Abstract
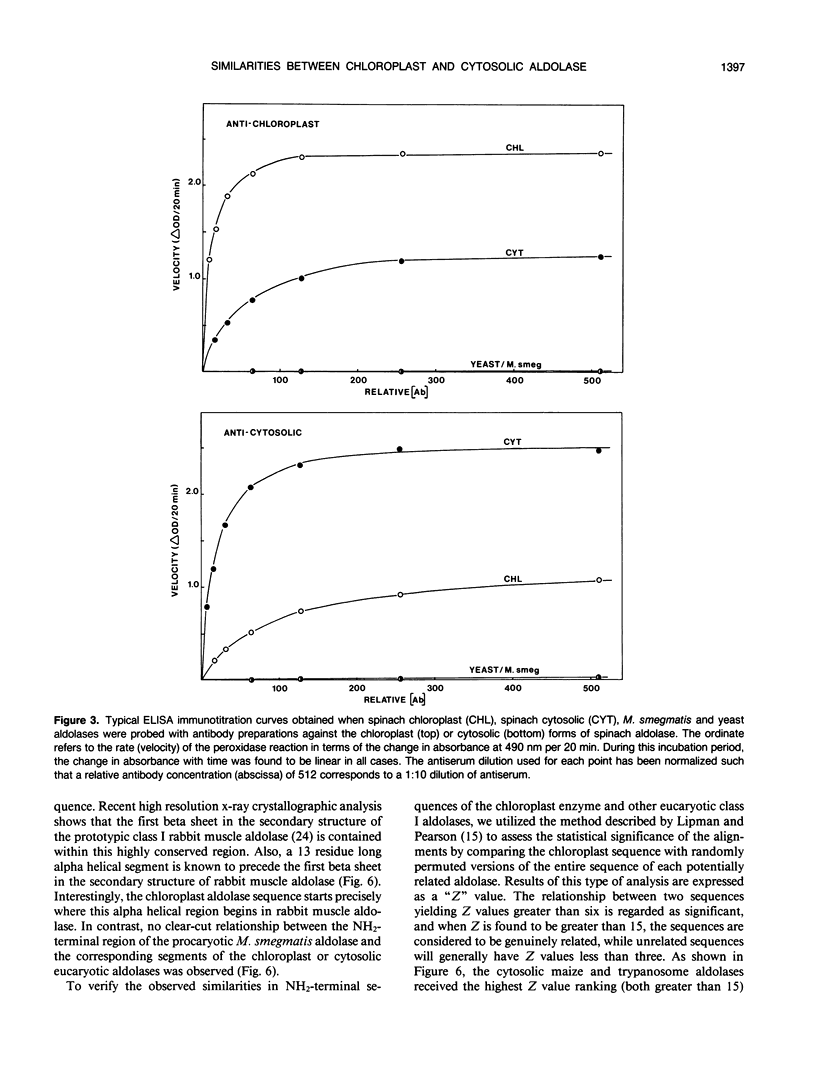
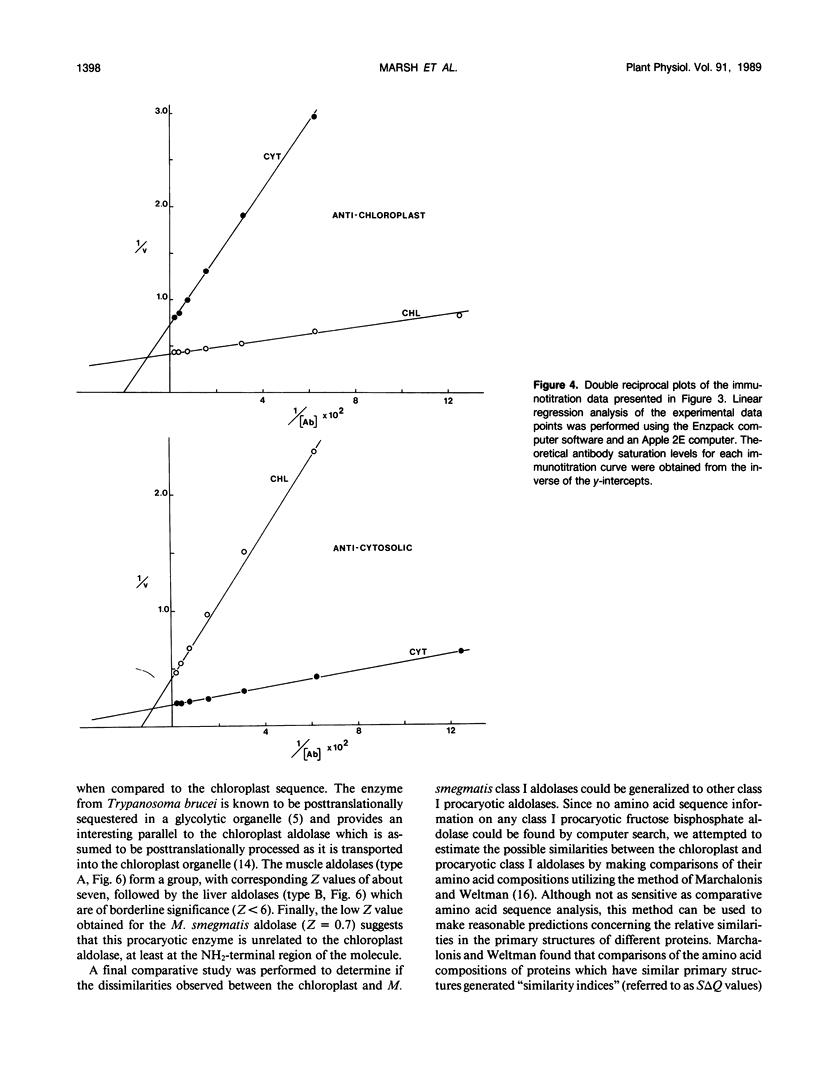
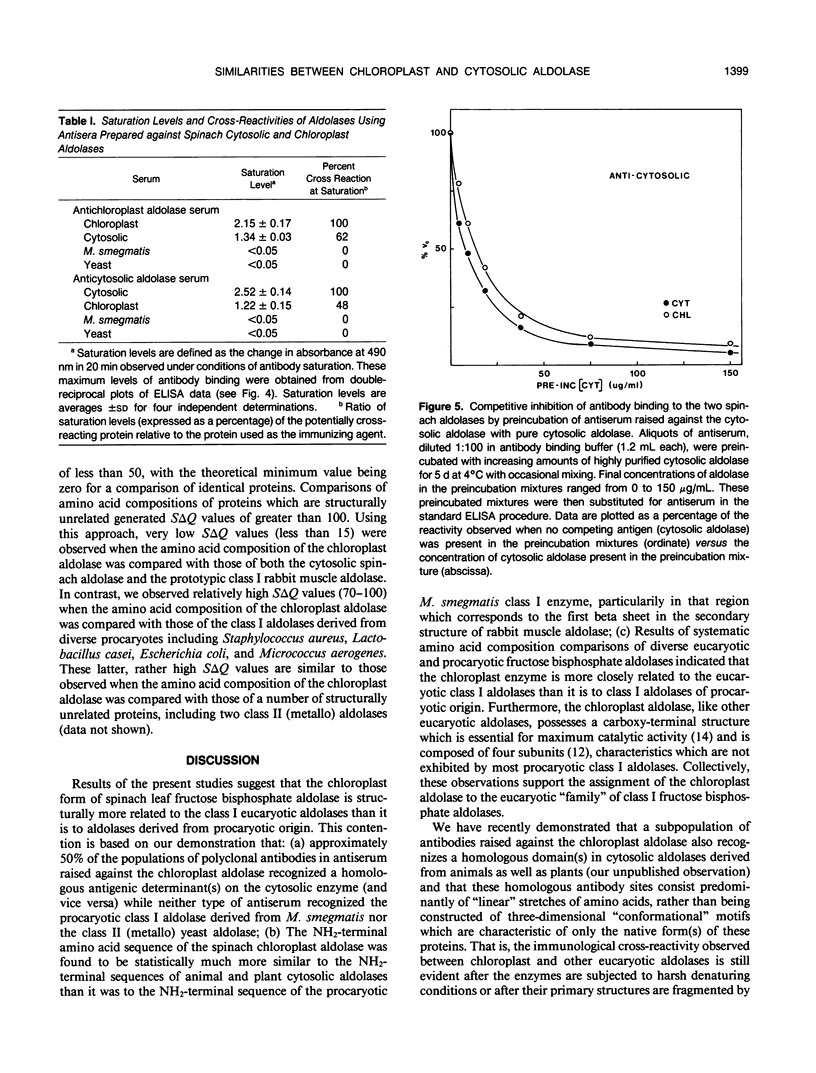
Immunochemical studies using polyclonal antisera prepared individually against highly purified cytosolic and chloroplast spinach leaf (Spinacia oleracea) fructose bisphosphate aldolases showed significant cross reaction between both forms of spinach aldolase and their heterologous antisera. The individual cross reactions were estimated to be approximately 50% in both cases under conditions of antibody saturation using a highly sensitive enzyme-linked immunosorbent assay. In contrast, the class I procaryotic aldolase from Mycobacterium smegmatis and the class II aldolase from yeast (Saccharomyces cerevisiae) did not cross-react with either type of antiserum. The 29 residue long amino-terminal amino acid sequences of the procaryotic M. smegmatis and the spinach chloroplast aldolases were determined. Comparisons of these sequences with those of other aldolases showed that the amino-terminal primary structure of the chloroplast aldolase is much more similar to the amino-terminal structures of class I cytosolic eucaryotic aldolases than it is to the corresponding region of the M. smegmatis enzyme, especially in that region which forms the first “beta sheet” in the secondary structure of the eucaryotic aldolases. Moreover, results of a systematic comparison of the amino acid compositions of a number of diverse eucaryotic and procaryotic fructose bisphosphate aldolases further suggest that the chloroplast aldolase belongs to the eucaryotic rather than the procaryotic “family” of class I aldolases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E. Chloroplast and Cytoplasmic Enzymes: VIII. Amino Acid Composition of the Pea Leaf Aldolases. Plant Physiol. 1979 Sep;64(3):404–405. doi: 10.1104/pp.64.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Heinrikson R. L., Nyes C. Chloroplast and cytoplasmic enzymes 1, 2, 3. Subunit structure of pea leaf aldolases. Arch Biochem Biophys. 1975 Jul;169(1):262–268. doi: 10.1016/0003-9861(75)90340-9. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Levin D. A. Chloroplast aldolase is controlled by a nuclear gene. Plant Physiol. 1970 Dec;46(6):819–820. doi: 10.1104/pp.46.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Pacold I. Chloroplast and Cytoplasmic Enzymes: IV. Pea Leaf Fructose 1,6-Diphosphate Aldolases. Plant Physiol. 1972 Mar;49(3):393–397. doi: 10.1104/pp.49.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E. Structure and regulated expression of genes encoding fructose biphosphate aldolase in Trypanosoma brucei. EMBO J. 1985 Nov;4(11):2997–3003. doi: 10.1002/j.1460-2075.1985.tb04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabius H. J., Engelhardt R., Schröder F. R., Cramer F. Evolutionary aspects of accuracy of phenylalanyl-tRNA synthetase. Accuracy of fungal and animal mitochondrial enzymes and their relationship to their cytoplasmic counterparts and a prokaryotic enzyme. Biochemistry. 1983 Nov 8;22(23):5306–5315. doi: 10.1021/bi00292a009. [DOI] [PubMed] [Google Scholar]
- Gottlieb L. D. Conservation and duplication of isozymes in plants. Science. 1982 Apr 23;216(4544):373–380. doi: 10.1126/science.216.4544.373. [DOI] [PubMed] [Google Scholar]
- Jayanthi Bia N., Ramachandra Pai M., Suryanarayana Murthy P., Venkitasubramanian T. A. Fructose diphosphate aldolase from Mycobacterium smegmatis. Purification and properties. Arch Biochem Biophys. 1975 May;168(1):230–234. doi: 10.1016/0003-9861(75)90245-3. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Tolan D. R. The complete amino Acid sequence for the anaerobically induced aldolase from maize derived from cDNA clones. Plant Physiol. 1986 Dec;82(4):1076–1080. doi: 10.1104/pp.82.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., de Vos A., Ogata C. Crystal structures of two intensely sweet proteins. Trends Biochem Sci. 1988 Jan;13(1):13–15. doi: 10.1016/0968-0004(88)90011-4. [DOI] [PubMed] [Google Scholar]
- Krüger I., Schnarrenberger C. Purification, subunit structure and immunological comparison of fructose-bisphosphate aldolases from spinach and corn leaves. Eur J Biochem. 1983 Oct 17;136(1):101–106. doi: 10.1111/j.1432-1033.1983.tb07711.x. [DOI] [PubMed] [Google Scholar]
- Lebherz H. G., Leadbetter M. M., Bradshaw R. A. Isolation and characterization of the cytosolic and chloroplast forms of spinach leaf fructose diphosphate aldolase. J Biol Chem. 1984 Jan 25;259(2):1011–1017. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- RUTTER W. J. EVOLUTION OF ALDOLASE. Fed Proc. 1964 Nov-Dec;23:1248–1257. [PubMed] [Google Scholar]
- Raff R. A., Mahler H. R. The non symbiotic origin of mitochondria. Science. 1972 Aug 18;177(4049):575–582. doi: 10.1126/science.177.4049.575. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Krüger I. Distinction between Cytosol and Chloroplast Fructose-Bisphosphate Aldolases from Pea, Wheat, and Corn Leaves. Plant Physiol. 1986 Feb;80(2):301–304. doi: 10.1104/pp.80.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C. Regulation and structure of isozymes of sugar phosphate metabolism in plants. Isozymes Curr Top Biol Med Res. 1987;16:223–240. [PubMed] [Google Scholar]
- Shih M. C., Lazar G., Goodman H. M. Evidence in favor of the symbiotic origin of chloroplasts: primary structure and evolution of tobacco glyceraldehyde-3-phosphate dehydrogenases. Cell. 1986 Oct 10;47(1):73–80. doi: 10.1016/0092-8674(86)90367-3. [DOI] [PubMed] [Google Scholar]
- Sygusch J., Beaudry D., Allaire M. Molecular architecture of rabbit skeletal muscle aldolase at 2.7-A resolution. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7846–7850. doi: 10.1073/pnas.84.22.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeden N. F. Evolutionary affinities of plant phosphoglucose isomerase and fructose-1,6-bisphosphatase isozymes. Isozymes Curr Top Biol Med Res. 1983;8:53–66. [PubMed] [Google Scholar]
- Weeden N. F. Genetic and biochemical implications of the endosymbiotic origin of the chloroplast. J Mol Evol. 1981;17(3):133–139. doi: 10.1007/BF01733906. [DOI] [PubMed] [Google Scholar]
- Willard J. M., Gibbs M. Role of Aldolase in Photosynthesis. II Demonstration of Aldolase Types in Photosynthetic Organisms. Plant Physiol. 1968 May;43(5):793–798. doi: 10.1104/pp.43.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]