Abstract
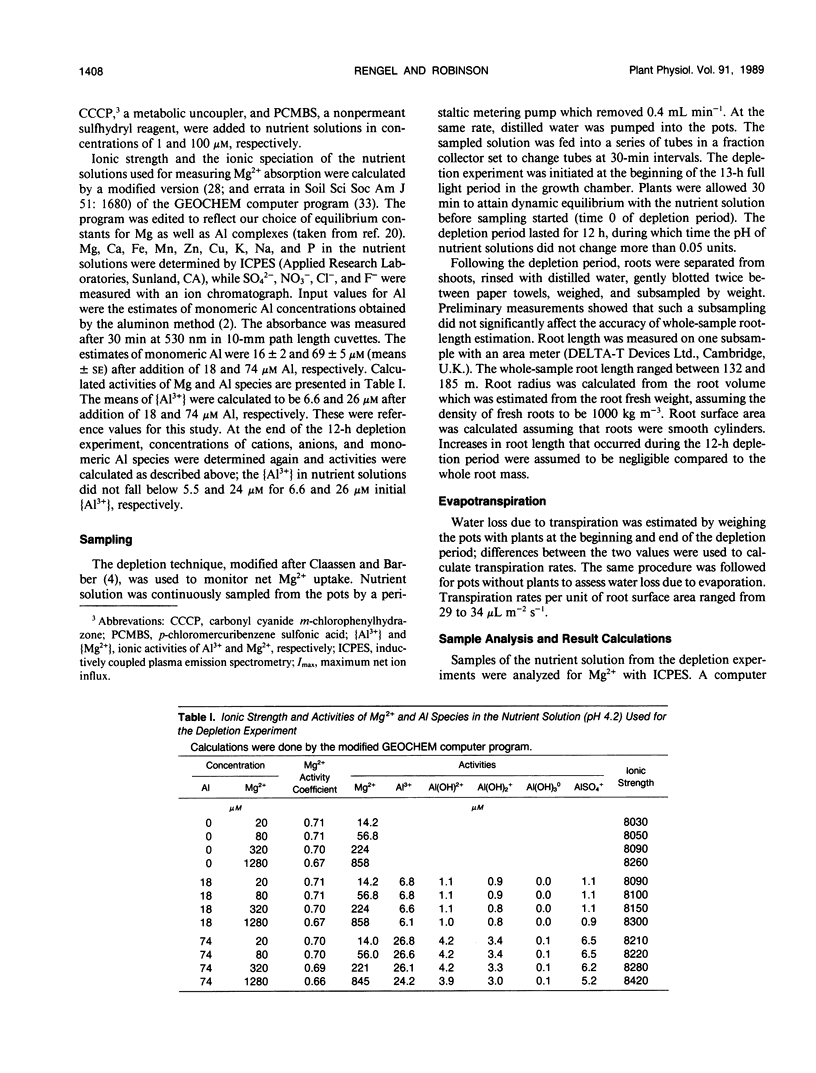
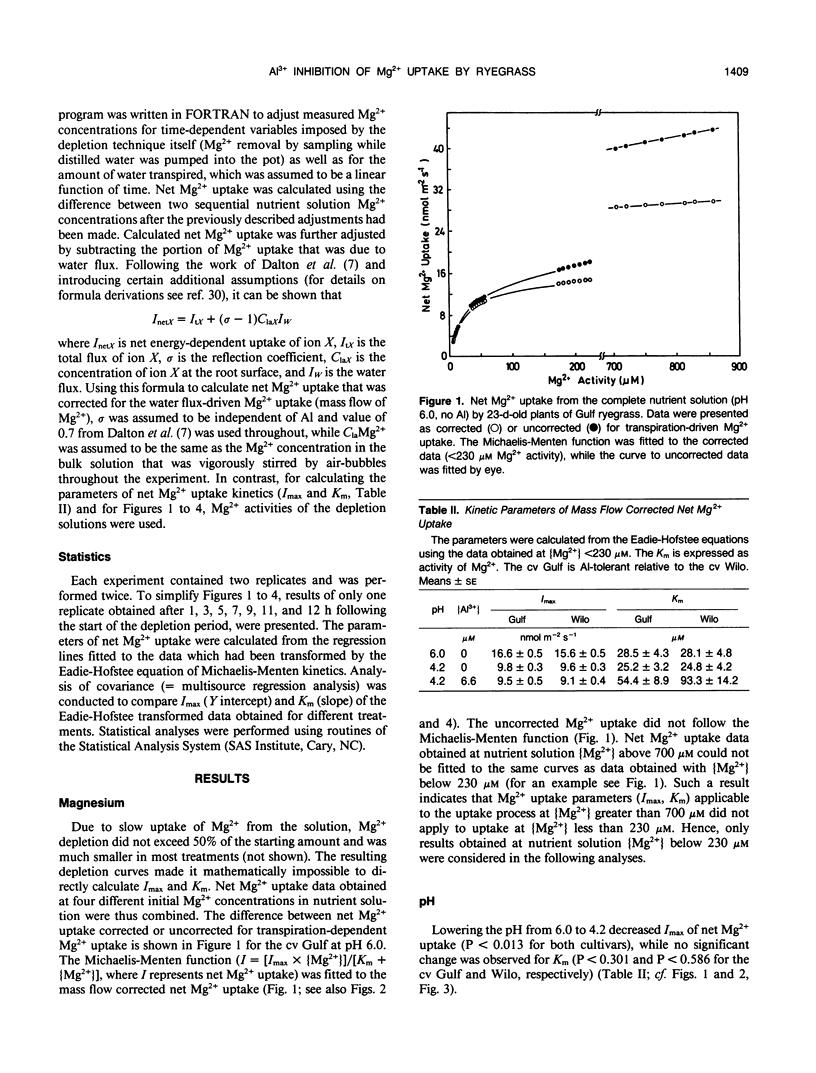
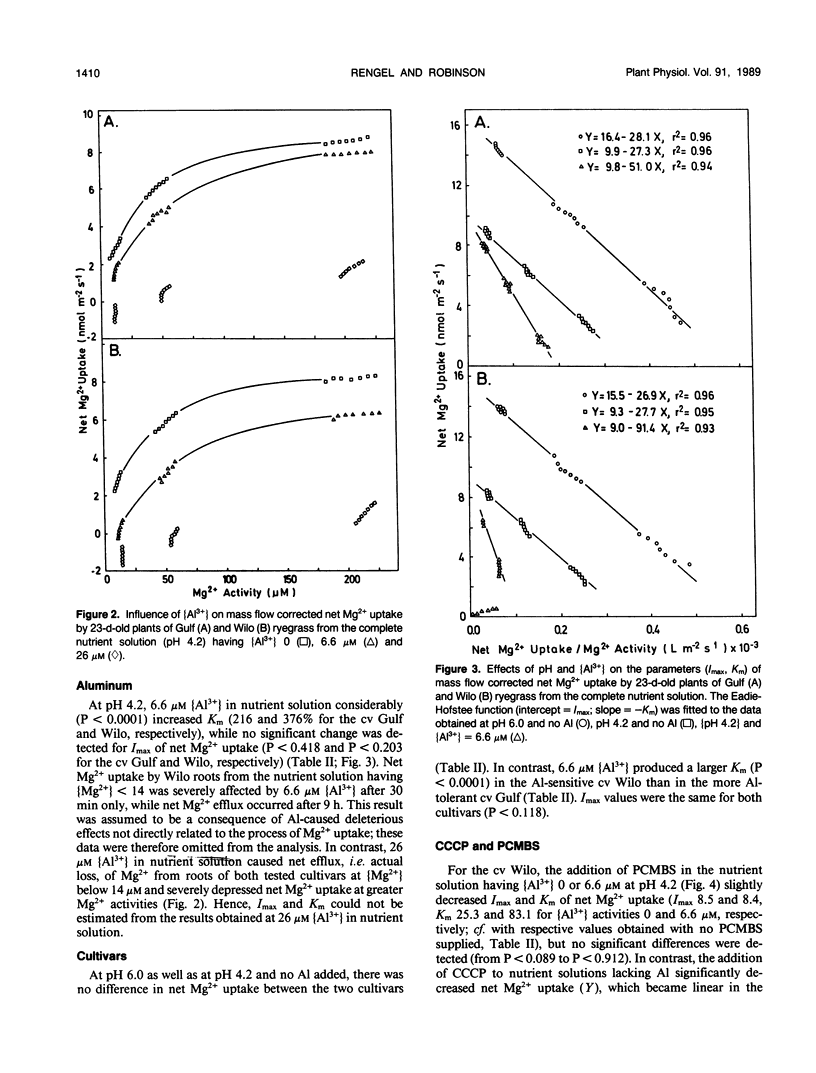
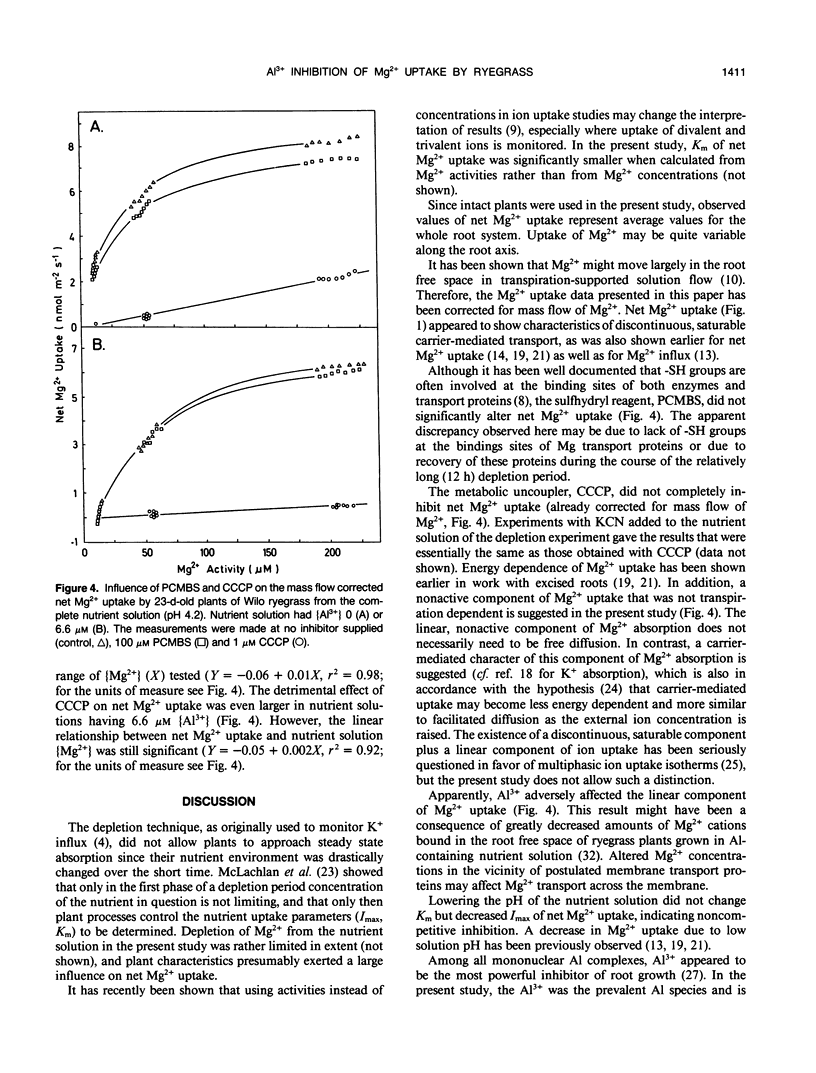
Aluminum impairs uptake of Mg2+, but the mechanisms of this inhibition are not understood. The depletion technique was used to monitor net Mg2+ uptake from nutrient solution by intact, 23-day-old plants of ryegrass (Lolium multiflorum Lam., cv Gulf and Wilo). Activities of Mg2+ and monomeric Al species in nutrient solution were calculated and used as the basis for expressing the results. The kinetics of net Mg2+ absorption was resolved into (a) a transpiration-dependent uptake component, (b) a metabolically mediated, discontinuous saturable component that is Al3+ sensitive and p-chloromercuribenzene sulfonic acid (PCMBS) resistant, and (c) a linear, carbonyl cyanide m-chlorophenylhydrazone resistant, Al3+ sensitive component that might be a type of facilitated diffusion. Lowering the pH from 6.0 to 4.2 exerted a noncompetitive inhibition of net Mg2+ uptake, while aluminum at 6.6 micromolar Al3+ activity exerted competitive inhibition of net Mg2+ uptake at pH 4.2. The Al3+-induced effect was obvious after 30 minutes. Cultivar-specific ability to retain a higher affinity for Mg2+ by postulated transport proteins in the presence of Al3+ might be one of the mechanisms of differential Al tolerance among ryegrass cultivars.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bock J. L. The binding of metal ions to ATP: a proton and phosphorus nmr investigation of diamagnetic metal--ATP complexes. J Inorg Biochem. 1980 Apr;12(2):119–130. doi: 10.1016/s0162-0134(00)80123-3. [DOI] [PubMed] [Google Scholar]
- Claassen N., Barber S. A. A Method for Characterizing the Relation between Nutrient Concentration and Flux into Roots of Intact Plants. Plant Physiol. 1974 Oct;54(4):564–568. doi: 10.1104/pp.54.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Parker D. R. Cation amelioration of aluminum toxicity in wheat. Plant Physiol. 1987 Mar;83(3):546–551. doi: 10.1104/pp.83.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B. Proton extrusion by wheat roots exhibiting severe aluminum toxicity symptoms. Plant Physiol. 1988 Oct;88(2):418–423. doi: 10.1104/pp.88.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L. V., Lucas W. J. Potassium transport in corn roots : I. Resolution of kinetics into a saturable and linear component. Plant Physiol. 1982 Dec;70(6):1723–1731. doi: 10.1104/pp.70.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett J. E., Gilbert W. A. Magnesium uptake by soybeans. Plant Physiol. 1969 Aug;44(8):1182–1186. doi: 10.1104/pp.44.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas E. V., Ogata G. Absorption of magnesium and chloride by excised corn root. Plant Physiol. 1971 Mar;47(3):357–360. doi: 10.1104/pp.47.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald T. L., Martin R. B. Aluminum ion in biological systems. Trends Biochem Sci. 1988 Jan;13(1):15–19. doi: 10.1016/0968-0004(88)90012-6. [DOI] [PubMed] [Google Scholar]
- Pfeffer P. E., Tu S. I., Gerasimowicz W. V., Cavanaugh J. R. In VivoP NMR Studies of Corn Root Tissue and Its Uptake of Toxic Metals. Plant Physiol. 1986 Jan;80(1):77–84. doi: 10.1104/pp.80.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. J., Sucoff E., Stadelmann E. J. Al and Ca Alteration of Membrane Permeability of Quercus rubra Root Cortex Cells. Plant Physiol. 1987 Jan;83(1):159–162. doi: 10.1104/pp.83.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]


