Abstract
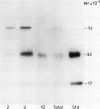
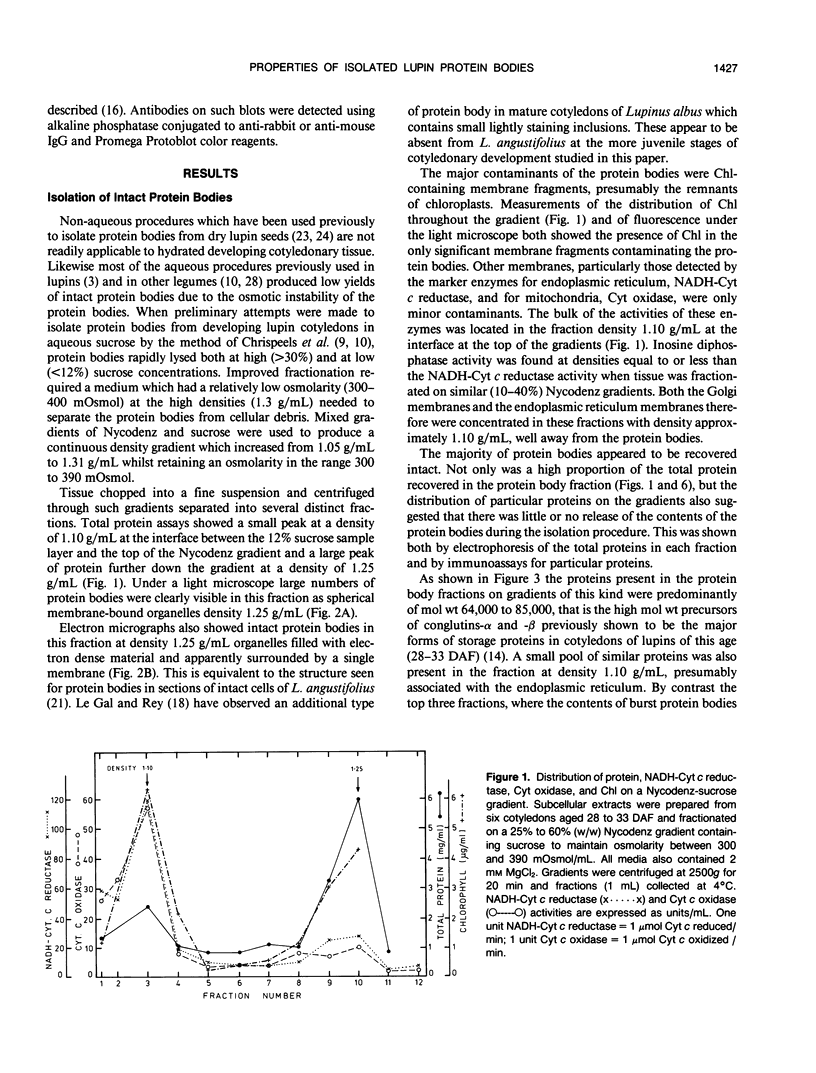
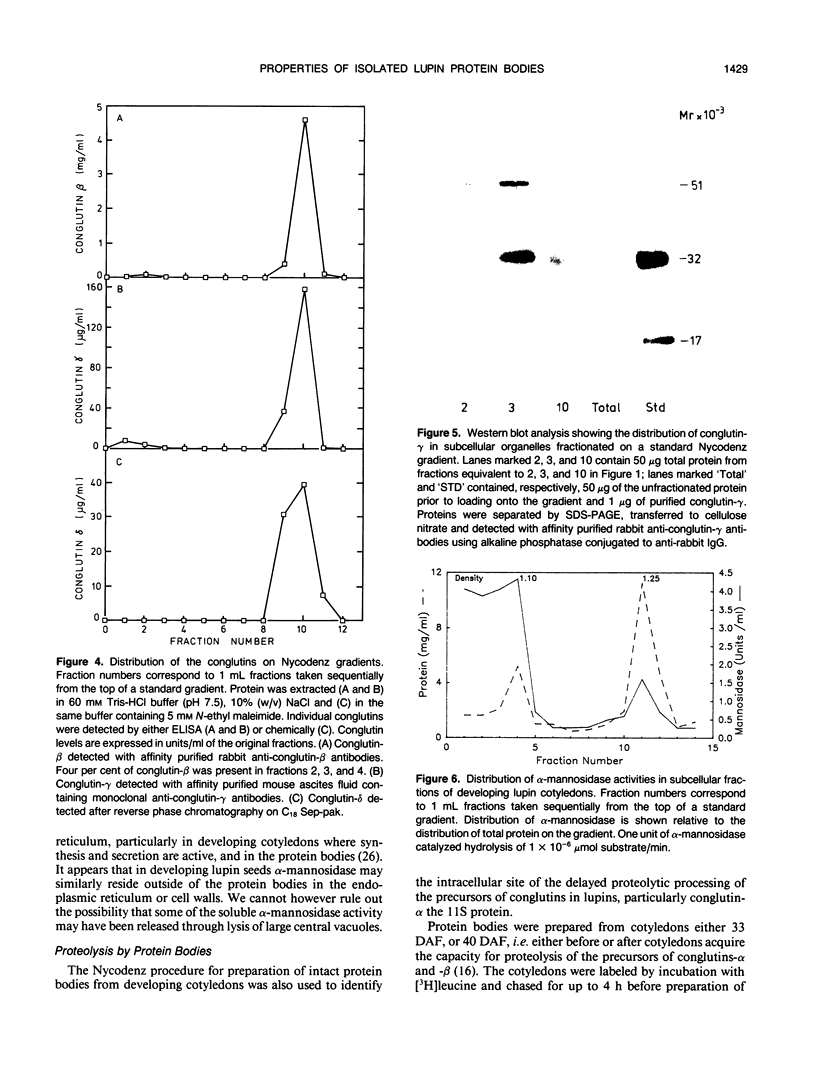
Using Nycodenz, a novel density gradient medium, we isolated intact protein bodies from developing seeds of Lupinus angustifolius L. (cultivar Unicrop) and achieved excellent separation from the endoplasmic reticulum, mitochondria, and other organelles. The distribution of the storage protein conglutin-β was taken as evidence that up to 96% of the protein bodies remained intact on the gradients and banded at 1.25 grams per milliliter. The protein bodies also contained the three other abundant proteins present in L. angustifolius seeds: conglutins-α, -γ, and -δ. Pulse labeling experiments were carried out to determine the site of proteolytic processing of conglutin-α, a legumin-like 11Svedberg unit storage protein. Cotyledons aged either 33 or 40 days after flowering were pulsed with [3H]leucine. Protein bodies obtained from the cotyledons aged 33 days after flowering contained only the labeled precursors of conglutin-α with molecular weights 85,000, 72,000, and 64,000, even after a 4 hour chase of the radioactivity. Protein bodies obtained from the cotyledons aged 40 days after flowering contained the same radioactive precursors if the tissue had been pulsed for 2 hours, and the processing products of these precursors when the tissue had been chased for 4 hours. These studies confirm that the subcellular location of proteolytic cleavage of this legumin-like protein is the protein body, that this activity is detected only in protein bodies from lupin seeds aged between 33 and 40 days of seed development after flowering and that protein bodies from seeds younger than this contain only unprocessed conglutin-α.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton K. A., Thompson J. F., Madison J. T., Rosenthal R., Jarvis N. P., Beachy R. N. The biosynthesis and processing of high molecular weight precursors of soybean glycinin subunits. J Biol Chem. 1982 Jun 10;257(11):6089–6095. [PubMed] [Google Scholar]
- Bowles D. J., Kauss H. Characterization, enzymatic and lectin properties of isolated membranes from Phaseolus aureus. Biochim Biophys Acta. 1976 Sep 7;443(3):360–374. doi: 10.1016/0005-2736(76)90456-9. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Craig S., Spencer D. Role of the endoplasmic reticulum in the synthesis of reserve proteins and the kinetics of their transport to protein bodies in developing pea cotyledons. J Cell Biol. 1982 Apr;93(1):5–14. doi: 10.1083/jcb.93.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Spencer D. Assembly of storage protein oligomers in the endoplasmic reticulum and processing of the polypeptides in the protein bodies of developing pea cotyledons. J Cell Biol. 1982 May;93(2):306–313. doi: 10.1083/jcb.93.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. L., Rödin J., Lenman M., Glimelius K., Josefsson L. G., Rask L. Structure of the rapeseed 1.7 S storage protein, napin, and its precursor. J Biol Chem. 1986 Nov 5;261(31):14576–14581. [PubMed] [Google Scholar]
- Ford T. C., Rickwood D. Formation of isotonic Nycodenz gradients for cell separations. Anal Biochem. 1982 Aug;124(2):293–298. doi: 10.1016/0003-2697(82)90041-0. [DOI] [PubMed] [Google Scholar]
- Gayler K. R., Boadle B. G., Snook M., Johnson E. D. Precursors of storage proteins in Lupinus angustifolius. Biochem J. 1984 Jul 15;221(2):333–341. doi: 10.1042/bj2210333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura I., Nishimura M. Proglobulin processing enzyme in vacuoles isolated from developing pumpkin cotyledons. Plant Physiol. 1987 Oct;85(2):440–445. doi: 10.1104/pp.85.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Chrispeels M. J., Chandler P. M., Spencer D. Intracellular sites of synthesis and processing of lectin in developing pea cotyledons. J Biol Chem. 1983 Aug 10;258(15):9550–9552. [PubMed] [Google Scholar]
- Johnson E. D., Knight J., Gayler K. R. Biosynthesis and processing of legumin-like storage proteins in Lupinus angustifolius (lupin). Biochem J. 1985 Dec 15;232(3):673–679. doi: 10.1042/bj2320673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Tulloch P. A., Blagrove R. J. Electron microscopy of seed-storage globulins. Arch Biochem Biophys. 1985 Sep;241(2):521–532. doi: 10.1016/0003-9861(85)90577-6. [DOI] [PubMed] [Google Scholar]
- Van Der Wilden W., Chrispeels M. J. Characterization of the Isozymes of alpha-Mannosidase Located in the Cell Wall, Protein Bodies, and Endoplasmic Reticulum of Phaseolus vulgaris Cotyledons. Plant Physiol. 1983 Jan;71(1):82–87. doi: 10.1104/pp.71.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walburg G., Larkins B. A. Oat seed globulin: subunit characterization and demonstration of its synthesis as a precursor. Plant Physiol. 1983 May;72(1):161–165. doi: 10.1104/pp.72.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]