Abstract
Introduction
Parkinson’s disease is neurodegenerative, complex and progressive, manifesting in a slow and irreversible way. Physical exercise has been proposed as therapeutic alternative to people with Parkinson´s disease.
Objective
To synthesize knowledge about the effects of physical exercise on people with Parkinson´s Disease as presented by published systematic reviews.
Methods
Nine electronic databases and two grey literature databases were searched for systematic reviews reporting the effects of physical exercises on people with Parkinson´s Disease. Searches involved a two-phase process, by, at least, two independent reviewers. Methodological quality of the included systematic reviews was assessed using AMSTAR-2.
Results
From 2,122 systematic reviews, 139 were included. Motor outcomes were assessed in 91% of the studies, with balance being the most studied. Non-motor outcomes were assessed in 68% of the studies, with emphasis on quality of life. Physical exercises were classified into five categories: aerobic exercises, strength, combined, sensorimotor activities and other activity protocols. Findings of the systematic reviews suggest that all exercise categories can be prescribed to improve balance and mobility, while combined exercises, strength, and specific activities improve both motor and non-motor outcomes, and aerobic exercise and sensorimotor activities improve motor outcomes.
Conclusion
Current evidence from systematic reviews suggests that physical exercises impacts both motor and non-motor outcomes in people with Parkinson´s Disease. Limits in evidence provided by the systematic reviews were related to methodological issues and to the description of the interventions and must be considered to improve decision-making and clinical application.
Introduction
Neurological disorders are the main cause of morbidities and functional disability in the world [1]. Parkinson´s disease (PD) is one of the most common neurological disorders, along with Alzheimer’s disease (AD), Huntington’s disease, amyotrophic lateral sclerosis and frontotemporal dementia [2]. These diseases may vary in their pathophysiology, but they have in common their association with population aging, and the common presence of aggregated protein forms in the brain of affected individuals [3].
PD presents important motor symptoms, namely bradykinesia, muscle rigidity, bent posture, motor blocking, postural instability and tremor. The level of functional disability may be determined by the 5-point Hoehn and Yahr scale, which consider stages 1 to 3 as minimally disabled, meaning that they are still able to live independently. Stages 4 and 5, on the other hand, characterize severely disabled people and has been associated with highly compromised neurocognitive issues [4,5]. Physical impairment caused by PD may be potentiated by sarcopenia (loss of muscle mass) and osteoporosis (loss of bone mass), conditions common to old people that often coexist [6].
According to the Global Burden of Diseases [1], PD is the fastest growing, as the population ages and life expectancy increases. In 2016, PD caused more than 211 thousand deaths (93.5 thousand women and 117.5 thousand men), in addition to 3.2 million cases of functional disability (1.4 million women and 1 .8 million in men) [1]. Expectations are that the number of individuals with PD, as well as the duration of the disease will continue to increase, demanding effective prevention and treatment strategies [7].
In general, physical exercises have been proposed as an efficient intervention in the treatment of several chronic conditions, with positive responses on blood pressure [8], prevention and treatment of diabetes [9], improvement of lipoprotein profile, increase of insulin sensitivity, help on weight control [10], prevention and improvement of mild conditions of depressive disorders, anxiety, dyspnea, and quality of life [11], improvement of physical fitness, cognitive functioning, and mind-body connection [12] and production of neurogenesis and neuroprotection [13]. Although there is no cure for PD, exercise protocols involving gait training, balance and muscle strengthening have shown important effects on the physical capacity of patients [14], being considered a safe and effective approach [15].
Regular physical exercise, especially aerobic exercise is beneficial for patients with PD, as it reduces hypokinesia, bradykinesia, gait disturbances, neuronal degeneration, loss of independence to perform activities of daily living (ADLs) and maintains the cardiovascular capacity in individuals classified with mild and moderate PD [16]. The literature presents dozens of systematic reviews (SR) that analyze the effect of physical exercise in people with PD. Several types of exercises are found along with a diversity of parameters for the application of these exercises, which, in turn, can make their prescription difficult. There are several exercise protocols and an enormous variability of elements that make up the exercise dose, such as type, weekly frequency, volume, intensity, duration of intervention, among others, making it difficult to establish a more adequate protocol. Considering the complexity of this scenario, this umbrella review aimed to collect the evidence of exercise in PD.
Methodology
Study design
This study was first conceived as a scoping review of SR, therefore, an umbrella review [17], and had its protocol registered in Open Science Framework (https://osf.io/knjuq/). The research question was structured based on PCC acronym: P (population) = people with Parkinson´s disease; C (concept) = physical exercise; C (context) = global context/impact on motor and non-motor symptoms.
Eligibility criteria and search
Systematic reviews of physical exercises as interventions to people with PD, regardless of the level of disability were considered eligible and were searched in the following electronic databases: Cochrane Library, CINAHL, EMBASE, PEDro, Medline via PubMed, LILACS via BVS, DARE, Scopus, SPORTDiscus and Web of Science. Grey literature was also searched in Grey Matters and national and international universities and research centers catalogs containing theses and dissertations. There was no restriction based on place (origin), language, date, and age/sex of participants. The references of included studies were also searched. Studies that did not fulfill the criteria for population, intervention and type of study were excluded.
The search strategy was built with the aid of an experienced librarian (CSS) using the MeSH terms (Medical Subject Headings) (https://www.ncbi.nlm.nih.gov/mesh/), DeCS (https://decs.bvsalud.org/) and EMTREE (https://www-embase.ez224.periodicos.capes.gov.br/emtree) as indexed terms, along with terms from natural language and Boolean operators to combine them. The search strategy was first built in PubMed and was repeated in the other databases respecting their own syntax rules, to ensure that no relevant study was lost. The search strategy was built from inception and the later search was performed on September 9th, 2022. Table 1 presents the search strategy developed for PubMed. The search strategies applied to the other databases may be found in S1 Table.
Table 1. Search strategy applied to PubMed database.
| ("Parkinson’s Disease" OR "Idiopathic Parkinson’s Disease" OR "Lewy Body Parkinson Disease" OR "Lewy Body Parkinson’s Disease" OR "Primary Parkinsonism" OR "Parkinsonism, Primary" OR "Parkinson Disease, Idiopathic" OR "Parkinson’s Disease" OR "Parkinson’s Disease, Idiopathic" OR "Parkinson’s Disease, Lewy Body" OR "Idiopathic Parkinson Disease" OR "Paralysis Agitans") AND (Exercise* OR "Physical Activity" OR "Activities, Physical" OR "Activity, Physical" OR "Physical Activities" OR "Exercise, Physical" OR "Exercises, Physical" OR "Physical Exercise" OR "Physical Exercises" OR "Acute Exercise" OR "Acute Exercises" OR "Exercise, Acute" OR "Exercises, Acute" OR "Exercise, Isometric" OR "Exercises, Isometric" OR "Isometric Exercises" OR "Isometric Exercise" OR "Exercise, Aerobic" OR "Aerobic Exercise" OR "Aerobic Exercises" OR "Exercises, Aerobic" OR "Exercise Training" OR "Exercise Trainings" OR "Training, Exercise" OR "Trainings, Exercise") AND ("systematic review" OR "systematic literature review" OR "systematic scoping review" OR "systematic narrative review" OR "systematic qualitative review" OR "systematic evidence review" OR "systematic meta-review" OR "systematic critical review" OR "systematic mixed studies review" OR "systematic mapping review" OR "systematic cochrane review" OR "systematic search and review" OR "systematic integrative review" OR "scoping review") |
Study selection
The studies were selected in a two-phase process. First, they were selected by reading of title and abstract. For that, the references databanks were uploaded to MyEndNote Desktop where duplicates were removed. Following, the resulting dataset was uploaded to Rayyan, where the first phase took place. The second phase involved full-text reading for selection. Both were conducted by two previously trained reviewers (CP and RS) and when there were divergencies, they were solved by consensus involving two other reviewers (CAS and SARJ).
Data extraction
Data were extracted from the studies independently by four reviewers (CP, RS, FGS and APG) and filled in a previously prepared Excel worksheet (Microsoft Excel 2013, Microsoft Corporation), which was tested and revised by other researchers (CAS and SARJ). The following information was extracted: regarding the studies (authors, year of publication, country of origin, title, purpose, study design, conclusions), the population (sample size, age, sex, diagnostic classification), related to the interventions (type of training, session duration, training time, intensity, frequency and control groups), outcomes (motor and non-motor outcomes, outcome measures and main results). The methodological quality of the study was assessed by four reviewers (CP, RS, FGS and APG) using the A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2). AMSTAR 2 is a 16-item, domain-based instrument meant to critically appraise systematic reviews of randomized controlled trials [18]. Seven domains of the instrument are considered critical to rate the methodological quality of the study, namely protocol registration before commencement of the review, adequacy of the literature search, justification for excluding individual studies, risk of bias from individual studies included in the review, appropriateness of meta-analytical methods, consideration of risk of bias when interpreting the results of the review and assessment of the likely impact of publication bias [18,19]. The identification of weaknesses in these critical domains allows one to correctly interpret the information provided by the systematic reviews, and provides four classification levels: ‘high quality’, ‘moderate quality’, ‘low quality’ and ‘critically low quality’ evidence [18].
Synthesis
Data were synthetized based on descriptive mapping of the breadth of research on the issue and a narrative summary.
Results
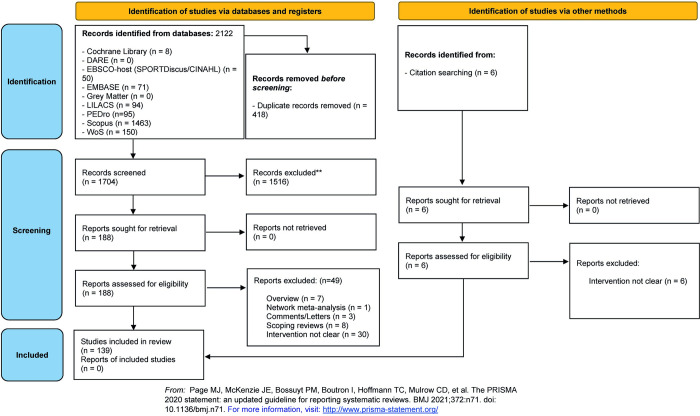
The study flow diagram is presented in Fig 1. Database records totalized 2,122 references, which were reduced to 1704 studies after duplicate removal. A total of 188 studies were analyzed by full-text reading from which 49 studies were excluded. The reasons for exclusion are depicted in the study flow diagram. Finally, 139 studies were included and had data extracted for the review.
Fig 1. Study flow diagram.
A significant number of physical exercise interventions was identified in the included systematic reviews and was categorized into five distinct categories: aerobic exercise, strength exercise, combined exercise, sensory-motor activities and other protocols. These categories are described in Table 2, along with the interventions classified in each category.
Table 2. Categorization of the physical exercises identified.
| Category | Physical exercises |
|---|---|
| AEROBIC EXERCISE: Involves movements of the large muscles of the body in a rhythmic manner for prolonged periods [20] | Cycling, treadmill, walking, Qigong, cross training, rowing ergometer, robot-assisted gait training, Nordic walking, endurance, rhythm retraining, treadmill walking, HIIT-LOW on treadmill, resistance training, elliptic, bike exercise, jogging, gait training with Body Weight Support (BWS), interval training, forced cycling exercise, Sitting and Standing; functional exercise; circuit training, vigorous intensity exercise, split belt treadmill (SBT). |
| STRENGTH EXERCISE: Activities in which muscles work or hold against an applied force or weight to improve muscle fitness (i.e., functional parameters of strength, endurance, and power) [20] | Whole body resistance training, strengthening exercises, functional training, Power Yoga, resistance training intensity, machine, weight training, strength training, lower or whole-body strength, resistance training program, Progressive Resistance Training (PRT), Inspiratory Muscle Strength Training (IMST), Expiratory Muscle Strength Training (EMST). |
| COMBINED EXERCISE: Consists of planned activities, structured and repetitive body movements that are performed to improve or maintain one or more components of physical fitness [20] | Walking training, strength, flexibility, balance, aerobic, agility, virtual reality, Tango, breathing, bodyweight supported, Tai Chi, Qigong, Brazilian samba, resistance, stretching, physiotherapy, Hatha Yoga, functional bodyweight, power, Pilates, Movement Strategy Group, dance, high-intensity physical training, ParkFit, treadmill training, stationary bike, cycling, rowing, multimodal exercises, coordination, motor therapy, respiratory muscle strength training, exergaming, LSVT BIG, boxing, task-specific training. |
| SENSORY-MOTOR ACTIVITIES: Combine exercises with interactions of emotional, mental, social, spiritual and behavioral factors directly affect our health. Mind-body interventions increase the body’s self-awareness, thereby increasing energy, mental clarity, concentration, and an individual’s ability to tolerate physical discomfort [21] | Neuro Proprioceptive Function, Qigong, Tai Chi, virtual reality, Exergame (WII, KINETIC, WIImove), mind-body, Yoga, LSVT-BIG, Pilates, Traditional Chinese Medical Exercise. |
| OTHER PROTOCOLS: Exercises that do not have volume and intensity control, or which are not classified as previous exercises (i.e. aquatic exercises, dance or any other physical activities) | Aquatic exercise, aerobic capacity, balance and postural control, mobility and strength, whole body stretching, cardiovascular activities and relaxation, resistance exercise, water walking, aquatic therapeutic exercise, hydrotherapy. Tango, Waltz, Foxtrot, Contemporary dance, Irish dance, Modern dance, PD-specific dance, Tango-based dance, Ballet, Music-based Movement therapy (MbM), improvisation, American Ballroom, RGRhythm, Jazz, Turo Dance. Exercise and motor training, highly challenging balance training, cueing, exercise training, task training (motion strategy), strength training, stretching exercise/ROM, ground walking/exercise, treadmill walking, dance, hydrotherapy, Tai Chi, virtual reality, mental practice, aquatic exercise, aerobic exercise, robotic gait training, boxing, whole body vibration, Nordic walking training, cardiovascular warm-up, functional exercises, treadmill using external auditory cues, Occupational Therapy (TO), walking, aerobic training, cycling, LSVT, Qigong; progressive resistance exercise training, Tango, home-exercise program; physiotherapy, Ronnie Gardiner Rhythm, Tai Chi; Nordic walking, body weight support, dual task training, whole body, vibration, Qigong, home-based, mobility, multi-component exercise program (home), cycling, training program involving daily activities, sensory therapy with external cues, therapy physical, boxing, multimodal, WII, goal-based exercise, proprioceptive, dual-task, virtual reality balance training, LSVT-BIG, breathing exercises, enhanced exercise therapy for PD (EXCEED). |
Table 3 presents the characteristics of participants, interventions and outcomes involved in the systematic reviews. Complementary characterization of these elements is found in S2 Table. Twenty-two studies published from 2009 to 2022 addressed the effect of aerobic exercises on seventeen different outcomes. Thirteen studies (2013:2021) accounted for the effect of strength exercises on fourteen outcomes. Nineteen studies (2014:2021) were dedicated to the effect of combined exercises on seventeen outcomes. Thirty studies published from 2008 to 2022 synthesized the effect of sensory-motor activities on seventeen outcomes. Fifty-four studies, from 2005 to 2022, addressed the effect of other exercise protocols on seventeen outcomes.
Table 3. Summary of participants characteristics, interventions and outcomes.
| Type of exercise intervention | Number of reporting SR (%) | Lower count | Higher count |
|---|---|---|---|
| Aerobic | |||
| Time range (year:year) | 22 (100%) | 2009 | 2022 |
| Number of RCT | 22 (100%) | 3 | 27 |
| Number of participants | 22 (100%) | 44 | 1,210 |
| Age range (years) | 18 (81.8%) | 30 | 80 |
| PD level (H & Y– 1–4 scale) | 19 (86.4%) | 1 | 4 |
| Duration range (years) | 14 (63.6%) | 0.3 | 18.1 |
| Session time (min) | 22 (100%) | 2 | 90 |
| Duration (weeks) | 21 (95.5%) | 1 | 64 |
| Frequency (days) | 19 (86.4%) | 1 | 7 |
| Intensity | 21 (95.5%) | SF* | SF |
| Control group | 19 (86.4%) | SF | SF |
| Outcomes assessed | 22 (100%) | QOL; FAT; BAL; MOV; MOT; MOB; FALL; CARD; WLK; COG; ANX/DEP; BDNF; ADL; DIS; APA; STR; HUM | |
| Strength | |||
| Time range (year:year) | 13 (100%) | 2013 | 2021 |
| Number of RCT | 13 (100%) | 4 | 31 |
| Number of participants | 13 (100%) | 92 | 1,239 |
| Age range (years) | 12 (92.3%) | 45 | 90 |
| PD level (H & Y– 1–4 scale) | 10 (76.9%) | 1 | 4 |
| Duration range (years) | 6 (46.2%) | 1.1 | 12.3 |
| Session time (min) | 11 (84.6%) | 15 | 90 |
| Duration (weeks) | 13 (100%) | 1 | 104 |
| Frequency (days) | 12 (92.3%) | 1 | 6 |
| Intensity | 11 (84.6%) | SF | SF |
| Control group | 12 (92.3%) | SF | SF |
| Outcomes assessed | 22 (100%) | MOT; WLK; STR; MOB; CARD; QOL; BAL; HUM; MOV; FLX; COG; FAT; FALL; BRE | |
| Combined | |||
| Time range (year:year) | 19 (100%) | 2014 | 2021 |
| Number of RCT | 19 (100%) | 9 | 50 |
| Number of participants | 19 (100%) | 79 | 2,972 |
| Age range (years) | 17 (89.5%) | 29 | 89 |
| PD level (H & Y– 1–4 scale) | 17 (89.5%) | 1 | 4 |
| Duration range (years) | 11 (57.9%) | 0.1 | 13.3 |
| Session time (min) | 19 (100%) | 15 | 120 |
| Duration (weeks) | 19 (100%) | 2 | 104 |
| Frequency (days) | 19 (100%) | 1 | 7 |
| Intensity | 17 (89.5%) | SF | SF |
| Control group | 19 (100%) | SF | SF |
| Outcomes assessed | 19 (100%) | QOL; COG; ANX/DEP; BAL; MOV; MOT; FALL; STR; CARD; ADL; WLK; HUM; SLP; BDNF; DIS; BRE; MOB | |
| Sensory-motor activities | |||
| Time range (year:year) | 31 (100%) | 2008 | 2022 |
| Number of RCT | 31 (100%) | 4 | 35 |
| Number of participants | 27 (87.1%) | 84 | 1,210 |
| Age range (years) | 27 (87.1%) | 40 | 86 |
| PD level (H & Y– 1–4 scale) | 24 (77.4%) | 1 | 4 |
| Duration range (years) | 9 (29.0%) | 2 | 10 |
| Session time (min) | 26 (83.9%) | 5 | 120 |
| Duration (weeks) | 29 (93.6%) | 1 | 52 |
| Frequency (days) | 29 (93.6%) | 1 | 7 |
| Intensity | 4 (12.9%) | SF | SF |
| Control group | 29 (93.6%) | SF | SF |
| Outcomes assessed | 31 (100%) | QOL; ANX/DEP; BAL; MOV; MOT; FALL; CARD; ADL; MOB; WLK; STR; COG; BRE; FLX; HUM; SLP; WLB | |
| Other protocols | |||
| Time range (year:year) | 54 (100%) | 2005 | 2022 |
| Number of RCT | 54 (100%) | 2 | 191 |
| Number of participants | 54 (100%) | 37 | 7,998 |
| Age range (years) | 48 (88.9%) | 18 | 90 |
| PD level (H & Y– 1–4 scale) | 43 (79.6%) | 1 | 4 |
| Duration range (years) | 16 (29.6%) | 0.3 | 18.7 |
| Session time (min) | 49 (90.7%) | 15 | 135 |
| Duration (weeks) | 53 (98.2%) | 2 | 104 |
| Frequency (days) | 53 (98.2%) | 1 | 7 |
| Intensity | 3 (5.6%) | SF | SF |
| Control group | 48 (88.9%) | SF | SF |
| Outcomes assessed | 54 (100%) | BAL; MOV; WLK; MOB; STR; ADL; FLX; ANX/DEP; MOT; CARD; FALL; COG; DIS; HUM; FAT; SLP; APA | |
NR–not reported.
QOF–Quality of life; FAT–Fatigue; BAL–Balance; MOV–Movement/walking; MOT–Motor function; FALL–Falls; CARD–cardiorespiratory; MOB–mobility; WLK–walking; COG–cognitive function; ANX/DEP–anxiety/depression; BNDF–brain-derived neurotrophic factor; ADL–activities of daily living; DIS–disease severity; APA–apathy; STR–strength; HUM–humor; SLP–sleep; WLB–well-being; FLX–flexibility.
*SF–Supplementary file.
Information of participants such as age range, PD level and duration were available in most, but not all studies. The duration, frequency, intensity and the presence of a control group were also missing in some studies, regardless of the exercise intervention being assessed.
Table 4 indicates the non-motor and motor outcomes improved by the physical exercises and the AMSTAR classification of the evidence provided by the systematic reviews to recommend them. S3 Table reveals the classification of each study considering the AMSTAR questions and domains.
Table 4. Non-motor and motor outcomes improved by the physical exercises and methodological quality of the systematic reviews.
| Author, year | Non-motor outcomes | Motor outcomes | AMSTAR |
|---|---|---|---|
| AEROBIC EXERCISES | |||
| Mehrholz, 2015 [15] | - | MOV; WLK | High |
| Robinson, 2019 [22] | - | MOB; WLK | High |
| Lorenzo Garcia, 2021 [23] | - | BAL; MOT; WLK | High |
| Mackay, 2017 [24] | BNDF | - | Moderate |
| Li, 2020 [25] | QOL | BAL; MOV | Moderate |
| Tiihonen, 2021 [26] | QOL | MOV; MOT | Moderate |
| De Almeida, 2022 [27] | - | MOT | Moderate |
| Shu, 2014 [28] | - | BAL; MOV; MOT | Low |
| Cascaes da Silva, 2016 [29] | QOL | BAL; MOV; MOT; MOB | Low |
| Alwadat, 2018 [30] | - | BAL; MOV; MOB | Low |
| De Santis, 2020 [31] | QOL; COG; HUM; FAT; APA | BAL; MOB; WLK | Low |
| Rodríguez, 2020 [32] | - | CARD | Low |
| Salse-Batán, 2022 [33] | QOL | MOV | Low |
| Herman, 2009 [34] | QOL | MOV; MOT | Critically low |
| Jambeau, 2011 [35] | - | MOT; MOB; WLK | Critically low |
| Lamotte, 2015 [36] | - | MOV; CARD | Critically low |
| Bombieri, 2017 [37] | QOL | MOV; MOB; WLK | Critically low |
| Flach, 2017 [38] | - | MOT | Critically low |
| Seuthe, 2019 [39] | - | MOV; WLK | Critically low |
| Aburub, 2020 [40] | - | CARD | Critically low |
| Miner, 2020 [41] | - | MOT; MOB | Critically low |
| Braz, 2021 [42] | - | MOV; MOB; STR | Critically low |
| STRENGTH EXERCISES | |||
| Saltychev, 2016 [43] | - | WLK; STR; CARD | High |
| Lima, 2013 [44] | - | WLK; STR | Moderate |
| Cruickshank, 2015 [45] | - | MOT; MOB; STR | Moderate |
| Li, 2020 [46] | QOL | BAL; STR | Moderate |
| Brienesse, 2014 [47] | - | MOT; MOB; STR; CARD | Low |
| Chung, 2016 [48] | - | MOT; STR | Low |
| Roeder, 2015 [49] | - | STR | Low |
| Tillman, 2015 [50] | - | STR | Low |
| Ramazzina, 2017 [51] | QOL | STR | Low |
| Van de Wetering-van Dongen, 2020 [52] | QOL; BRE | STR | Low |
| Rodríguez, 2020 [53] | QOL; BRE | STR | Critically low |
| Braz, 2021 [54] | - | BAL; MOV; STR; CARD | Critically low |
| De Lima, 2022 [55] | QOL | BAL; STR | Critically low |
| COMBINED EXERCISES | |||
| Choi, 2020 [56] | QOL | - | High |
| Cristini, 2021 [57] | SLP | - | High |
| Uhrbrand, 2015 [58] | - | STR; CARD | Moderate |
| Ni, 2018 [59] | - | BAL; MOV; WLK | Moderate |
| Stuckenschneider, 2019 [60] | COG | - | Moderate |
| Choi, 2020 [61] | - | BAL; MOV; MOT; WLK | Moderate |
| Johansson, 2020 [62] | BDNF | - | Moderate |
| McMahon, 2020 [63] | BRE | WLK | Moderate |
| Gilat, 2021 [64] | - | MOV | Moderate |
| Ruiz-Gonzalez, 2021 [65] | BDNF | - | Moderate |
| Da Silva, 2016 [28] | QOL | - | Low |
| Da Silva, 2018 [66] | COG | - | Low |
| Hirsch, 2018 [67] | BDNF | MOT | Low |
| Da Costa, 2020 [68] | QOL | BAL; MOT | Low |
| Gamborg, 2022 [69] | QOL; ANX/DEP | BAL; MOV; MOT; WLK; STR; CARD | Low |
| Tambosco, 2014 [70] | QOL | BAL; MOV; MOT; STR; CARD | Critically low |
| Reynolds, 2016 [71] | COG; HUM; SLP | - | Critically low |
| Smith, 2020 [72] | - | MOB | Critically low |
| Molina, 2021 [73] | - | MOT | Critically low |
| SENSORY-MOTOR ACTIVITIES | |||
| Ni, 2014 [74] | - | BAL; MOB | High |
| Dockx, 2016 [75] | - | WLK | Moderate |
| Song, 2017 [76] | QOL; ANX/DEP | BAL; MOT; FALL; STR | Moderate |
| McDonell, 2018 [77] | - | MOV; MOT; WLK | Moderate |
| Liu, 2019 [78] | - | BAL; FALL; MOB | Moderate |
| Alexandre, 2020 [79] | - | MOV; WLK | Moderate |
| Chen, 2020 [80] | - | BAL; MOT; WLK | Moderate |
| Jin, 2019 [81] | QOL; ANX/DEP | BAL; MOT | Moderate |
| Yu, 2020 [82] | - | BAL; MOV; MOT | Moderate |
| Cugusi, 2021 [83] | QOL | - | Moderate |
| Elena, 2021 [84] | QOL | BAL; MOV | Moderate |
| Wang, 2021 [85] | QOL | BAL; MOV | Moderate |
| Yang, 2014 [86] | - | BAL; MOT; MOB | Low |
| Harris, 2015 [87] | - | BAL; MOT | Low |
| Yang, 2015 [88] | - | BAL; MOT | Low |
| Kwok, 2016 [20] | ANX/DEP | BAL; MOT; MOB; WLK; STR; CARD; ADL; FLX | Low |
| Winser, 2018 [89] | - | BAL; MOT; FALL | Low |
| Santos, 2019 [90] | QOL | BAL | Low |
| Suárez-Iglesias, 2019 [91] | - | BAL; MOB; STR; CARD | Low |
| Suárez-Iglesias, 2022 [92] | - | MOT | Low |
| Garcia-Lopez, 2021 [93] | - | BAL; FALL | Low |
| Lee, 2008 [94] | - | MOT; FALL | Critically low |
| Toh, 2013 [95] | - | - | Critically low |
| Zhou, 2015 [96] | - | BAL; MOT | Critically low |
| Cwiekala-Lewis, 2016 [97] | - | BAL; MOV; MOT; FALL; ADL; FLX | Critically low |
| Stickdorn, 2018 [98] | - | - | Critically low |
| Garcia-Agunde, 2019 [99] | COG | BAL; MOT | Critically low |
| Kamieniarz, 2020 [100] | QOL | BAL; MOV; FALL; WLK | Critically low |
| Mailankody, 2021 [101] | ANX/DEP | BAL | Critically low |
| Campo-Prieto, 2021 [102] | - | MOT | Critically low |
| Sevcenko, 2022 [103] | QOL; COG | BAL; MOV; MOT; MOB; ADL | Critically low |
| OTHER PROTOCOLS | |||
| Perry, 2019 [104] | - | MOT | High |
| Cugusi, 2019 [105] | QOL | BAL; FALL | High |
| Pinto, 2019 [106] | - | BAL; MOB | High |
| Abou, 2021 [107] | - | FALL | High |
| Goodwin, 2008 [108] | QOL | BAL; MOV; MOB | Moderate |
| Allen, 2011 [109] | - | BAL | Moderate |
| Shen, 2015 [110] | - | BAL; MOV; FALL | Moderate |
| Wang, 2016 [111] | - | BAL; MOV | Moderate |
| Klamroth, 2016 [112] | - | BAL | Moderate |
| Delabary, 2017 [113] | - | MOT; MOB | Moderate |
| Flynn, 2019 [114] | - | BAL; MOV | Moderate |
| Kalyani, 2019 [115] | COG | MOV; WLK | Moderate |
| Zhang, 2019 [116] | COG | - | Moderate |
| Li, 2020 [25] | - | BAL; MOV; MOT | Moderate |
| Miller, 2020 [117] | - | MOV | Moderate |
| Ismail, 2021 [118] | - | BAL; MOT | Moderate |
| Okada, 2021 [119] | - | MOT; ADL | Moderate |
| Zhou, 2021 [120] | QOL; ANX/DEP | BAL; MOV; MOT | Moderate |
| Ayán Pérez, 2014 [121] | QOL | MOT; MOB | Low |
| Sharp, 2014 [122] | QOL | BAL; MOV; MOT | Low |
| Lötzke, 2015 [123] | QOL; FAT | BAL; MOV; MOT | Low |
| Cassimatis, 2016 [124] | - | ADL | Low |
| Cusso, 2016 [125] | COG; ANX/DEP; FAT; SLP; APA | - | Low |
| Yitayeh, 2016 [126] | - | BAL | Low |
| Mazzarin, 2017 [127] | - | MOB | Low |
| Connors, 2018 [128] | QOL; COG | BAL; MOV | Low |
| Carroll, 2020 [129] | - | BAL; MOV; MOB | Low |
| Morris, 2019 [130] | QOL | BAL; MOB | Low |
| Pritchard, 2019 [131] | QOL | BAL | Low |
| Barnish, 2020 [132] | QOL | MOT | Low |
| Gomes Neto, 2020 [133] | QOL | BAL; MOB | Low |
| Hidalgo-Agudo, 2020 [134] | - | BAL | Low |
| Cosentino, 2020 [135] | - | MOV | Low |
| Oh, 2021 [136] | QOL | BAL; MOT | Low |
| Hasan, 2022 [137] | COG | BAL; MOV; MOT | Low |
| Lim, 2005 [138] | - | WLK | Critically low |
| Crizzle, 2006 [139] | QOL | BAL; STR; ADL | Critically low |
| Kwakkel, 2007 [140] | - | BAL; MOV | Critically low |
| Dibble, 2009 [141] | - | BAL | Critically low |
| De Dreu, 2012 [142] | - | BAL; WLK | Critically low |
| Foster, 2014 [143] | - | BAL; MOT; MOB | Critically low |
| Mandelbaum, 2014 [144] | QOL | BAL; MOV; MOT | Critically low |
| Murray, 2014 [145] | COG | BAL | Critically low |
| Alves da Rocha, 2015 [146] | QOL | BAL; MOB | Critically low |
| Shanahan, 2015 [147] | QOL | BAL; MOT; MOV; CARD | Critically low |
| Aguiar, 2016 [148] | QOL | MOV; MOB; WLK | Critically low |
| McNeely, 2015 [149] | - | MOV; MOT; MOB; CARD | Critically low |
| Wu, 2017 [150] | QOL; ANX/DEP | MOT | Critically low |
| Costa, 2018 [151] | - | MOV; MOB | Critically low |
| De Freitas, 2020 [152] | - | BAL; MOV | Critically low |
| Chiong, 2019 [153] | - | BAL; MOV | Critically low |
| Pupíková, 2019 [154] | - | - | Critically low |
| Radder, 2020 [155] | QOL | BAL; MOV; MOT | Critically low |
| Foster, 2021 [156] | - | ADL | Critically low |
Colors in the fourth column indicate the level of evidence provided by the systematic review, considering its methodological quality: Green means high level of evidence, yellow means moderate level of evidence, orange means low level of evidence and red means critically low level of evidence.
Abbreviations represent the outcomes improved by physical exercise, as follows: QOF–Quality of life; BRE–Breath; FAT–Fatigue; BAL–Balance; MOV–Movement/walking; MOT–Motor function; FALL–Falls; CARD–cardiorespiratory; MOB–mobility; WLK–walking; COG–cognitive function; ANX/DEP–anxiety/depression; BNDF–brain-derived neurotrophic factor; ADL–activities of daily living; DIS–disease severity; APA–apathy; STR–strength; HUM–humor; SLP–sleep; WLB–well-being; FLX–flexibility.
Discussion
An expressive amount of physical exercise intervention protocols focusing on the population with PD has been proposed, assessed and synthesized in scientific literature. This umbrella review found one hundred and thirty-nine SR dedicated to synthesizing the evidence from clinical trials (Fig 1). It also pointed out the outcomes improved by the practice of each category of exercise and determined, based on AMSTAR 2, the level of evidence available to recommend such protocols to people with PD (Table 4). High quality evidence revealed that movement, walking, mobility, motor function and equilibrium benefit from aerobic exercises, while strength exercises improve strength, walking and cardiorespiratory function. Also, it was found that combined exercises improve sleep and the quality of life, and the sensory-motor activities improve balance and mobility. Finally, other protocols, such as aquatic exercises, music-based and dance exercises improve motor function, balance, mobility and prevent falls of people with PD (Table 4).
Still, a low number of high-quality studies provided evidence for such conclusions (Table 4). The methodological quality of the systematic reviews, as assessed by AMSTAR 2 [18], was classified as low or critically low in 63% of the cases. Only 64% of the reviews provided quantitative measures of effect across interventions via meta-analysis. A well-conducted, quality meta-analysis relies on consistent primary data and is supported by consistent statistical inferences, improving the decision-making process toward the most effective intervention [157]. Besides, the lack of a priori protocol registration, lack of justification for exclusion of individual studies and possible publication bias were identified as methodological drawbacks (S3 Table). The method for the conduct of systematic reviews has undergone changes and improvements that should be extensively acknowledged by authors and journal revisors aiding at the publication of high standard reviews that aid robust evidence [158].
The pooled number of participants with PD was 54,501, including populations from all the continents. The lowest number of participants included in a systematic review was 37, while the highest was 7,998 (Table 3). Sex of the participants was informed by 65% of the studies, with the presence of both sexes. Males have been shown with a higher risk of developing PD compared to females [1]. Also, age ranged from 18 to 90 years, with 91% of the participants older than 50 years. PD is age-related, with prevalence peaking in 85-89-years range. Moreover, its lethality has been shown to raise with age [1]. The Global Burden of Diseases revealed that age-standardized prevalence of PD in 2016 maintained the 1990 pattern, with men presenting a 1.40 (95% uncertainty interval 1.36–1.43) times higher prevalence than women [1].
Most studies did not report the time elapsed since the onset of PD (Table 3). For the studies that reported, a variation of 0.3 to 17 years was observed. Characteristically, the progression of PD in terms of symptoms and severity may oscillate [159]. The staging of the functional disability caused by PD may be assessed and monitored using the 5-point Hoehn & Yahr scale [4]. The level of functional compromise was expressed by 82% of the studies and varied from 1 (unilateral involvement only) to 4 (severe disability; still able to walk or stand unassisted) (Table 3).
Motor outcomes were assessed in 91% of the studies. Balance was the most studied motor outcome and was determined using the Berg Balance Scale (BBS) and the Activities-Specific Balance Confidence Scale (ABC). The BBS scale measures balance skills in sitting, standing and changing positions, while the ABC scale assesses balance confidence and fear of falling that may be protective for dangerous activities [160]. Non-motor outcomes were assessed in 68% of the studies. Quality of life was the most assessed non-motor outcome (92%), mainly with the Parkinson´s Disease Questionnaire (PDQ-39). Thirty-nine items address difficulties facing eight domains of daily living, which involve mobility, activities of daily living (ADL) emotional well-being, stigma, social support, cognition, communication and physical discomfort [161].
Aerobic exercises
Twenty-four different interventions were categorized as aerobic exercises in the included systematic reviews (Table 2). High-quality studies revealed benefit on balance, movement/walking, motor function, mobility, walking and endurance/cardiorespiratory function by aerobic exercises [21,22,23] (Table 4). Aerobic exercises also have been shown to reduce cardiovascular diseases, to improve bone health and to lower mortality rates in people with PD [162]. Additionally, they improve VO2max performance and attenuate motor symptoms without the use of medication. No high-quality systematic review evidenced benefit of non-motor outcomes by aerobic exercises (Table 4). Even so, moderate quality studies found improvement in quality of life after performing aerobic exercise protocols [25,26]. Protocol registration prior to commencement of the review and adequate literature search were the critical domains that lowered rating of these studies. Exercise dosage, expressed by frequency, volume, intensity and duration of the protocol, among other factors, may be better reported in some of the critically low- and low-quality systematic reviews. Optimal exercise dosage remains a challenge for specialized professionals. Although high-intensity exercises are known as more effective than moderate-intensity exercises, evidence on dose-response relationship for people with PD is still required [162]. Even so, aerobic exercise has been shown to reduce long-term prodromal, early and intermediate stages of motor and non-motor symptoms in people with PD [159]. Therefore, reduction of tremor and bradykinesia, improvement in balance and gait, and improved quality of life are expected.
Strength exercises
Strength exercises improved quality of life in almost half of the studies (Table 4). Still, only one moderate-quality study addressed this outcome. Also, the only high-quality study and the other moderate-quality studies addressed walking, strength, endurance/cardiorespiratory function, motor function, mobility and balance, showing improved results. Lima et al. [44] found positive effects of strength exercises on walking and strength, but not on motor function. The authors recommended strength training to improve walking. The methodological ranking was moderate due to lack of protocol registration, justification for exclusion of primary studies and assessment of probable risk of publication bias. Saltychev et al. [43] was ranked with high evidence and found improvement of walking, muscle strength and cardiorespiratory endurance by strength training.
Combined exercises
Combined exercises are planned to impact more than one component of physical fitness, for instance, cardiorespiratory capacity, flexibility and strength, simultaneously [20]. Thirty-six interventions were categorized as combined exercises, involving Pilates, different types of dances, Yoga and cycling. Two high-quality studies observed improvement of non-motor outcomes, namely sleep [57] and quality of life [56]. Four moderate-quality studies showed improved cognitive function, breathing capacity and the presence of the Brain-Derived Neurotrophic Factor (BDNF), which is a modulator of neurodevelopment and neuroprotection [163]. Blood levels of BDNF decrease in people with PD [164], and the recovery of BDNF blood levels by combined exercises suggest possible non-pharmacological interventions to enhance neuroprotection. Muscle strength, cardiorespiratory capacity, balance, motor function, movement and walking were improved by combined exercises, as revealed by moderate-quality studies (Table 4). These studies were rated down due to one to two critical domains, including lack of protocol registration prior to commencement of the review, justification for excluding individual studies and assessment of likely impact of publication bias.
Sensory-motor activities
Sensory-motor activities promote emotional, mental, behavioral, social and spiritual interactions that affect one´s health [20]. This category involved ten types of exercises, including Tai Chi, virtual reality, exergames, Yoga and Pilates. Almost all studies of sensory-motor activities addressed some motor outcome. Quality of life and anxiety/depression were the non-motor outcomes analyzed. One high-quality study [74] revealed improvement of balance and mobility with sensory-motor activities. Besides, most moderate-quality studies observed positive effect of the exercise on motor function, fall prevention, mobility, walking, flexibility and, mainly, on balance (Table 4). Moderate quality rating was due to lack of protocol registration, adequacy of literature search and to lack of assessment of likely impact of publication bias.
Other protocols
A variety of exercise protocols were otherwise classified. The eighty-four types of exercises categorized as ’other protocols’ did not present sufficient data regarding the protocols such as volume and/or intensity, a fact that limits the analyses related to the exercise dose and the observed effect. Still, two high-quality studies revealed that water-based studies had a positive effect on quality of life, balance, fall prevention and mobility [105,106]. Also, that functional task training improved motor function [104] and that a combination of physical therapy, resistance, treadmill and strategy training, dance, aerobic exercise, balance and gait training and Tai Chi improved fall prevention [107]. Moderate quality studies, which may present more than one flawed non-critical domain [152] revealed benefits of cognition by dance-based exercises, such as Tango, Irish dance, Jazz and Ballet [115,116]. Different exercises, such as aerobic, force, balance Qigong and other exercises, and music-based movement improved quality of life [108,120]. Moderate-quality evidence of benefit of balance as the most assessed motor outcome was found by the several different exercises [25,108–112,114,118,120]. Mobility, movement/walking, fall prevention, mobility, walking, activities of daily living and motor function also improved with the different types of exercise proposed (Table 4).
Evidence on safety, adherence and maintenance of interventions based on physical exercise programs for patients with PD must be constantly reviewed. Concerns with the practice of physical exercises by people with PD involve musculoskeletal injuries and cardiovascular risks, which requires an individual prescription considering the possibilities and limitations of each patient [36,165].
Considering the studies analyzed in this umbrella review, it is not possible to indicate the best intervention protocol, mainly due to the diversity of physical exercise protocols and the characteristics of the patients included in the SR. It should also be noted that only 45% of the SR reported adverse effects related to the interventions (pain, injuries and worsening of general health conditions and comorbidities).
Even so, evidence suggests that exercise is a promising, economical, low-risk intervention, which improves motor and non-motor symptoms and may be prescribed and encouraged for people with PD. Based on that, research on the therapeutic efficacy of exercise is a growing and exciting area [166].
Implications for clinical practice
Health professionals may recommend physical exercises to people with PD, considering the specific characteristics and individualities of each one.
Any type of exercise may be indicated to improve balance and mobility. Aerobic exercises would be indicated to improve balance, movement, motor function, mobility, walking, cardiorespiratory fitness and BDNF. Strength exercises can improve strength and quality of life, while protocols that combine different types of exercises can improve quality of life, cognitive function, sleep, balance, movement, motor function, mobility, walking, cardiorespiratory fitness, strength, and BDNF.
Sensorimotor activities may be indicated to improve balance, motor function, mobility, walking and to reduce falls, the latter being the only intervention with positive results for this population. Various physical activities such as those performed in the aquatic environment or dancing are indicated to improve quality of life, cognitive function, sleep, balance, movement, mobility, motor function, walking and physical activities of specific tasks improve responses in activities of daily living.
Implications for research
The number of published and analyzed studies shows that the issue has been widely studied, due to its relevance and potential impact on the lives of patients with PD. On the other hand, a considerable part of the analyzed studies has important limitations that compromise the production of consistent scientific evidence to support professionals who work with the prescription of physical exercises for patients with PD.
The findings of this study reveal the need for greater methodological care in conducting RCTs, especially in relation to instruments for data collection and types of outcomes analyzed, description of exercise protocols and adverse effects related to interventions.
Conclusion
In conclusion, evidence suggests that physical exercise improves the motor outcomes of balance, mobility, movement, motor function and walking. Combined exercises may have positive effects on motor and non-motor outcomes. Exercise in PD has been studied because of its relevance and potential impact. However, the evidence-based decision process is hindered by the limited methodological quality of most reviews.
This study presents information and directions to produce evidence with better methodological quality in RCTs and RS for the effects of physical exercise to people with PD, aiding at formulation of policies and guidelines.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors thank the information specialist Cristiane Sinimbu Sanchez for the help building and testing the search strategies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by a Master of Science scholarship provided by the Community University of Chapecó Region – Unochapecó to the first author (C. P.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11): 939–953. doi: 10.1016/S1474-4422(18)30295-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovacs GG. Molecular Pathological Classification of Neurodegenerative Diseases: Turning towards Precision Medicine. Int J Mol Sci. 2016;17(2): 189. doi: 10.3390/ijms17020189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. 2022;7(1): 391. doi: 10.1038/s41392-022-01251-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5): 427–442. doi: 10.1212/wnl.17.5.427 . [DOI] [PubMed] [Google Scholar]
- 5.Modestino EJ, Reinhofer AM, Blum K, Amenechi C, Toole P. Hoehn and Yahr staging of Parkinson´s disease in relation to neuropsychological measures. Front Biosci. 2018;23(7): 1370–1379. doi: 10.2741/4649 . [DOI] [PubMed] [Google Scholar]
- 6.Clynes MA, Gregson CL, Bruyère O, Cooper C, Dennison EM. Osteosarcopenia: where osteoporosis and sarcopenia colide. Rheumatology. 2021;60(2): 529–537. doi: 10.1093/rheumatology/keaa755 . [DOI] [PubMed] [Google Scholar]
- 7.Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5): 384–386. doi: 10.1212/01.wnl.0000247740.47667.03 . [DOI] [PubMed] [Google Scholar]
- 8.Cezar MA, de Sá CA, Corralo VS, Grigoletto MES, Copatti SL, Gonzaga dos Santos GA. Effects of exercise training with blood flow restriction on blood pressure in medicated hypertensive patients. Motriz: Rev Educ Fis. 2016;(22): 9–17. doi: org/10.1590/S1980-6574201600020002 [Google Scholar]
- 9.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39(11): 2065–2079. doi: 10.2337/dc16-1728 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciolac EG, Guimarães GV. Physical exercise and metabolic syndrome. Rev Bras Med Esporte. 2004;(10): 325–330. [Google Scholar]
- 11.Lin FL, Yeh ML, Lai YH, Lin KC, Yu CJ, Chang JS. Two-month breathing-based walking improves anxiety, depression, dyspnoea and quality of life in chronic obstructive pulmonary disease: A randomised controlled study. J Clin Nurs. 2019;28(19–20): 3632–3640. doi: 10.1111/jocn.14960 . [DOI] [PubMed] [Google Scholar]
- 12.Song D, Yu DSF. Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: A randomised controlled trial. Int J Nurs Stud. 2019;93: 97–105. doi: 10.1016/j.ijnurstu.2019.02.019 . [DOI] [PubMed] [Google Scholar]
- 13.Marinus N, Hansen D, Feys P, Meesen R, Timmermans A, Spildooren J. The Impact of Different Types of Exercise Training on Peripheral Blood Brain-Derived Neurotrophic Factor Concentrations in Older Adults: A Meta-Analysis. Sports Med. 2019;49(10): 1529–1546. doi: 10.1007/s40279-019-01148-z . [DOI] [PubMed] [Google Scholar]
- 14.Cabreira V, Massano J. Doença de Parkinson: Revisão Clínica e Atualização [Parkinson’s Disease: Clinical Review and Update]. Acta Med Port. 2019;32(10): 661–670. doi: 10.20344/amp.11978 . [DOI] [PubMed] [Google Scholar]
- 15.Mehrholz J, Kugler J, Storch A, Pohl M, Hirsch K, Elsner B. Treadmill training for patients with Parkinson’s disease. Cochrane Database Syst Rev. 2015;2015(9): CD007830. doi: 10.1002/14651858.CD007830.pub4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira de Carvalho A, Filho ASS, Murillo-Rodriguez E, Rocha NB, Carta MG, Machado S. Physical Exercise For Parkinson’s Disease: Clinical And Experimental Evidence. Clin Pract Epidemiol Ment Health. 2018;14: 89–98. doi: 10.2174/1745017901814010089 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aromataris E, Fernandez R, Godfrey C, Holly C, Khalil H, Tungpunkom P. Chapter 10: Umbrella Reviews. In: Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. JBI, 2020. doi: 10.46658/JBIMES-20-11 [DOI] [Google Scholar]
- 18.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358: j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liguori S, Young VM, Arienti C, Pollini E, Patrini M, Gimigliano F, et al. Overview of Cochrane systematic reviews for rehabilitation interventions in individuals with cerebral palsy: A mapping synthesis. Dev Med Child Neurol. 2023;65(10): 1280–1291. doi: 10.1111/dmcn.15572 . [DOI] [PubMed] [Google Scholar]
- 20.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. ; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7): 1334–1359. doi: 10.1249/MSS.0b013e318213fefb . [DOI] [PubMed] [Google Scholar]
- 21.Kwok JY, Choi KC, Chan HY. Effects of mind-body exercises on the physiological and psychosocial well-being of individuals with Parkinson’s disease: A systematic review and meta-analysis. Complement Ther Med. 2016;29: 121–131. doi: 10.1016/j.ctim.2016.09.016 . [DOI] [PubMed] [Google Scholar]
- 22.Robinson AG, Dennett AM, Snowdon DA. Treadmill training may be an effective form of task-specific training for improving mobility in people with Parkinson’s disease and multiple sclerosis: a systematic review and meta-analysis. Physiotherapy. 2019;105(2): 174–186. doi: 10.1016/j.physio.2018.11.007 . [DOI] [PubMed] [Google Scholar]
- 23.Lorenzo-García P, Cavero-Redondo I, Torres-Costoso AI, Guzmán-Pavón MJ, Núñez de Arenas-Arroyo S, Álvarez-Bueno C. Body Weight Support Gait Training for Patients With Parkinson Disease: A Systematic Review and Meta-analyses. Arch Phys Med Rehabil. 2021;102(10): 2012–2021. doi: 10.1016/j.apmr.2021.02.016 . [DOI] [PubMed] [Google Scholar]
- 24.Mackay CP, Kuys SS, Brauer SG. The Effect of Aerobic Exercise on Brain-Derived Neurotrophic Factor in People with Neurological Disorders: A Systematic Review and Meta-Analysis. Neural Plast. 2017;2017: 4716197. doi: 10.1155/2017/4716197 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Wang T, Liu H, Jiang Y, Wang Z, Zhuang J. Dual-task training on gait, motor symptoms, and balance in patients with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil. 2020;34(11): 1355–1367. doi: 10.1177/0269215520941142 . [DOI] [PubMed] [Google Scholar]
- 26.Tiihonen M, Westner BU, Butz M, Dalal SS. Parkinson’s disease patients benefit from bicycling—a systematic review and meta-analysis. NPJ Parkinsons Dis. 2021;7(1): 86. doi: 10.1038/s41531-021-00222-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Almeida FO, Santana V, Corcos DM, Ugrinowitsch C, Silva-Batista C. Effects of Endurance Training on Motor Signs of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Sports Med. 2022;52(8): 1789–1815. doi: 10.1007/s40279-022-01650-x . [DOI] [PubMed] [Google Scholar]
- 28.Shu HF, Yang T, Yu SX, Huang HD, Jiang LL, Gu JW, et al. Aerobic exercise for Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(7): e100503. doi: 10.1371/journal.pone.0100503 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cascaes da Silva F, Iop Rda R, Domingos Dos Santos P, Aguiar Bezerra de Melo LM, Barbosa Gutierres Filho PJ, da Silva R. Effects of Physical-Exercise-Based Rehabilitation Programs on the Quality of Life of Patients With Parkinson’s Disease: A Systematic Review of Randomized Controlled Trials. J Aging Phys Act. 2016;24(3): 484–96. doi: 10.1123/japa.2015-0162 . [DOI] [PubMed] [Google Scholar]
- 30.Alwardat M, Etoom M, Al Dajah S, Schirinzi T, Di Lazzaro G, Sinibaldi Salimei P, et al. Effectiveness of robot-assisted gait training on motor impairments in people with Parkinson’s disease: a systematic review and meta-analysis. Int J Rehabil Res. 2018;41(4): 287–296. doi: 10.1097/MRR.0000000000000312 . [DOI] [PubMed] [Google Scholar]
- 31.De Santis KK, Kaplan I. The motor and the non-motor outcomes of Nordic Walking in Parkinson’s disease: A systematic review. J Bodyw Mov Ther. 2020;24(2): 4–10. doi: 10.1016/j.jbmt.2020.01.003 . [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez MÁ, Crespo I, Del Valle M, Olmedillas H. Should respiratory muscle training be part of the treatment of Parkinson’s disease? A systematic review of randomized controlled trials. Clin Rehabil. 2020;34(4): 429–437. doi: 10.1177/0269215519896054 . [DOI] [PubMed] [Google Scholar]
- 33.Salse-Batán J, Sanchez-Lastra MA, Suarez-Iglesias D, Varela S, Ayán C. Effects of Nordic walking in people with Parkinson’s disease: A systematic review and meta-analysis. Health Soc Care Community. 2022;30(5): e1505–e1520. doi: 10.1111/hsc.13842 . [DOI] [PubMed] [Google Scholar]
- 34.Herman T, Giladi N, Hausdorff JM. Treadmill training for the treatment of gait disturbances in people with Parkinson’s disease: a mini-review. J Neural Transm (Vienna). 2009;116(3): 307–318. doi: 10.1007/s00702-008-0139-z . [DOI] [PubMed] [Google Scholar]
- 35.Jambeau M, Chauvière C, Cordier JP. La thérapie par la contrainte pour améliorer la marche du parkinsonien: Treadmill training to improve parkinsonian gait. Kinésithérapie, la Revue 2011;(11): 35–45. doi: 10.1016/S1779-0123(11)75113-0 [DOI] [Google Scholar]
- 36.Lamotte G, Rafferty MR, Prodoehl J, Kohrt WM, Comella CL, Simuni T, et al. Effects of endurance exercise training on the motor and non-motor features of Parkinson’s disease: a review. J Parkinsons Dis. 2015; 5(1): 21–41. doi: 10.3233/JPD-140425 Erratum in: J Parkinsons Dis. 2015; 5(3): 621. Erratum in: J Parkinsons Dis. 2015; 5(4): 993. . [DOI] [PubMed] [Google Scholar]
- 37.Bombieri F, Schena F, Pellegrini B, Barone P, Tinazzi M, Erro R. Walking on four limbs: A systematic review of Nordic Walking in Parkinson disease. Parkinsonism Relat Disord. 2017; 38: 8–12. doi: 10.1016/j.parkreldis.2017.02.004 . [DOI] [PubMed] [Google Scholar]
- 38.Flach A, Jaegers L, Krieger M, Bixler E, Kelly P, Weiss EP, et al. Endurance exercise improves function in individuals with Parkinson’s disease: A meta-analysis. Neurosci Lett. 2017; 659: 115–119. doi: 10.1016/j.neulet.2017.08.076 . [DOI] [PubMed] [Google Scholar]
- 39.Seuthe J D’Cruz N, Ginis P, Weisser B, Berg D, Deuschl G, et al. Split-belt treadmill walking in patients with Parkinson’s disease: A systematic review. Gait Posture. 2019; 69: 187–194. doi: 10.1016/j.gaitpost.2019.01.032 . [DOI] [PubMed] [Google Scholar]
- 40.Aburub A, Ledger SJ, Sim J, Hunter SM. Cardiopulmonary Function and Aerobic Exercise in Parkinson’s: A Systematic Review of the Literature. Mov Disord Clin Pract. 2020;7(6): 599–606. doi: 10.1002/mdc3.13011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miner DG, Aron A, DiSalvo E. Therapeutic effects of forced exercise cycling in individuals with Parkinson’s disease. J Neurol Sci. 2020; 410: 116677. doi: 10.1016/j.jns.2020.116677 . [DOI] [PubMed] [Google Scholar]
- 42.de Oliveira MPB, Lobato DFM, Smaili SM, Carvalho C, Borges JBC. Effect of aerobic exercise on functional capacity and quality of life in individuals with Parkinson’s disease: A systematic review of randomized controlled trials. Arch Gerontol Geriatr. 2021; 95: 104422. doi: 10.1016/j.archger.2021.104422 . [DOI] [PubMed] [Google Scholar]
- 43.Saltychev M, Bärlund E, Paltamaa J, Katajapuu N, Laimi K. Progressive resistance training in Parkinson’s disease: a systematic review and meta-analysis. BMJ Open. 2016; 6(1): e008756. doi: 10.1136/bmjopen-2015-008756 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lima LO, Scianni A, Rodrigues-de-Paula F. Progressive resistance exercise improves strength and physical performance in people with mild to moderate Parkinson’s disease: a systematic review. J Physiother. 2013; 59(1):7–13. doi: 10.1016/S1836-9553(13)70141-3 . [DOI] [PubMed] [Google Scholar]
- 45.Cruickshank TM, Reyes AR, Ziman MR. A systematic review and meta-analysis of strength training in individuals with multiple sclerosis or Parkinson disease. Medicine (Baltimore). 2015; 94(4): e411. doi: 10.1097/MD.0000000000000411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, He J, Yun J, Qin H. Lower Limb Resistance Training in Individuals With Parkinson’s Disease: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Neurol. 2020; 11: 591605. doi: 10.3389/fneur.2020.591605 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brienesse LA, Emerson MN. Effects of resistance training for people with Parkinson’s disease: a systematic review. J Am Med Dir Assoc. 2013; 14(4): 236–241. doi: 10.1016/j.jamda.2012.11.012 . [DOI] [PubMed] [Google Scholar]
- 48.Chung CL, Thilarajah S, Tan D. Effectiveness of resistance training on muscle strength and physical function in people with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil. 2016; 30(1): 11–23. doi: 10.1177/0269215515570381 . [DOI] [PubMed] [Google Scholar]
- 49.Roeder L, Costello JT, Smith SS, Stewart IB, Kerr GK. Effects of Resistance Training on Measures of Muscular Strength in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. PLoS One. 2015; 10(7): e0132135. doi: 10.1371/journal.pone.0132135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tillman A, Muthalib M, Hendy AM, Johnson LG, Rantalainen T, Kidgell DJ, et al. Lower limb progressive resistance training improves leg strength but not gait speed or balance in Parkinson’s disease: a systematic review and meta-analysis. Front Aging Neurosci. 2015; 7: 40. doi: 10.3389/fnagi.2015.00040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramazzina I, Bernazzoli B, Costantino C. Systematic review on strength training in Parkinson’s disease: an unsolved question. Clin Interv Aging. 2017; 12: 619–628. doi: 10.2147/CIA.S131903 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van de Wetering-van Dongen VA, Kalf JG, van der Wees PJ, Bloem BR, Nijkrake MJ. The Effects of Respiratory Training in Parkinson’s Disease: A Systematic Review. J Parkinsons Dis. 2020; 10(4): 1315–1333. doi: 10.3233/JPD-202223 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodríguez MÁ, Crespo I, Del Valle M, Olmedillas H. Should respiratory muscle training be part of the treatment of Parkinson’s disease? A systematic review of randomized controlled trials. Clin Rehabil. 2020; 34(4): 429–437. doi: 10.1177/0269215519896054 . [DOI] [PubMed] [Google Scholar]
- 54.Braz de Oliveira MP, Maria Dos Reis L, Pereira ND. Effect of Resistance Exercise on Body Structure and Function, Activity, and Participation in Individuals With Parkinson Disease: A Systematic Review. Arch Phys Med Rehabil. 2021; 102(10): 1998–2011. doi: 10.1016/j.apmr.2021.01.081 . [DOI] [PubMed] [Google Scholar]
- 55.de Lima KP, da Silva CN, de Seixas NF, Maneschy MS, Lima BN, Junior GV, et al. Effect of resistance training on balance and postural control in people with Parkinson’s: A systematic review. Rev. Científica de la Soc. Española de Enfermería Neurológica 2022; (56): 18–28. doi: 10.1016/j.sedeng.2021.05.002 [DOI] [Google Scholar]
- 56.Chen K, Tan Y, Lu Y, Wu J, Liu X, Zhao Y. Effect of Exercise on Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Parkinsons Dis. 2020;2020: 3257623. doi: 10.1155/2020/3257623 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cristini J, Weiss M, De Las Heras B, Medina-Rincón A, Dagher A, Postuma RB, et al. The effects of exercise on sleep quality in persons with Parkinson’s disease: A systematic review with meta-analysis. Sleep Med Rev. 2021;55: 101384. doi: 10.1016/j.smrv.2020.101384 . [DOI] [PubMed] [Google Scholar]
- 58.Uhrbrand A, Stenager E, Pedersen MS, Dalgas U. Parkinson’s disease and intensive exercise therapy—a systematic review and meta-analysis of randomized controlled trials. J Neurol Sci. 2015; 353(1–2): 9–19. doi: 10.1016/j.jns.2015.04.004 . [DOI] [PubMed] [Google Scholar]
- 59.Ni M, Hazzard JB, Signorile JF, Luca C. Exercise Guidelines for Gait Function in Parkinson’s Disease: A Systematic Review and Meta-analysis. Neurorehabil Neural Repair. 2018; 32(10): 872–886. doi: 10.1177/1545968318801558 . [DOI] [PubMed] [Google Scholar]
- 60.Stuckenschneider T, Askew CD, Menêses AL, Baake R, Weber J, Schneider S. The Effect of Different Exercise Modes on Domain-Specific Cognitive Function in Patients Suffering from Parkinson’s Disease: A Systematic Review of Randomized Controlled Trials. J Parkinsons Dis. 2019; 9(1): 73–95. doi: 10.3233/JPD-181484 . [DOI] [PubMed] [Google Scholar]
- 61.Choi HY, Cho KH, Jin C, Lee J, Kim TH, Jung WS, et al. Exercise Therapies for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Parkinsons Dis. 2020;2020: 2565320. doi: 10.1155/2020/2565320 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johansson H, Hagströmer M, Grooten WJA, Franzén E. Exercise-Induced Neuroplasticity in Parkinson’s Disease: A Metasynthesis of the Literature. Neural Plast. 2020;2020: 8961493. doi: 10.1155/2020/8961493 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McMahon L, Blake C, Lennon O. Nonpharmacological interventions for respiratory health in Parkinson’s disease: A systematic review and meta-analysis. Eur J Neurol. 2021; 28(3): 1022–1040. doi: 10.1111/ene.14605 . [DOI] [PubMed] [Google Scholar]
- 64.Gilat M, Ginis P, Zoetewei D, De Vleeschhauwer J, Hulzinga F, D’Cruz N, et al. A systematic review on exercise and training-based interventions for freezing of gait in Parkinson’s disease. NPJ Parkinsons Dis. 2021;7(1): 81. doi: 10.1038/s41531-021-00224-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruiz-González D, Hernández-Martínez A, Valenzuela PL, Morales JS, Soriano-Maldonado A. Effects of physical exercise on plasma brain-derived neurotrophic factor in neurodegenerative disorders: A systematic review and meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2021; 128: 394–405. doi: 10.1016/j.neubiorev.2021.05.025 . [DOI] [PubMed] [Google Scholar]
- 66.da Silva FC, Iop RDR, de Oliveira LC, Boll AM, de Alvarenga JGS, Gutierres Filho PJB, et al. Effects of physical exercise programs on cognitive function in Parkinson’s disease patients: A systematic review of randomized controlled trials of the last 10 years. PLoS One. 2018; 13(2): e0193113. doi: 10.1371/journal.pone.0193113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirsch MA, van Wegen EEH, Newman MA, Heyn PC. Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson’s disease: a systematic review and meta-analysis. Transl Neurodegener. 2018; 7: 7. doi: 10.1186/s40035-018-0112-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Costa RM, Sisconeto TM, Sousa LR, Tavarez GH, Kanitz AC. Contributions of physical exercise to motor symptoms and the balance of people with Parkinson’s Disease: a systematic review. Rev Andal Med Deporte 2020; (13): 235–240. [Google Scholar]
- 69.Gamborg M, Hvid LG, Dalgas U, Langeskov-Christensen M. Parkinson’s disease and intensive exercise therapy—An updated systematic review and meta-analysis. Acta Neurol Scand. 2022; 145(5): 504–528. doi: 10.1111/ane.13579 . [DOI] [PubMed] [Google Scholar]
- 70.Tambosco L, Percebois-Macadré L, Rapin A, Nicomette-Bardel J, Boyer FC. Effort training in Parkinson’s disease: a systematic review. Ann Phys Rehabil Med. 2014; 57(2): 79–104. doi: 10.1016/j.rehab.2014.01.003 . [DOI] [PubMed] [Google Scholar]
- 71.Reynolds GO, Otto MW, Ellis TD, Cronin-Golomb A. The Therapeutic Potential of Exercise to Improve Mood, Cognition, and Sleep in Parkinson’s Disease. Mov Disord. 2016; 31(1): 23–38. doi: 10.1002/mds.26484 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith M, Barker R, Williams G, Carr J, Gunnarsson R. The effect of exercise on high-level mobility in individuals with neurodegenerative disease: a systematic literature review. Physiotherapy. 2020; 106: 174–193. doi: 10.1016/j.physio.2019.04.003 . [DOI] [PubMed] [Google Scholar]
- 73.Molina Palomino FM, López López L, Rodríguez Torres J, Granados Santiago M, Martos IC, Valenza MC. Efectividad del trabajo de resistencia y de cicloergómetro a alta velocidad sobre la bradicinesia en la enfermedad de Parkinson: revisión sistemática. Fisioterapia 2021; (43): 230–238. doi: 10.1016/j.ft.2021.01.005 [DOI] [Google Scholar]
- 74.Ni X, Liu S, Lu F, Shi X, Guo X. Efficacy and safety of Tai Chi for Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014; 9(6): e99377. doi: 10.1371/journal.pone.0099377 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dockx K, Bekkers EM, Van den Bergh V, Ginis P, Rochester L, Hausdorff JM, et al. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst Rev. 2016; 12(12): CD010760. doi: 10.1002/14651858.CD010760.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song R, Grabowska W, Park M, Osypiuk K, Vergara-Diaz GP, Bonato P, et al. The impact of Tai Chi and Qigong mind-body exercises on motor and non-motor function and quality of life in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat Disord. 2017; 41: 3–13. doi: 10.1016/j.parkreldis.2017.05.019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McDonnell MN, Rischbieth B, Schammer TT, Seaforth C, Shaw AJ, Phillips AC. Lee Silverman Voice Treatment (LSVT)-BIG to improve motor function in people with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil. 2018; 32(5): 607–618. doi: 10.1177/0269215517734385 . [DOI] [PubMed] [Google Scholar]
- 78.Liu HH, Yeh NC, Wu YF, Yang YR, Wang RY, Cheng FY. Effects of Tai Chi Exercise on Reducing Falls and Improving Balance Performance in Parkinson’s Disease: A Meta-Analysis. Parkinsons Dis. 2019;2019: 9626934. doi: 10.1155/2019/9626934 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alexandre de Assis IS, Luvizutto GJ, Bruno ACM, Sande de Souza LAP. The Proprioceptive Neuromuscular Facilitation Concept in Parkinson Disease: A Systematic Review and Meta-Analysis. J Chiropr Med. 2020; 19(3): 181–187. doi: 10.1016/j.jcm.2020.07.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen S, Zhang Y, Wang YT, Liu X, Song W, Du X. The effect of Qigong-based therapy on patients with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil. 2020; 34(12): 1436–1448. doi: 10.1177/0269215520946695 . [DOI] [PubMed] [Google Scholar]
- 81.Jin X, Wang L, Liu S, Zhu L, Loprinzi PD, Fan X. The Impact of Mind-body Exercises on Motor Function, Depressive Symptoms, and Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-analysis. Int J Environ Res Public Health. 2019; 17(1): 31. doi: 10.3390/ijerph17010031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu X, Wu X, Hou G, Han P, Jiang L, Guo Q. The Impact of Tai Chi on Motor Function, Balance, and Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2021;2021: 6637612. doi: 10.1155/2021/6637612 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cugusi L, Prosperini L, Mura G. Exergaming for Quality of Life in Persons Living with Chronic Diseases: A Systematic Review and Meta-analysis. PM R. 2021; 13(7): 756–780. doi: 10.1002/pmrj.12444 . [DOI] [PubMed] [Google Scholar]
- 84.Elena P, Demetris S, Christina M, Marios P. Differences Between Exergaming Rehabilitation and Conventional Physiotherapy on Quality of Life in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front Neurol. 2021; 12: 683385. doi: 10.3389/fneur.2021.683385 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lei C, Sunzi K, Dai F, Liu X, Wang Y, Zhang B, et al. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: A systematic review. PLoS One. 2019; 14(11): e0224819. doi: 10.1371/journal.pone.0224819 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Y, Li XY, Gong L, Zhu YL, Hao YL. Tai Chi for improvement of motor function, balance and gait in Parkinson’s disease: a systematic review and meta-analysis. PLoS One. 2014; 9(7): e102942. doi: 10.1371/journal.pone.0102942 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harris DM, Rantalainen T, Muthalib M, Johnson L, Teo WP. Exergaming as a Viable Therapeutic Tool to Improve Static and Dynamic Balance among Older Adults and People with Idiopathic Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front Aging Neurosci. 2015; 7: 167. doi: 10.3389/fnagi.2015.00167 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang Y, Qiu WQ, Hao YL, Lv ZY, Jiao SJ, Teng JF. The efficacy of traditional Chinese Medical Exercise for Parkinson’s disease: a systematic review and meta-analysis. PLoS One. 2015; 10(4): e0122469. doi: 10.1371/journal.pone.0122469 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Winser SJ, Tsang WW, Krishnamurthy K, Kannan P. Does Tai Chi improve balance and reduce falls incidence in neurological disorders? A systematic review and meta-analysis. Clin Rehabil. 2018; 32(9): 1157–1168. doi: 10.1177/0269215518773442 . [DOI] [PubMed] [Google Scholar]
- 90.Santos P, Scaldaferri G, Santos L, Ribeiro N, Neto M, Melo A. Effects of the Nintendo Wii training on balance rehabilitation and quality of life of patients with Parkinson’s disease: A systematic review and meta-analysis. NeuroRehabilitation. 2019; 44(4): 569–577. doi: 10.3233/NRE-192700 . [DOI] [PubMed] [Google Scholar]
- 91.Suárez-Iglesias D, Miller KJ, Seijo-Martínez M, Ayán C. Benefits of Pilates in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Medicina (Kaunas). 2019; 55(8): 476. doi: 10.3390/medicina55080476 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suárez-Iglesias D, Santos L, Sanchez-Lastra MA, Ayán C. Systematic review and meta-analysis of randomised controlled trials on the effects of yoga in people with Parkinson’s disease. Disabil Rehabil. 2022; 44(21): 6210–6229. doi: 10.1080/09638288.2021.1966522 . [DOI] [PubMed] [Google Scholar]
- 93.García-López H, Obrero-Gaitán E, Castro-Sánchez AM, Lara-Palomo IC, Nieto-Escamez FA, Cortés-Pérez I. Non-Immersive Virtual Reality to Improve Balance and Reduce Risk of Falls in People Diagnosed with Parkinson’s Disease: A Systematic Review. Brain Sci. 2021; 11(11): 1435. doi: 10.3390/brainsci11111435 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee MS, Lam P, Ernst E. Effectiveness of tai chi for Parkinson’s disease: a critical review. Parkinsonism Relat Disord. 2008; 14(8): 589–594. doi: 10.1016/j.parkreldis.2008.02.003 . [DOI] [PubMed] [Google Scholar]
- 95.Toh SFM. A systematic review on the effectiveness of Tai Chi exercise in individuals with Parkinson’s disease from 2003 to 2013. Hong Kong Journal of Occupational Therapy 2013; (13): 69–81. doi: 10.1016/j.hkjot.2013.11.001 [DOI] [Google Scholar]
- 96.Zhou J, Yin T, Gao Q, Yang XC. A Meta-Analysis on the Efficacy of Tai Chi in Patients with Parkinson’s Disease between 2008 and 2014. Evid Based Complement Alternat Med. 2015; 2015: 593263. doi: 10.1155/2015/593263 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ćwiękała-Lewis KJ, Gallek M, Taylor-Piliae RE. The effects of Tai Chi on physical function and well-being among persons with Parkinson’s Disease: A systematic review. J Bodyw Mov Ther. 2017; 21(2): 414–421. doi: 10.1016/j.jbmt.2016.06.007 . [DOI] [PubMed] [Google Scholar]
- 98.Stickdorn I, Marks D, Thiel C, Braun T. Effects of Lee Silverman Voice Treatment (LSVT)-BIG Training on Motor Functioning of People with Parkinson’s Disease–A Systematic Review. Physioscience 2018; (14): 153–160. doi: 10.1055/a-0749-0818 [DOI] [Google Scholar]
- 99.Garcia-Agundez A, Folkerts AK, Konrad R, Caserman P, Tregel T, Goosses M, et al. Recent advances in rehabilitation for Parkinson’s Disease with Exergames: A Systematic Review. J Neuroeng Rehabil. 2019; 16(1): 17. doi: 10.1186/s12984-019-0492-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kamieniarz A, Milert A, Grzybowska-Ganszczyk D, Opara J, Juras G. Tai Chi and Qi Gong therapies as a complementary treatment in Parkinson’s disease—a systematic review. Complement Ther Med. 2021; 56: 102589. doi: 10.1016/j.ctim.2020.102589 . [DOI] [PubMed] [Google Scholar]
- 101.Mailankody P, Varambally S, Thennarasu K, Pal PK. The Rationale of Yoga in Parkinson’s Disease: A Critical Review. Neurol India. 2021; 69(5): 1165–1175. doi: 10.4103/0028-3886.329545 . [DOI] [PubMed] [Google Scholar]
- 102.Campo-Prieto P, Santos-García D, Cancela-Carral JM, Rodríguez-Fuentes G. Estado actual de la realidad virtual inmersiva como herramienta de rehabilitación física y funcional en pacientes con enfermedad de Parkinson: revisión sistemática [Current status of immersive virtual reality as a tool for physical and functional rehabilitation in patients with Parkinson´s disease: systematic review]. Rev Neurol. 2021; 73(10): 358–367. doi: 10.33588/rn.7310.2021330 . [DOI] [PubMed] [Google Scholar]
- 103.Sevcenko K, Lindgren I. The effects of virtual reality training in stroke and Parkinson’s disease rehabilitation: a systematic review and a perspective on usability. Eur Rev Aging Phys Act. 2022; 19(1): 4. doi: 10.1186/s11556-022-00283-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perry SIB, Nelissen PM, Siemonsma P, Lucas C. The effect of functional-task training on activities of daily living for people with Parkinson`s disease, a systematic review with meta-analysis. Complement Ther Med. 2019; 42: 312–321. doi: 10.1016/j.ctim.2018.12.008 . [DOI] [PubMed] [Google Scholar]
- 105.Cugusi L, Manca A, Bergamin M, Di Blasio A, Monticone M, Deriu F, et al. Aquatic exercise improves motor impairments in people with Parkinson’s disease, with similar or greater benefits than land-based exercise: a systematic review. J Physiother. 2019; 65(2): 65–74. doi: 10.1016/j.jphys.2019.02.003 . [DOI] [PubMed] [Google Scholar]
- 106.Pinto C, Salazar AP, Marchese RR, Stein C, Pagnussat AS. The Effects of Hydrotherapy on Balance, Functional Mobility, Motor Status, and Quality of Life in Patients with Parkinson Disease: A Systematic Review and Meta-analysis. PM R. 2019; 11(3): 278–291. doi: 10.1016/j.pmrj.2018.09.031 . [DOI] [PubMed] [Google Scholar]
- 107.Abou L, Alluri A, Fliflet A, Du Y, Rice LA. Effectiveness of Physical Therapy Interventions in Reducing Fear of Falling Among Individuals With Neurologic Diseases: A Systematic Review and Meta-analysis. Arch Phys Med Rehabil. 2021; 102(1): 132–154. doi: 10.1016/j.apmr.2020.06.025 . [DOI] [PubMed] [Google Scholar]
- 108.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2008; 23(5): 631–640. doi: 10.1002/mds.21922 . [DOI] [PubMed] [Google Scholar]
- 109.Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson’s disease: a meta-analysis of the effect of exercise and motor training. Mov Disord. 2011; 26(9): 1605–1615. doi: 10.1002/mds.23790 . [DOI] [PubMed] [Google Scholar]
- 110.Shen X, Wong-Yu IS, Mak MK. Effects of Exercise on Falls, Balance, and Gait Ability in Parkinson’s Disease: A Meta-analysis. Neurorehabil Neural Repair. 2016; 30(6): 512–527. doi: 10.1177/1545968315613447 . [DOI] [PubMed] [Google Scholar]
- 111.Wang XQ, Pi YL, Chen BL, Wang R, Li X, Chen PJ. Cognitive motor intervention for gait and balance in Parkinson’s disease: systematic review and meta-analysis. Clin Rehabil. 2016; 30(2): 134–144. doi: 10.1177/0269215515578295 . [DOI] [PubMed] [Google Scholar]
- 112.Klamroth S, Steib S, Devan S, Pfeifer K. Effects of Exercise Therapy on Postural Instability in Parkinson Disease: A Meta-analysis. J Neurol Phys Ther. 2016; 40(1): 3–14. doi: 10.1097/NPT.0000000000000117 . [DOI] [PubMed] [Google Scholar]
- 113.Dos Santos Delabary M, Komeroski IG, Monteiro EP, Costa RR, Haas AN. Effects of dance practice on functional mobility, motor symptoms and quality of life in people with Parkinson’s disease: a systematic review with meta-analysis. Aging Clin Exp Res. 2018; 30(7): 727–735. doi: 10.1007/s40520-017-0836-2 . [DOI] [PubMed] [Google Scholar]
- 114.Flynn A, Allen NE, Dennis S, Canning CG, Preston E. Home-based prescribed exercise improves balance-related activities in people with Parkinson’s disease and has benefits similar to centre-based exercise: a systematic review. J Physiother. 2019; 65(4): 189–199. doi: 10.1016/j.jphys.2019.08.003 . [DOI] [PubMed] [Google Scholar]
- 115.Kalyani HHN, Sullivan K, Moyle G, Brauer S, Jeffrey ER, Roeder L, et al. Effects of Dance on Gait, Cognition, and Dual-Tasking in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J Parkinsons Dis. 2019; 9(2): 335–349. doi: 10.3233/JPD-181516 . [DOI] [PubMed] [Google Scholar]
- 116.Zhang Q, Hu J, Wei L, Jia Y, Jin Y. Effects of dance therapy on cognitive and mood symptoms in people with Parkinson’s disease: A systematic review and meta-analysis. Complement Ther Clin Pract. 2019; 36: 12–17. doi: 10.1016/j.ctcp.2019.04.005 . [DOI] [PubMed] [Google Scholar]
- 117.Miller KJ, Suárez-Iglesias D, Seijo-Martínez M, Ayán C. Physiotherapy for freezing of gait in Parkinson’s disease: a systematic review and meta-analysis. Rev Neurol. 2020; 70(5): 161–170. doi: 10.33588/rn.7005.2019417 . [DOI] [PubMed] [Google Scholar]
- 118.Ismail SR, Lee SWH, Merom D, Megat Kamaruddin PSN, Chong MS, Ong T, et al. Evidence of disease severity, cognitive and physical outcomes of dance interventions for persons with Parkinson’s Disease: a systematic review and meta-analysis. BMC Geriatr. 2021; 21(1): 503. doi: 10.1186/s12877-021-02446-w . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okada Y, Ohtsuka H, Kamata N, Yamamoto S, Sawada M, Nakamura J, et al. Effectiveness of Long-Term Physiotherapy in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J Parkinsons Dis. 2021; 11(4): 1619–1630. doi: 10.3233/JPD-212782 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou Z, Zhou R, Wei W, Luan R, Li K. Effects of music-based movement therapy on motor function, balance, gait, mental health, and quality of life for patients with Parkinson’s disease: A systematic review and meta-analysis. Clin Rehabil. 2021; 35(7): 937–951. doi: 10.1177/0269215521990526 . [DOI] [PubMed] [Google Scholar]
- 121.Ayán Pérez C, Cancela JM. Effectiveness of water-based exercise in people living with Parkinson’s disease: a systematic review. Eur Rev Aging Phys. Activity 2014;11: 107–118. doi: 10.1007/s11556-013-0135-7 [DOI] [Google Scholar]
- 122.Sharp K, Hewitt J. Dance as an intervention for people with Parkinson’s disease: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2014;47: 445–456. doi: 10.1016/j.neubiorev.2014.09.009 . [DOI] [PubMed] [Google Scholar]
- 123.Lötzke D, Ostermann T, Büssing A. Argentine tango in Parkinson disease—a systematic review and meta-analysis. BMC Neurol. 2015;15: 226. doi: 10.1186/s12883-015-0484-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cassimatis C, Liu KP, Fahey P, Bissett M. The effectiveness of external sensory cues in improving functional performance in individuals with Parkinson’s disease: a systematic review with meta-analysis. Int J Rehabil Res. 2016;39(3): 211–8. doi: 10.1097/MRR.0000000000000171 . [DOI] [PubMed] [Google Scholar]
- 125.Cusso ME, Donald KJ, Khoo TK. The Impact of Physical Activity on Non-Motor Symptoms in Parkinson’s Disease: A Systematic Review. Front Med (Lausanne). 2016;3: 35. doi: 10.3389/fmed.2016.00035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yitayeh A, Teshome A. The effectiveness of physiotherapy treatment on balance dysfunction and postural instability in persons with Parkinson’s disease: a systematic review and meta-analysis. BMC Sports Sci Med Rehabil. 2016;8: 17. doi: 10.1186/s13102-016-0042-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mazzarin CM, Valderramas SR, Ferreira MP, Tiepolo E, Guérios L, Parissoto D, et al. Effects of Dance and of Tai Chi on Functional Mobility, Balance, and Agility in Parkinson Disease A Systematic Review and Meta-analysis. Topics in Geriatric Rehab. 2017;(33): 262–272. doi: 10.1097/TGR.0000000000000163 [DOI] [Google Scholar]
- 128.Connors MH, Quinto L, McKeith I, Brodaty H, Allan L, Bamford C, et al. Non-pharmacological interventions for Lewy body dementia: a systematic review. Psychol Med. 2018;48(11): 1749–1758. doi: 10.1017/S0033291717003257 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carroll LM, Morris ME, O’Connor WT, Clifford AM. Is Aquatic Therapy Optimally Prescribed for Parkinson’s Disease? A Systematic Review and Meta-Analysis. J Parkinsons Dis. 2020;10(1): 59–76. doi: 10.3233/JPD-191784 . [DOI] [PubMed] [Google Scholar]
- 130.Morris ME, Ellis TD, Jazayeri D, Heng H, Thomson A, Balasundaram AP, et al. Boxing for Parkinson’s Disease: Has Implementation Accelerated Beyond Current Evidence? Front Neurol. 2019;10: 1222. doi: 10.3389/fneur.2019.01222 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pritchard M, Laster J, Rogers L, Schultz M, Babl R. The Effects of Aquatic Therapy on Gait, Balance, and Quality of Life in Patients with Parkinson’s Disease. J Aquatic Phys Ther. 2019;27(2): 10–24. [Google Scholar]
- 132.Barnish MS, Barran SM. A systematic review of active group-based dance, singing, music therapy and theatrical interventions for quality of life, functional communication, speech, motor function and cognitive status in people with Parkinson’s disease. BMC Neurol. 2020;20(1): 371. doi: 10.1186/s12883-020-01938-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gomes Neto M, Pontes SS, Almeida LO, da Silva CM, da Conceição Sena C, Saquetto MB. Effects of water-based exercise on functioning and quality of life in people with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil. 2020;34(12): 1425–1435. doi: 10.1177/0269215520943660 . [DOI] [PubMed] [Google Scholar]
- 134.Hidalgo-Agudo RD, Lucena-Anton D, Luque-Moreno C, Heredia-Rizo AM, Moral-Munoz JA. Additional Physical Interventions to Conventional Physical Therapy in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J Clin Med. 2020;9(4): 1038. doi: 10.3390/jcm9041038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cosentino C, Baccini M, Putzolu M, Ristori D, Avanzino L, Pelosin E. Effectiveness of Physiotherapy on Freezing of Gait in Parkinson’s Disease: A Systematic Review and Meta-Analyses. Mov Disord. 2020;35(4): 523–536. doi: 10.1002/mds.27936 . [DOI] [PubMed] [Google Scholar]
- 136.Oh S, Lee S. Effect of aquatic exercise on physical function and QOL in individuals with neurological disorder: A systematic review and meta-analysis. J Bodyw Mov Ther. 2021;27: 67–76. doi: 10.1016/j.jbmt.2021.01.009 . [DOI] [PubMed] [Google Scholar]
- 137.Hasan SM, Alshafie S, Hasabo EA, Saleh M, Elnaiem W, Qasem A, et al. Efficacy of dance for Parkinson’s disease: a pooled analysis of 372 patients. J Neurol. 2022;269(3): 1195–1208. doi: 10.1007/s00415-021-10589-4 . [DOI] [PubMed] [Google Scholar]
- 138.Lim I, van Wegen E, de Goede C, Deutekom M, Nieuwboer A, Willems A, et al. Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: a systematic review. Clin Rehabil. 2005; 19(7): 695–713. doi: 10.1191/0269215505cr906oa . [DOI] [PubMed] [Google Scholar]
- 139.Crizzle AM, Newhouse IJ. Is physical exercise beneficial for persons with Parkinson’s disease? Clin J Sport Med. 2006;16(5): 422–425. doi: 10.1097/01.jsm.0000244612.55550.7d . [DOI] [PubMed] [Google Scholar]
- 140.Kwakkel G, de Goede CJ, van Wegen EE. Impact of physical therapy for Parkinson’s disease: a critical review of the literature. Parkinsonism Relat Disord. 2007;13 Suppl 3: S478–487. doi: 10.1016/S1353-8020(08)70053-1 . [DOI] [PubMed] [Google Scholar]
- 141.Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson’s disease: a systematic review across the disability spectrum. J Neurol Phys Ther. 2009;33(1): 14–26. doi: 10.1097/NPT.0b013e3181990fcc . [DOI] [PubMed] [Google Scholar]
- 142.de Dreu MJ, van der Wilk AS, Poppe E, Kwakkel G, van Wegen EE. Rehabilitation, exercise therapy and music in patients with Parkinson’s disease: a meta-analysis of the effects of music-based movement therapy on walking ability, balance and quality of life. Parkinsonism Relat Disord. 2012;18 Suppl 1: S114–119. doi: 10.1016/S1353-8020(11)70036-0 . [DOI] [PubMed] [Google Scholar]
- 143.Foster ER, Bedekar M, Tickle-Degnen L. Systematic review of the effectiveness of occupational therapy-related interventions for people with Parkinson’s disease. Am J Occup Ther. 2014;68(1): 39–49. doi: 10.5014/ajot.2014.008706 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mandelbaum R, Lo AC. Examining dance as an intervention in Parkinson´s disease: a systematic review. Am J Dance Ther. 2014;(36): 160–175. doi: 10.1007/s10465-014-9181-6 [DOI] [Google Scholar]
- 145.Murray DK, Sacheli MA, Eng JJ, Stoessl AJ. The effects of exercise on cognition in Parkinson’s disease: a systematic review. Transl Neurodegener. 2014;3(1): 5. doi: 10.1186/2047-9158-3-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alves Da Rocha P, McClelland J, Morris ME. Complementary physical therapies for movement disorders in Parkinson’s disease: a systematic review. Eur J Phys Rehabil Med. 2015;51(6): 693–704. . [PubMed] [Google Scholar]
- 147.Shanahan J, Morris ME, Bhriain ON, Saunders J, Clifford AM. Dance for people with Parkinson disease: what is the evidence telling us? Arch Phys Med Rehabil. 2015;96(1): 141–153. doi: 10.1016/j.apmr.2014.08.017 . [DOI] [PubMed] [Google Scholar]
- 148.Aguiar C, da Rocha PA, Morris M. Therapeutic Dancing for Parkinson’s Disease. Int. J. Gerodontology 2016;(10): 64–70. doi: 10.1016/j.ijge.2016.02.002 [DOI] [Google Scholar]
- 149.McNeely ME, Duncan RP, Earhart GM. A comparison of dance interventions in people with Parkinson disease and older adults. Maturitas. 2015;81(1): 10–16. doi: 10.1016/j.maturitas.2015.02.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu PL, Lee M, Huang TT. Effectiveness of physical activity on patients with depression and Parkinson’s disease: A systematic review. PLoS One. 2017; 12(7): e0181515. doi: 10.1371/journal.pone.0181515 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Costa PS, Villas Bôas ECC, Fonseca EP. Efetividade do treino de marcha na água para pacientes com Doença de Parkinson: revisão sistemática. Rev Pesq Fisioter. 2018;(8): 551–557. doi: 10.17267/2238-2704rpf.v8i4.2034 [DOI] [Google Scholar]
- 152.De Freitas TB MS, PT, Leite PHW BS, Doná F PhD, PT, Pompeu JE PhD, PT, Swarowsky A PhD, PT, Torriani-Pasin C PhD, PT. The effects of dual task gait and balance training in Parkinson’s disease: a systematic review. Physiother Theory Pract. 2020;36(10): 1088–1096. doi: 10.1080/09593985.2018.1551455 . [DOI] [PubMed] [Google Scholar]
- 153.Chiong JD, Patel V, Tairan HM. Effects of Physical Exercise on Gait and Balance in Patients with Parkinson’s Disease. Crit Rev Phys Rehabil Med. 2019;31(3): 217–232. doi: 10.1615/CritRevPhysRehabilMed.2019032612 [DOI] [Google Scholar]
- 154.Pupíková M, Rektorová I. Non-pharmacological management of cognitive impairment in Parkinson’s disease. J Neural Transm (Vienna). 2020;127(5): 799–820. doi: 10.1007/s00702-019-02113-w . [DOI] [PubMed] [Google Scholar]
- 155.Radder DLM, Lígia Silva de Lima A, Domingos J, Keus SHJ, van Nimwegen M, Bloem BR, et al. Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities. Neurorehabil Neural Repair. 2020;34(10): 871–880. doi: 10.1177/1545968320952799 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Foster ER, Carson LG, Archer J, Hunter EG. Occupational Therapy Interventions for Instrumental Activities of Daily Living for Adults With Parkinson’s Disease: A Systematic Review. Am J Occup Ther. 2021;75(3): 7503190030p1–7503190030p24. doi: 10.5014/ajot.2021.046581 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. New Jersey: John & Sons, Ltda; 2009. [Google Scholar]
- 158.Li X, Li Y, Guo K, Chen N, Cui X, Bai Z, et al. Evidence Based Social Science in China Paper 3: The quality of social science RCTs published from 2000–2020. J Clin Epidemiol. 2022;141: 64–73. doi: 10.1016/j.jclinepi.2021.09.014 . [DOI] [PubMed] [Google Scholar]
- 159.Cheng YC, Su CH. Evidence Supports PA Prescription for Parkinson’s Disease: Motor Symptoms and Non-Motor Features: A Scoping Review. Int J Environ Res Public Health. 2020;17(8): 2894. doi: 10.3390/ijerph17082894 Erratum in: Int J Environ Res Public Health. 2020;17(14): . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Franchignoni F, Giordano A, Ronconi G, Rabini A, Ferriero G. Rasch validation of the Activities-specific Balance Confidence Scale and its short versions in patients with Parkinson’s disease. J Rehabil Med. 2014;46(6): 532–539. doi: 10.2340/16501977-1808 . [DOI] [PubMed] [Google Scholar]
- 161.Morley D, Dummett S, Kelly L, Dawson J, Jenkinson C. Evaluating the psychometric properties of an e-based version of the 39-item Parkinson’s Disease Questionnaire. Health Qual Life Outcomes. 2015;13: 5. doi: 10.1186/s12955-014-0193-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schootemeijer S, van der Kolk NM, Bloem BR, de Vries NM. Current Perspectives on Aerobic Exercise in People with Parkinson’s Disease. Neurotherapeutics. 2020;17(4): 1418–1433. doi: 10.1007/s13311-020-00904-8 Erratum in: Neurotherapeutics. 2022;19(2): 683–685. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5(6): 311–22. doi: 10.1038/nrneurol.2009.54 . [DOI] [PubMed] [Google Scholar]
- 164.Jiang L, Zhang H, Wang C, Ming F, Shi X, Yang M. Serum level of brain-derived neurotrophic factor in Parkinson’s disease: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88: 168–174. doi: 10.1016/j.pnpbp.2018.07.010 . [DOI] [PubMed] [Google Scholar]
- 165.Bouça-Machado R, Rosário A, Caldeira D, Castro Caldas A, Guerreiro D, Venturelli M, et al. Physical Activity, Exercise, and Physiotherapy in Parkinson’s Disease: Defining the Concepts. Mov Disord Clin Pract. 2019;7(1): 7–15. doi: 10.1002/mdc3.12849 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bhalsing KS, Abbas MM, Tan LCS. Role of Physical Activity in Parkinson’s Disease. Ann Indian Acad Neurol. 2018;21(4): 242–249. doi: 10.4103/aian.AIAN_169_18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.