or therapeutic, such as a lumbar drain or external ventricular drain (EVD) to drain CSF and reduce ICP, or decompressive craniectomy to reduce both ICP and mass effect. Early invasive ICP monitoring may also be beneficial in providing objective (numerical) data to guide ICP-based treatment pathways.24 This allows for the judicious use of interventions with a defined target point, thereby avoiding potentially harmful and/or unnecessary treatments. Owing to limited published experience with neurosurgical management of cases of autoimmune-mediated elevated ICP, decisions relating to ICP monitor placement or initiation of medical therapies to manage ICP are mainly based on the basic principles of reducing secondary brain injury mentioned above and extrapolation from the ICP and mortality benefit described in the literature on TBI and cerebral infarction, where neurosurgical intervention has been most extensively studied.25,26 In our practice, for acute diffuse intracranial processes causing a depressed neurologic examination (GCS ≤8) with signs of diffuse cerebral edema, herniation, or mass effect on imaging, placement of an ICP monitor or EVD is preferred. If the cerebral ventricles are small, an ICP transducer is implanted unless the ventricles are large enough to accurately place an EVD, which allows the therapeutic option of CSF drainage to reduce ICP. We target an ICP <20–25 mm Hg and use age-based cerebral perfusion pressure thresholds similar to published guidelines for severe TBI in children.27 The use of lumbar drainage to treat ICP can be effective, but it usually requires a functional EVD, is case-specific, and is contraindicated if imaging shows an intracranial mass lesion, herniation, or complete cisternal effacement.28 Ultimately, if ICP is refractory to medical management or if lateralizing signs due to brainstem compression or mass effect are present, decompressive craniectomy is an option; there have been case reports describing good outcomes for ADEM.29-37 None of the previously published cases, however, were tested for or reported to be positive for anti-MOG-IgG. In our cohort, none underwent decompressive craniectomy, and it remains controversial whether they would have benefited from this intervention. Although beyond the scope of our study, continuous EEG monitoring may also be a tool to monitor the severity of encephalopathy and quantify seizures that could portend severe neuronal injury.
The MOG protein is a minor component of myelin on the outermost myelin sheath layers and oligodendrocyte cell surface, making it directly accessible to the MOG antibody. The exact role of MOG antibody in the pathophysiology of MOGAD is still unclear, and there are currently no studies on the proposed mechanisms of elevated ICP in MOGAD. Because these autoantibodies do not target structures involved in the circulation of the CSF, the effect on ICP is likely indirect. Other cohort studies have shown increased ICP in CNS-demyelinating disorders, including ADEM (with anti-MOG-IgG serostatus not specified), MS, and clinically isolated syndrome.38-40 It may be that the inflammatory process itself alters the CSF flow dynamics, such as increasing viscosity of CSF and thereby impairing reabsorption at the arachnoid villi.38,39 Notably, our cohort with increased ICP had increased CSF white blood cell count compared with those with normal ICP. Future studies are needed to further understand the pathophysiology of increased ICP in MOGAD and other CNS-demyelinating disorders.
Our study has several limitations. First, it follows a retrospective design. Second, data were collected from a single academic pediatric hospital; therefore, our management practices may not be applicable to other centers. In addition, the frequency of ICP may be influenced by a major number of severe cases seen at our tertiary care center compared with other hospitals. We also reviewed patients seen in our Demyelinating Disease Clinic; it is possible that we missed potential patients who were not followed up or saved in our database. Third, only half of our cohort had OP measurements, creating a possible selection bias. We, however, found no significant difference between those who did and did not have OP measurements when examining age, sex, presence of headache on admission, GCS ≤12 on admission, ADEM presentation, or ICU stay. Fourth, while all 3 of the cases who had both OP documented and required ICP-directed care had significantly elevated OP (all >42 mm H2O), there are several inherent limitations in the use of LP OP as a proxy for ICP, including variation in pressure with patient position, patient body habitus, level of manometer, straining by the patient during the procedure, and the inability to correlate pressures with symptoms over time.41,42 As such, ICP and LP OP are not always concordant, with risk of false-negative and false-positive results. In our group analysis, we used mRS as the marker of neurologic disability, which is highly dependent on gross motor outcomes. The mRS does not account well for other aspects of long-term symptoms, such as behavior or cognition, which may have been better assessed using alternative functional scoring scales. Finally, neurocritical care management of patients with CNS autoimmune disease is not formally protocolized at our institution and individual patient care decisions are made by the attending neurosurgeon and critical care team, which likely introduced care variability.
Increased ICP complicating pediatric MOGAD is associated with greater use of hospital resources and a greater risk of neurologic disability. We suggest measuring OP as part of the standard of care when CSF analysis is performed for all potential patients with MOGAD, especially those with an ADEM phenotype, because this provides an early indicator to stratify risk of requiring ICU admission and need for medical/surgical ICP-directed care. Further studies are needed to determine which patients would benefit from ICP monitoring. Early institution of aggressive monitoring and treatment measures for increased ICP may improve long-term outcomes.
Acknowledgment
The authors thank all the patients and their caregivers.
Glossary
- ADEM
acute disseminated encephalomyelitis
- AQP4
anti-aquaporin-4
- EVD
external ventricular drain
- GCS
Glasgow Coma Scale
- HD
hospital day
- ICP
intracranial pressure
- IQR
interquartile range
- LP
lumbar puncture
- MOG
myelin oligodendrocyte glycoprotein
- mRS
modified Rankin Scale
- MS
multiple sclerosis
- OP
opening pressure
- TBI
traumatic brain injury
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
L. Nguyen, D.K. Miles, L.L. Harder, S. Singh, B.A. Whittemore, B.M. Greenberg, and C.X. Wang report no disclosures relevant to the manuscript. Unrelated to the current work, L.L. Harder has served as a consultant for Ionis Pharmaceuticals and is a member of the Medical and Scientific Council and Board of Directors for the Siegel Rare Neuroimmune Association. B.M. Greenberg has received consulting fees from Alexion, Novartis, EMD Serono, Horizon Therapeutics, Genentech/Roche, Signant, IQVIA, Sandoz, Sanofi, TG Therapeutics, Cycle Pharma, and PRIME Education. He has received grant funding from NIH, Anokion, Clene Nanomedicine, and Regeneron. He serves as an unpaid member of the board of the Siegel Rare Neuroimmune Association. He receives royalties from UpToDate. Go to Neurology.org/NN for full disclosures.
References
- 1.Narayan RN, Wang C, Greenberg BM. Acute disseminated encephalomyelitis (ADEM) and increased intracranial pressure associated with anti–myelin oligodendrocyte glycoprotein antibodies. Pediatr Neurol. 2019;99:64-68. doi: 10.1016/j.pediatrneurol.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 2.Valdrighi A, Russ J, Waubant E, Rasool N, Francisco C. Atypical myelin oligodendrocyte glycoprotein antibody disease presenting with isolated elevated intracranial pressure. Neuroimmunol Rep. 2021;1:100028. doi: 10.1016/j.nerep.2021.100028 [DOI] [Google Scholar]
- 3.Chaudhuri JR, Bagul JJ, Swathi A, Singhal BS, Reddy NC, Vallam KK. Myelin oligodendrocyte glycoprotein antibody-associated disease presenting as intracranial hypertension: a case report. Neurol Neuroimmunol Neuroinflamm. 2022;9(6):e200020. doi: 10.1212/NXI.0000000000200020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, ZhangBao J, Zhou L, et al. Encephalitis is an important clinical component of myelin oligodendrocyte glycoprotein antibody associated demyelination: a single-center cohort study in Shanghai, China. Eur J Neurol. 2019;26(1):168-174. doi: 10.1111/ene.13790 [DOI] [PubMed] [Google Scholar]
- 5.Jeantin L, Hesters A, Fournier D, et al. Anti-MOG associated disease with intracranial hypertension after COVID-19 vaccination. J Neurol. 2022;269(10):5647-5650. doi: 10.1007/s00415-022-11130-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin S, Long W, Wen J, Su Q, Liao J, Hu Z. Myelin oligodendrocyte glycoprotein antibody-associated aseptic meningitis without neurological parenchymal lesions: a novel phenotype. Mult Scler Relat Disord. 2022;68:104126. doi: 10.1016/j.msard.2022.104126 [DOI] [PubMed] [Google Scholar]
- 7.Wong WK, Troedson C, Peacock K, et al. Steroid‐responsive aseptic meningitis with raised intracranial pressure syndrome associated with myelin oligodendrocyte glycoprotein autoantibodies. J Paediatrics Child Health. 2022;58(12):2322-2326. doi: 10.1111/jpc.16189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotan I, Brody J, Hellmann MA, et al. Myelin oligodendrocyte glycoprotein-positive optic neuritis masquerading as pseudotumor cerebri at presentation. J Neurol. 2018;265(9):1985-1988. doi: 10.1007/s00415-018-8956-y [DOI] [PubMed] [Google Scholar]
- 9.Narayan RN, Wang C, Sguigna P, Husari K, Greenberg B. Atypical anti-MOG syndrome with aseptic meningoencephalitis and pseudotumor cerebri-like presentations. Mult Scler Relat Disord. 2019;27:30-33. doi: 10.1016/j.msard.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Rempe T, Tarhan B, Rodriguez E, et al. Anti-MOG associated disorder–clinical and radiological characteristics compared to AQP4-IgG+ NMOSD–a single-center experience. Mult Scler Relat Disord. 2021;48:102718. doi: 10.1016/j.msard.2020.102718 [DOI] [PubMed] [Google Scholar]
- 11.Armangue T, Olivé-Cirera G, Martínez-Hernandez E, et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: a multicentre observational study. Lancet Neurol. 2020;19(3):234-246. doi: 10.1016/S1474-4422(19)30488-0 [DOI] [PubMed] [Google Scholar]
- 12.Banwell B, Bennett JL, Marignier R, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: international MOGAD Panel proposed criteria. Lancet Neurol. 2023;22(3):268-282. doi: 10.1016/S1474-4422(22)00431-8 [DOI] [PubMed] [Google Scholar]
- 13.Bruijstens AL, Lechner C, Flet-Berliac L, et al. E.U. paediatric MOG consortium consensus: part 1–classification of clinical phenotypes of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatric Neurol. 2020;29:2-13. doi: 10.1016/j.ejpn.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 14.Avery RA. Reference range of cerebrospinal fluid opening pressure in children: historical overview and current data. Neuropediatrics. 2014;45(4):206-211. doi: 10.1055/s-0034-1376202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bigi S, Fischer U, Wehrli E, et al. Acute ischemic stroke in children versus young adults. Ann Neurol. 2011;70(2):245-254. doi: 10.1002/ana.22427 [DOI] [PubMed] [Google Scholar]
- 16.Kamate M. Acute leukoencephalopathy with restricted diffusion. Indian J Crit Care Med. 2018;22(7):519-523. doi: 10.4103/ijccm.IJCCM_139_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoeman JF. Childhood pseudotumor cerebri: clinical and intracranial pressure response to acetazolamide and furosemide treatment in a case series. J Child Neurol. 1994;9(2):130-134. doi: 10.1177/088307389400900205 [DOI] [PubMed] [Google Scholar]
- 18.Rubin RC, Henderson ES, Ommaya AK, Walker MD, Rall DP. The production of cerebrospinal fluid in man and its modification by acetazolamide. J Neurosurg. 1966;25(4):430-436. doi: 10.3171/jns.1966.25.4.0430 [DOI] [PubMed] [Google Scholar]
- 19.Dutra BG, da Rocha AJ, Nunes RH, Maia ACM. Neuromyelitis optica spectrum disorders: spectrum of MR imaging findings and their differential diagnosis. Radiographics. 2018;38(1):169-193. doi: 10.1148/rg.2018170141 [DOI] [PubMed] [Google Scholar]
- 20.Shahriari M, Sotirchos ES, Newsome SD, Yousem DM. MOGAD: how it differs from and resembles other neuroinflammatory disorders. AJR Am J Roentgenol. 2021;216(4):1031-1039. doi: 10.2214/AJR.20.24061 [DOI] [PubMed] [Google Scholar]
- 21.Fujimori J, Takai Y, Nakashima I, et al. Bilateral frontal cortex encephalitis and paraparesis in a patient with anti-MOG antibodies. J Neurol Neurosurg Psychiatry. 2017;88(6):534-536. doi: 10.1136/jnnp-2016-315094 [DOI] [PubMed] [Google Scholar]
- 22.Salama S, Khan M, Shanechi A, Levy M, Izbudak I. MRI differences between MOG antibody disease and AQP4 NMOSD. Mult Scler. 2020;26(14):1854-1865. doi: 10.1177/1352458519893093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budhram A, Mirian A, Sharma M. Meningo-cortical manifestations of myelin oligodendrocyte glycoprotein antibody-associated disease: review of a novel clinico-radiographic spectrum. Front Neurol. 2022;13:1044642. doi: 10.3389/fneur.2022.1044642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75(6):813-821. doi: 10.1136/jnnp.2003.033126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahuquillo J, Dennis JA. Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. Cochrane Database Syst Rev. 2019;12:CD003983. doi: 10.1002/14651858.CD003983.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dower A, Mulcahy M, Maharaj M, et al. Surgical decompression for malignant cerebral oedema after ischaemic stroke. Cochrane Database Syst Rev. 2022;11(11):CD014989. doi: 10.1002/14651858.CD014989.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kochanek PM, Tasker RC, Carney N, et al. Guidelines for the management of pediatric severe traumatic brain injury, third edition: update of the brain trauma foundation guidelines, executive summary. Neurosurgery. 2019;84(6):1169-1178. doi: 10.1093/neuros/nyz051 [DOI] [PubMed] [Google Scholar]
- 28.Stevens AR, Soon W, Chowdhury YA, et al. External lumbar drainage for refractory intracranial hypertension in traumatic brain injury: a systematic review. Cureus. 2022;14(10):e30033. doi: 10.7759/cureus.30033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alawadhi A, Saint-Martin C, Bhanji F, Srour M, Atkinson J, Sébire G. Acute hemorrhagic encephalitis responding to combined decompressive craniectomy, intravenous immunoglobulin, and corticosteroid therapies: association with novel RANBP2 variant. Front Neurol. 2018;9:130. doi: 10.3389/fneur.2018.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granget E, Milh M, Pech-Gourg G, et al. Life-saving decompressive craniectomy for acute disseminated encephalomyelitis in a child: a case report. Childs Nerv Syst. 2012;28(7):1121-1124. doi: 10.1007/s00381-012-1733-9 [DOI] [PubMed] [Google Scholar]
- 31.Pohl D, Tenembaum S. Treatment of acute disseminated encephalomyelitis. Curr Treat Options Neurol. 2012;14(3):264-275. doi: 10.1007/s11940-012-0170-0 [DOI] [PubMed] [Google Scholar]
- 32.Ahmed A, Eynon C, Kinton L, Nicoll J, Belli A. Decompressive craniectomy for acute disseminated encephalomyelitis. Neurocrit Care. 2010;13(3):393-395. doi: 10.1007/s12028-010-9420-8 [DOI] [PubMed] [Google Scholar]
- 33.Sekula RF, Marchan EM, Baghai P, Jannetta PJ, Quigley MR. Central brain herniation secondary to fulminant acute disseminated encephalomyelitis: implications for neurosurgical management. Case report. J Neurosurg. 2006;105(3):472-474. doi: 10.3171/jns.2006.105.3.472 [DOI] [PubMed] [Google Scholar]
- 34.Rabee H, Gharbeyah M, Abuhassan A, Ziyadeh J, Hamayel H, Adas A. Decompressive hemicraniectomy for severe acute disseminated encephalomyelitis: a case report. Ann Clin Case Rep. 2021;6:1972. [Google Scholar]
- 35.Dombrowski KE, Mehta AI, Turner DA, Mcdonagh DL. Life-saving hemicraniectomy for fulminant acute disseminated encephalomyelitis. Br J Neurosurg. 2011;25(2):249-252. doi: 10.3109/02688697.2010.544784 [DOI] [PubMed] [Google Scholar]
- 36.von Stuckrad–Barre S, Klippel E, Foerch C, Lang J-M, du Mesnil de Rochemont R, Sitzer M. Hemicraniectomy as a successful treatment of mass effect in acute disseminated encephalomyelitis. Neurology. 2003;61(3):420-421. doi: 10.1212/01.wnl.0000073540.35919.ae [DOI] [PubMed] [Google Scholar]
- 37.Mazzola MA, Yalcin N, Khadjvand M, Lerner D, Chaves C. Adult-Onset Recurrent Fulminant Acute Disseminated Encephalomyelitis (ADEM) Requiring Hemicraniectomy–A Case Report (2140). AAN Enterprises; 2020. [Google Scholar]
- 38.Narula S, Liu GT, Avery RA, Banwell B, Waldman AT. Elevated cerebrospinal fluid opening pressure in a pediatric demyelinating disease cohort. Pediatr Neurol. 2015;52(4):446-449. doi: 10.1016/j.pediatrneurol.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan-Followell B, Aylward SC. Comparison of cerebrospinal fluid opening pressure in children with demyelinating disease to children with primary intracranial hypertension. J Child Neurol. 2017;32(4):366-370. doi: 10.1177/0883073816681936 [DOI] [PubMed] [Google Scholar]
- 40.Orbach R, Schneebaum Sender N, Lubetzky R, Fattal-Valevski A. Increased intracranial pressure in acute disseminated encephalomyelitis. J Child Neurol. 2019;34(2):99-103. doi: 10.1177/0883073818811541 [DOI] [PubMed] [Google Scholar]
- 41.Cartwright C, Igbaseimokumo U. Lumbar puncture opening pressure is not a reliable measure of intracranial pressure in children. J Child Neurol. 2015;30(2):170-173. doi: 10.1177/0883073814533006 [DOI] [PubMed] [Google Scholar]
- 42.Pedersen SH, Andresen M, Lilja-Cyron A, Petersen LG, Juhler M. Lumbar puncture position influences intracranial pressure. Acta Neurochir (Wien). 2021;163(7):1997-2004. doi: 10.1007/s00701-021-04813-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.