Abstract
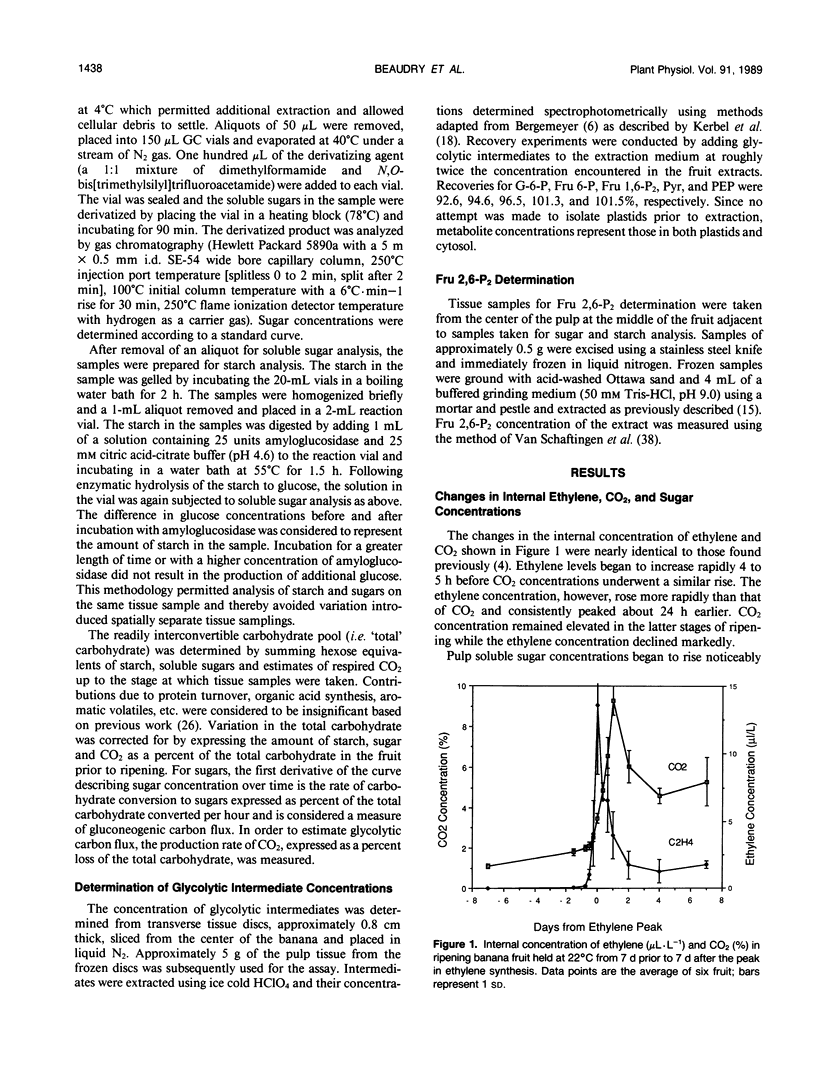
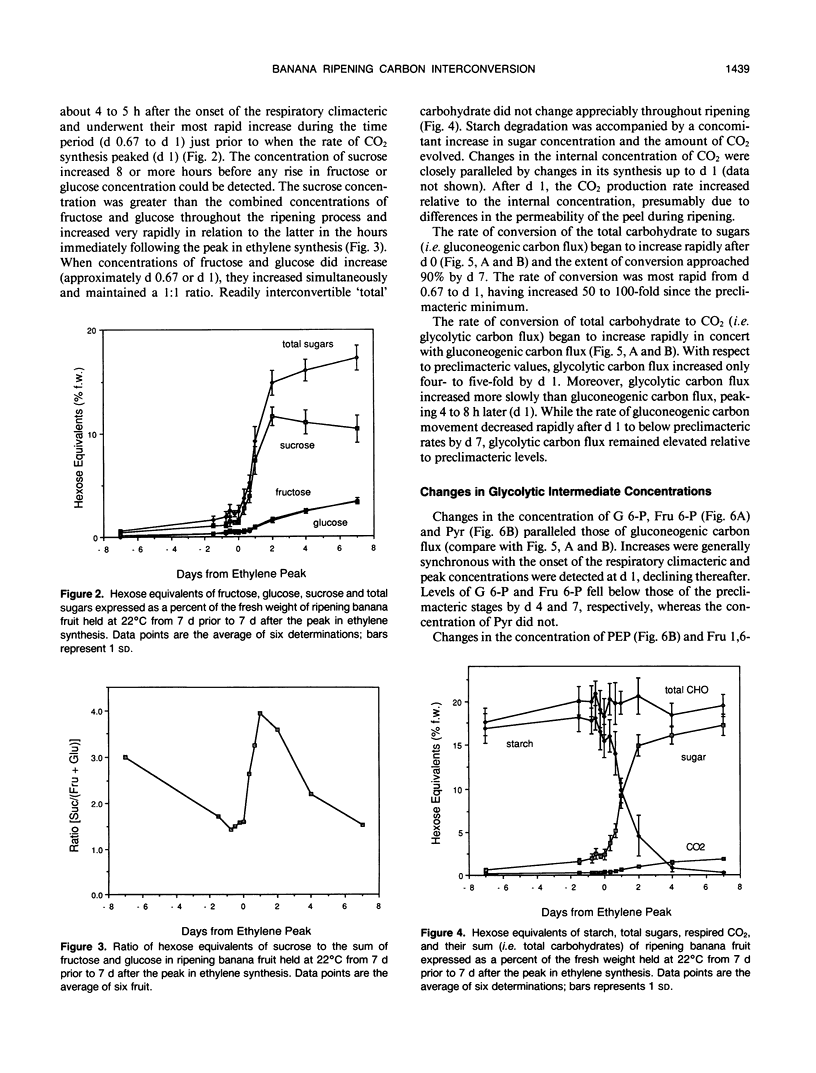
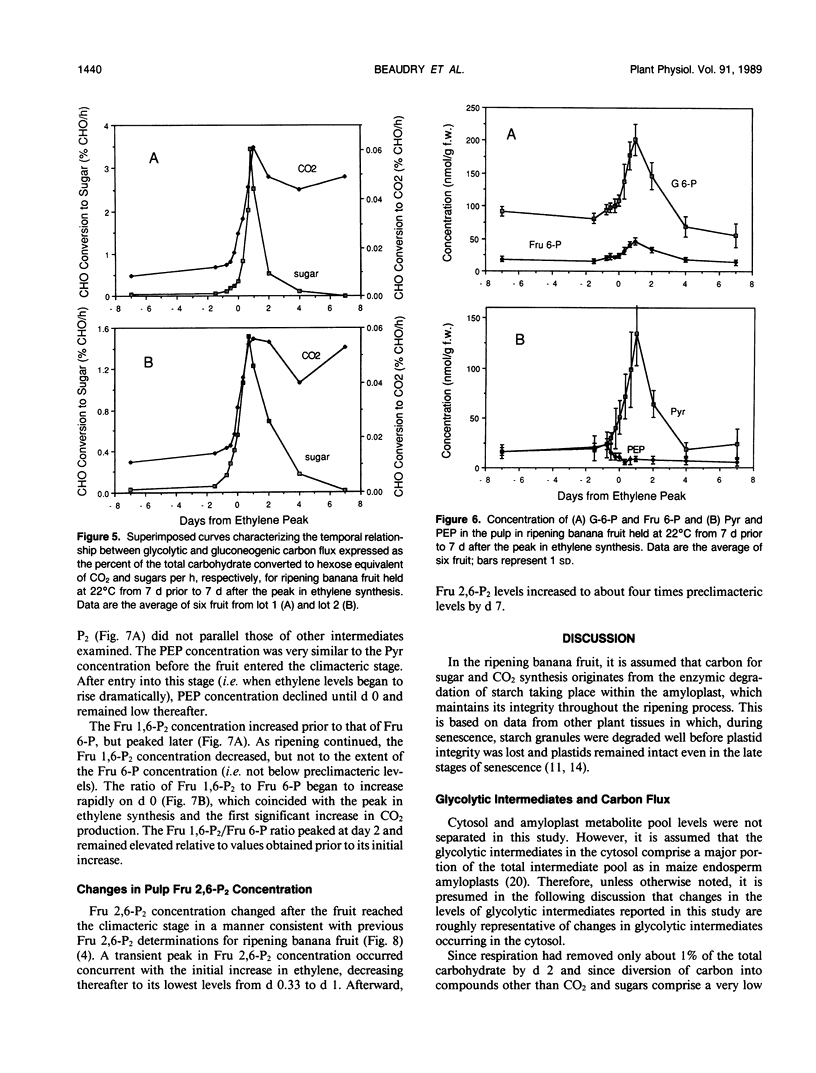
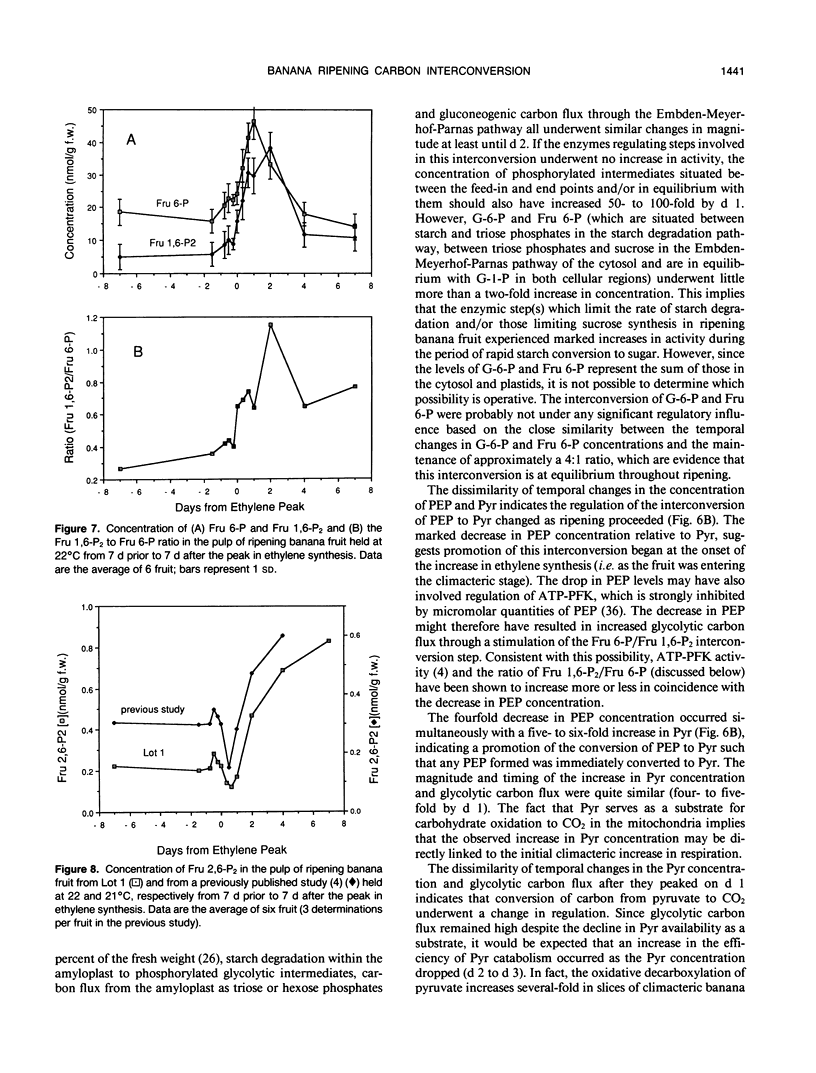
In ripening banana (Musa sp. [AAA group, Cavendish subgroup] cv Valery) fruit, the concentration of glycolytic intermediates increased in response to the rapid conversion of starch to sugars and CO2. Glucose 6-phosphate (G-6-P), fructose 6-phosphate (Fru 6-P), and pyruvate (Pyr) levels changed in synchrony, increasing to a maximum one day past the peak in ethylene synthesis and declining rapidly thereafter. Fructose 1,6-bisphosphate (Fru 1,6-P2) and phosphoenolpyruvate (PEP) levels underwent changes dissimilar to those of G 6-P, Fru 6-P, and Pyr, indicating that carbon was regulated at the PEP/Pyr and Fru 6-P/Fru 1,6-P2 interconversion sites. During the climacteric respiratory rise, gluconeogenic carbon flux increased 50- to 100-fold while glycolytic carbon flux increased only 4- to 5-fold. After the climacteric peak in CO2 production, gluconeogenic carbon flux dropped dramatically while glycolytic carbon flux remained elevated. The steady-state fructose 2,6-bisphosphate (Fru 2,6-P2) concentration decreased to ½ that of preclimacteric fruit during the period coinciding with the rapid increase in gluconeogenesis. Fru 2,6-P2 concentration increased thereafter as glycolytic carbon flux increased relative to gluconeogenic carbon flux. It appears likely that the initial increase in respiration in ripening banana fruit is due to the rapid influx of carbon into the cytosol as starch is degraded. As starch reserves are depleted and the levels of intermediates decline, the continued enhancement of respiration may, in part, be maintained by an increased steady-state Fru 2,6-P2 concentration acting to promote glycolytic carbon flux at the step responsible for the interconversion of Fru 6-P and Fru 1,6-P2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudry R. M., Paz N., Black C. C., Kays S. J. Banana Ripening: Implications of Changes in Internal Ethylene and CO(2) Concentrations, Pulp Fructose 2,6-Bisphosphate Concentration, and Activity of Some Glycolytic Enzymes. Plant Physiol. 1987 Sep;85(1):277–282. doi: 10.1104/pp.85.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. B., Smith G. M., Nichols B. G. Regulation of climacteric respiration in ripening avocado fruit. Plant Physiol. 1987 Apr;83(4):973–976. doi: 10.1104/pp.83.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biale J. B. Growth, Maturation, and Senescence in Fruits: Recent knowledge on growth regulation and on biological oxidations has been applied to studies with fruits. Science. 1964 Nov 13;146(3646):880–888. doi: 10.1126/science.146.3646.880. [DOI] [PubMed] [Google Scholar]
- Carnal N. W., Black C. C. Pyrophosphate-dependent 6-phosphofructokinase, a new glycolytic enzyme in pineapple leaves. Biochem Biophys Res Commun. 1979 Jan 15;86(1):20–26. doi: 10.1016/0006-291x(79)90376-0. [DOI] [PubMed] [Google Scholar]
- Chalmers D. J., Rowan K. S. The climacteric in ripening tomato fruit. Plant Physiol. 1971 Sep;48(3):235–240. doi: 10.1104/pp.48.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cséke C., Weeden N. F., Buchanan B. B., Uyeda K. A special fructose bisphosphate functions as a cytoplasmic regulatory metabolite in green leaves. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4322–4326. doi: 10.1073/pnas.79.14.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria E., Boyer C. D., Thomas P. A., Liu K. C., Shannon J. C. Enzyme activities associated with maize kernel amyloplasts. Plant Physiol. 1988 Mar;86(3):786–792. doi: 10.1104/pp.86.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey-Wyssling A., Schwegler F. Ultrastructure of the chromoplasts in the carrot root. J Ultrastruct Res. 1965 Dec;13(5):543–559. doi: 10.1016/s0022-5320(65)90013-4. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Bickett D. M. Evidence for control of carbon partitioning by fructose 2,6-bisphosphate in spinach leaves. Plant Physiol. 1984 Feb;74(2):445–447. doi: 10.1104/pp.74.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling P. L., Wood J. R., Tyson R. H., Bridges I. G. Starch Biosynthesis in Developing Wheat Grain : Evidence against the Direct Involvement of Triose Phosphates in the Metabolic Pathway. Plant Physiol. 1988 Jun;87(2):311–319. doi: 10.1104/pp.87.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel E. L., Kader A. A., Romani R. J. Effects of Elevated CO(2) Concentrations on Glycolysis in Intact ;Bartlett' Pear Fruit. Plant Physiol. 1988 Apr;86(4):1205–1209. doi: 10.1104/pp.86.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R. A., Rees T., Fuller W. A., Banfield J. The location of acid invertase activity and sucrose in the vacuoles of storage roots of beetroot (Beta vulgaris). Biochem J. 1979 Mar 15;178(3):539–547. doi: 10.1042/bj1780539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. T., Shannon J. C. Measurement of Metabolites Associated with Nonaqueously Isolated Starch Granules from Immature Zea mays L. Endosperm. Plant Physiol. 1981 Mar;67(3):525–529. doi: 10.1104/pp.67.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens E., Marcellin P., Van Schaftingen E., Hers H. G. Effect of ethylene treatment on the concentration of fructose-2,6-bisphosphate and on the activity of phosphofructokinase 2/fructose-2,6-bisphosphatase in banana. Eur J Biochem. 1987 Sep 15;167(3):579–583. doi: 10.1111/j.1432-1033.1987.tb13375.x. [DOI] [PubMed] [Google Scholar]
- Mohabir G., John P. Effect of temperature on starch synthesis in potato tuber tissue and in amyloplasts. Plant Physiol. 1988 Dec;88(4):1222–1228. doi: 10.1104/pp.88.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz N., Xu D. P., Black C. C. Rapid oscillations in fructose 2,6-bisphosphate levels in plant tissues. Plant Physiol. 1985 Dec;79(4):1133–1136. doi: 10.1104/pp.79.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer G., Heber U. Glucose transport into spinach chloroplasts. Plant Physiol. 1977 Aug;60(2):286–289. doi: 10.1104/pp.60.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Herzog B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : V. Modulation of the Spinach Leaf Cytosolic Fructose 1,6-Bisphosphatase Activity in Vitro by Substrate, Products, pH, Magnesium, Fructose 2,6-Bisphosphate, Adenosine Monophosphate, and Dihydroxyacetone Phosphate. Plant Physiol. 1985 Nov;79(3):590–598. doi: 10.1104/pp.79.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Wu M. X., Smyth D. A., Black C. C. Regulation of pea seed pyrophosphate-dependent phosphofructokinase: Evidence for interconversion of two molecular forms as a glycolytic regulatory mechanism. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5051–5055. doi: 10.1073/pnas.81.16.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]


