Abstract
An improved selective medium, cellobiose-colistin (CC) agar, gave a significantly higher (P < 0.05) isolation rate of Vibrio vulnificus from water and sediment samples than did modified cellobiose-polymyxin B-colistin (mCPC) agar. In a total of 446 alkaline peptone water preenrichments amended with polymyxin B, V. vulnificus was isolated from 154 preenrichments (35%) with mCPC agar and from 179 preenrichments (40%) with CC agar. CC agar gave a higher plating efficiency of V. vulnificus cells than did cellobiose-polymyxin B-colistin (CPC) agar, mCPC agar, or thiosulfate-citrate-bile salts-sucrose (TCBS) agar; the only significant difference was observed with TCBS agar, which gave much lower plating efficiencies than the other selective media. Determination of MICs demonstrated that the concentrations of colistin and polymyxin B in CPC agar inhibit growth of a proportion of V. vulnificus strains.
Vibrio vulnificus is an autochthonous bacterium of estuarine waters in both temperate and tropical climates which can cause septicemias and severe wound infections in humans (5, 8). Infection generally occurs through consumption of contaminated raw or minimally cooked shellfish or by direct invasion through wounds (13).
A great number and variety of bacteria are present in environmental samples; therefore, the use of selective media is necessary for the isolation of V. vulnificus. Preenrichment in alkaline peptone water (APW) (1% NaCl [pH 8.6]) in combination with thiosulfate-citrate-bile salts-sucrose (TCBS) agar has been routinely used for the isolation of pathogenic vibrios for many years. However, the use of TCBS agar for the isolation of pathogenic Vibrio spp. has been questioned, and several new media have been developed and recommended for isolation of V. vulnificus (2, 3, 10, 11).
The use of cellobiose-polymyxin B-colistin (CPC) agar has proven successful in the isolation and differentiation of V. vulnificus in laboratory testing (10). This medium takes advantage of the resistance of V. vulnificus to colistin and polymyxin B; in addition, high-temperature incubation, at 40°C, inhibits the growth of many marine bacteria, and the fermentation of cellobiose acts as a further differential criterion. In subsequent field studies, CPC agar was effective in environmental monitoring and was superior to TCBS, sodium dodecyl sulfate-polymyxin B-sucrose agar, and V. vulnificus enumeration agar in the isolation of V. vulnificus (14, 16). Tamplin et al. (18) used a modification of CPC agar, termed mCPC, with a reduced concentration of colistin. mCPC agar has also been reported to be effective for the isolation of V. vulnificus from environmental sources (6, 17, 18).
Colistin and polymyxin B are both fatty acyl decapeptide antibiotics with bactericidal activity against most gram-negative bacteria (15). The chemical compositions of colistin and polymyxin B differ only in a single amino acid, and their modes of action and microbiological activities are identical (15). Arguments for using both of these chemically related antibiotics in V. vulnificus-selective agars have not been provided (10, 18). Furthermore, no data have been presented to establish the optimal concentrations of colistin or polymyxin B in CPC or mCPC agar (10, 18), nor have the efficiencies of CPC and mCPC agars in the isolation of V. vulnificus been compared. Preliminary studies in our laboratory indicated that the growth of several V. vulnificus strains was inhibited on CPC and mCPC agars. Thus, we felt there was a need to investigate whether the concentrations of colistin and polymyxin B in CPC and mCPC agars are optimal for the isolation of V. vulnificus while inhibiting undesirable background flora. A less selective medium, cellobiose-colistin (CC) agar, was therefore tested in comparison studies.
MATERIALS AND METHODS
Bacterial strains.
Fifty clinical and environmental V. vulnificus strains from various sources and countries were used in plating efficiency experiments and MIC testing. The clinical strains originated from Denmark (n = 11), Sweden (n = 1), Germany (n = 2), Holland (n = 2), and the United States (n = 5). The majority of the environmental strains were isolated from seawater, sediment, wild and diseased eels in Denmark, and eel pouts in Denmark (n = 18), as previously described (9). Strains isolated from diseased eels (n = 4) and seawater (n = 1) in Holland, seawater from the Baltic Sea (n = 1), Gulf of Mexico oysters (n = 3), and shrimps imported to Denmark from Thailand (n = 2) were also tested (6). Fourteen strains had originally been isolated on mCPC agar, whereas 36 strains, to the best of our knowledge, had been isolated on a medium not containing any colistin or polymyxin B. An Escherichia coli K-12 strain (JEO 699) which is lactose positive, nalidixic acid resistant, and sensitive to colistin was included as a control in the MIC testing.
Media.
Four selective media were used in plating efficiency experiments: TCBS, CPC, mCPC, and CC agars. TCBS agar (Difco, Detroit, Mich.) was prepared according to the instructions of the manufacturer. The composition and preparation of CPC and mCPC agars have been described elsewhere (10, 18). CC agar has the same basic composition as the originally described CPC agar, but it contains no polymyxin B and the concentration of colistin is decreased from 1 × 106 U/liter to 4 × 105 U/liter, which is the same concentration of colistin as in mCPC agar (18). We chose this colistin concentration because we wanted to vary only one parameter compared to mCPC agar (18). Table 1 shows the concentrations of colistin and polymyxin B in CPC, mCPC, and CC agars.
TABLE 1.
Concentrations of colistin and polymyxin B in CPC, mCPC, and CC agars
| Antibiotic (unit) | Agar
|
||
|---|---|---|---|
| CPC | mCPC | CC | |
| Colistin (U/liter) | 1.4 × 106 | 4.0 × 105 | 4.0 × 105 |
| Polymyxin B (U/liter) | 1.0 × 105 | 1.0 × 105 | —a |
| Total colistin (mg/ml)b | 0.09 | 0.044 | 0.03 |
—, there is no polymyxin B in CC agar.
Polymyxin B units were converted to milligrams of colistin by means of activity per milligram.
Determination of MICs.
MICs of colistin (colistin sulfate, 12,990 U/mg; Dumex Alpharma, Copenhagen, Denmark) were determined by a broth microdilution method in 96-well plates. Since colistin and polymyxin B have identical microbiological activities, we determined MICs for only one of these antibiotics. Mueller-Hinton broth (Difco) with 1% NaCl was used as a test medium. Each strain was inoculated in duplicate wells to give a final inoculum concentration of 5 × 105 CFU/ml in 200 μl of test broth (19). We used the following concentrations of colistin: 0.72, 0.36, 0.18, 0.09, 0.08, 0.07, 0.06, 0.05, 0.04, 0.03, and 0.02 mg/ml. Two wells not containing colistin were inoculated with each strain as growth controls. The plates were covered with lids to prevent evaporation and were incubated at 37°C for 48 h. Bacterial growth was read after 24 and 48 h. The control wells were examined for growth after 24 h, and an aliquot was subcultured onto blood agar (BA) plates (blood agar base [Difco] with 5% citrated calf blood) to verify inoculum purity.
Plating efficiency.
The term plating efficiency can be defined as the percentage of CFU which can be recovered on a selective medium compared to the CFU encountered on a corresponding nonselective BA plate. Cultures were grown overnight with agitation at 37°C in veal infusion broth (Difco) and were 10-fold serially diluted in physiological saline solution (0.9% NaCl). An aliquot of 100 μl from each dilution was plated in duplicate onto CC, mCPC, CPC, TCBS, and BA plates. CC, mCPC, and CPC plates were incubated at 40°C, and TCBS and BA plates were incubated at 37°C. Plates with CFU ranging from 30 to 300 were counted after 24 h and again after 48 h for the CC, mCPC, and CPC agar plates. Recovery rates of V. vulnificus strains on the different selective media were compared by using Kruskal-Wallis one-way nonparametric analysis of variance (Statistix for Windows; Analytical Software, Tallahassee, Fla.).
Comparison of mCPC and CC agars for isolation of V. vulnificus from water and sediment samples.
Water and sediment samples were collected at various coastal sites in Denmark during the summers of 1996 and 1997 (9). Samples were analyzed by a three-tube most probable number method with an APW preenrichment supplemented with polymyxin B (APWP) (1% NaCl, 2.0 × 104 U of polymyxin B per liter [pH 8.6]) and incubated at 37°C for 18 to 24 h (9). We reported previously that the use of APWP yielded a higher recovery of V. vulnificus than did APW with no polymyxin B in analyses of water and sediment (4). A standardized loop (QuadLoop, Miniplast; EIN-SHEMER, Post Menashe, Israel) was used to streak 1 μl of each preenrichment tube onto mCPC and CC agar, respectively, followed by overnight incubation at 40°C. Two V. vulnificus-like colonies were selected and subcultured from each mCPC and CC agar plate. The sizes of the V. vulnificus colonies on both media were recorded. The identity of each isolate was verified by colony hybridization with a V. vulnificus-specific alkaline phosphatase-labeled (VVAP) DNA probe directed against a cytolysin-hemolysin gene (12). Data for the isolation of V. vulnificus were compared by using McNemar’s chi-square statistic (7). The growth and colony morphology of any background flora were described for each agar plate. Representative strains of the background flora were identified by routine tests for biochemical properties (1).
RESULTS
MIC testing.
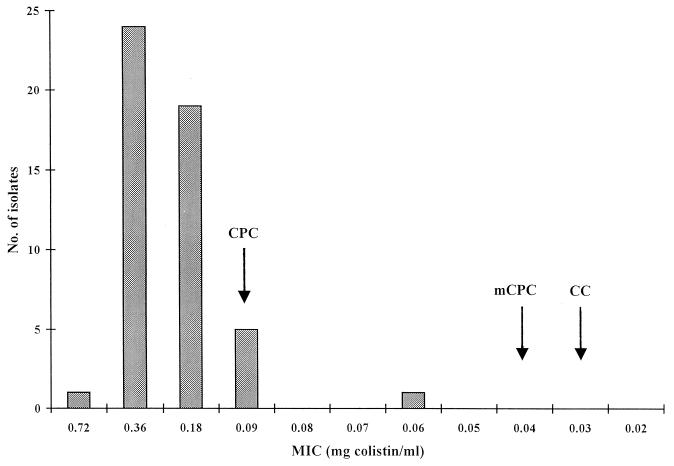
The distributions of MICs for the 50 V. vulnificus strains are shown in Fig. 1. Twenty-four strains (48%) had a MIC of 0.36 mg of colistin/ml, 19 strains (38%) had a MIC of 0.18 mg of colistin/ml, and 5 strains (10%) had a MIC of 0.09 mg of colistin/ml. Two strains showed MICs of 0.72 and 0.06 mg of colistin/ml. Most of the strains (88%) showed MICs above the concentration of colistin in CPC agar (0.090 mg/ml). However, six strains (12%) had MICs that were identical to or below the concentration of colistin in CPC agar. These six strains were isolated from a clinical case in Denmark (n = 1), diseased eels in Denmark and Holland (n = 3), seawater from the Baltic Sea (n = 1), and Gulf Coast oysters (n = 1). No strains had MICs similar to the concentrations of colistin in mCPC or CC agar.
FIG. 1.
Distribution of MICs of colistin for 50 V. vulnificus strains. Arrows indicate the concentrations of colistin in CPC, mCPC, and CC agars.
Plating efficiency testing.
CC agar yielded a mean recovery rate of V. vulnificus cells of 100%. CPC and mCPC agars showed recovery rates of 76% and 93%, respectively (Table 2). Plating efficiencies varied considerably among the 50 V. vulnificus strains tested; therefore, high standard deviations were observed. The differences in mean recovery rates between CPC, mCPC, and CC agars were not statistically significant. The plating efficiencies for the clinical strains were between 10 and 40% lower than for the environmental strains on CPC, mCPC, and CC agars (Table 2). Strains that had originally been isolated on a medium containing colistin or polymyxin B gave between 10 and 38% higher plating efficiencies on CPC, mCPC, and CC agars than strains originally isolated on a medium without the addition of these antibiotics; these differences were not statistically significant (data not shown). No increases in the number of CFU on CPC, mCPC, or CC agar plates were observed after 48 h of incubation, compared with 24 h of incubation. The mean plating efficiency on TCBS agar was 1%, which was significantly lower (P < 0.05) than the plating efficiencies observed on the other selective media.
TABLE 2.
Plating efficiencies on selective media for V. vulnificus strains
| Type of strain (n) | Plating efficiency (%) ± SD on selective agar medium
|
|||
|---|---|---|---|---|
| TCBS | CPC | mCPC | CC | |
| Clinical (21) | 1 ± 3 | 55 ± 47 | 88 ± 76 | 90 ± 70 |
| Environmental (29) | 1 ± 2 | 96 ± 130 | 98 ± 120 | 110 ± 130 |
| Total (50) | 1 ± 3 | 76 ± 108 | 93 ± 103 | 100 ± 111 |
Comparison of mCPC and CC agars for isolation of V. vulnificus from water and sediment samples.
A total of 26 water samples and 14 sediment samples were analyzed by a three-tube most probable number method with APWP. Between three and six dilutions were investigated for each sample, and only the contents of turbid APWP tubes were streaked onto CC and mCPC agars. In a total of 446 APWP preenrichments, V. vulnificus was isolated from 154 preenrichments (35%) with mCPC agar and from 179 preenrichments (40%) with CC agar (Table 3). The increased isolation rates of V. vulnificus on CC agar compared with mCPC agar were all statistically significant (P < 0.05). More than 95% of the presumptive isolates from mCPC and CC agar plates were identified as V. vulnificus when tested with the VVAP probe. For both sample types, the colony diameters of V. vulnificus varied from approximately 1 to 2 mm on mCPC agar to 2 to 3 mm on CC agar. No differences were seen in the growth or composition of the background flora on CC agar compared with mCPC agar. Growth of two other types of cellobiose-positive colonies, which were negative with the VVAP probe, was seen on both mCPC and CC agars. Preliminary identification of the two types showed a pin-point yellow colony type of gram-positive cocci and a pale yellow mucoid type belonging to a Vibrio sp. Both types of strains are being further identified.
TABLE 3.
Number of APWP preenrichments positive for V. vulnificus with CC and mCPC agars
| Type of sample (n) | APWP preenrichments
|
||
|---|---|---|---|
| No. investigated | No. (%) positive for V. vulnificus
|
||
| CC agara | mCPC agar | ||
| Water (26) | 344 | 123 (36) | 110 (32) |
| Sediment (14) | 102 | 56 (55) | 44 (43) |
| Total | 44 | 179 (40) | 154 (35) |
Significantly higher (P < 0.05) V. vulnificus recovery rates were obtained with CC agar (by McNemar’s chi-square statistic [7]).
DISCUSSION
In the present study, we have shown that the use of an improved selective and differential agar, CC agar, with a reduced concentration of colistin is superior to selective agar media previously used for the isolation of V. vulnificus.
Although V. vulnificus has been described as being resistant to colistin and polymyxin B (10), the plating efficiency experiment showed that CC agar, which has the lowest concentration of colistin of the media tested, gave the best recovery of V. vulnificus cells. Further, our results showed that V. vulnificus was inhibited by increasing concentrations of colistin and polymyxin B. Surprisingly, TCBS agar gave a very low plating efficiency (1%) of both clinical and environmental V. vulnificus strains and therefore cannot be recommended for the isolation of V. vulnificus.
Compared with clinical strains, the majority of the environmental strains, which were originally isolated on mCPC agar, showed high plating efficiencies. This may have been due to acquired resistance to colistin and polymyxin B, which has been reported to occur (15). Induced changes in the phospholipid content of the bacterial outer membrane can decrease the binding capacity for colistin and polymyxin B. However, the nature of this resistance is adaptive and cultures have reverted to normal susceptibility levels when colistin and polymyxin B were removed (15). Therefore, we suggest that a heterogeneity may exist as to colistin-polymyxin B susceptibility within an environmental population of V. vulnificus and that the least susceptible strains will be favored on a medium containing colistin and/or polymyxin B.
Six of 50 V. vulnificus strains had MICs which indicated that they would be inhibited by the total concentration of colistin and polymyxin B in CPC agar. No strains had MICs within the concentration ranges of colistin and polymyxin B in mCPC or CC agar; therefore, the MIC testing did not reveal any differences between these two media. However, the MIC testing was done with pure cultures under optimal growth conditions, which do not resemble the conditions V. vulnificus is exposed to in the marine environment. When we compared mCPC and CC agars for the isolation of V. vulnificus from water and sediment samples, we found that CC agar was significantly better than mCPC agar for both sample types when used in combination with APWP preenrichment. The use of polymyxin B in a low concentration (2.0 × 104 U/liter) in the preenrichment step may inhibit the background flora to such an extent that even small numbers of V. vulnificus organisms are permitted to grow. Furthermore, the use of APWP may decrease the need for a highly selective agar, which would inhibit susceptible V. vulnificus strains. In future testing, there is no reason to use polymyxin B in the preenrichment broth and colistin in the agar, since these antibiotics have identical microbiological activities (15). We suggest that either colistin or polymyxin B be used in both the preenrichment broth and the selective medium at the same concentrations (in units per liter) as described in the present study. The need for only one antibiotic will make the procedure both cheaper and simpler.
Other types of cellobiose-positive colonies were observed on both mCPC and CC agars, with the same background microflora levels on the two agars. The reduced concentration of antibiotics in CC agar did not result in overgrowth of unwanted bacteria, and by using the distinct colony morphology of V. vulnificus, CC agar provided optimal differentiation of V. vulnificus from other organisms present.
Our results clearly demonstrate that a proportion of the V. vulnificus strains present in seawater and sediments are inhibited by the concentrations of colistin and polymyxin B in CPC and mCPC agars and that the use of CC agar increases the isolation rate of V. vulnificus. Further research is needed to determine whether the use of APWP in combination with CC agar enhances the recovery of V. vulnificus from oysters compared to current methods.
ACKNOWLEDGMENTS
This work received financial support from The Ministry of Food, Agriculture and Fisheries and The Danish Environmental Protection Agency, Ministry of Environment and Energy. Lise Høi was supported by a fellowship from The Royal Veterinary and Agricultural University.
We thank Jens Laurits Larsen for critical review of the manuscript. The technical assistance of Anita Forslund, Lotte Hein, Mahawash Houssain, Kirsten Kaas, and Suzanne Skytte is highly appreciated. We thank the following persons for providing strains for our study: B. Bruun, Statens Serum Institut, Copenhagen, Denmark; J. E. Olsen, The Royal Veterinary and Agricultural University, Copenhagen, Denmark; R. J. Siebeling, Louisiana State University, Baton Rouge, La.; A. DePaola, FDA Gulf Coast Seafood Laboratory; J. Veenstra, Academish Medisch Centrum, Amsterdam, Holland; Å. Melhus, Malmø General Hospital, Malmø, Sweden; and R. Stephan, Robert Koch Institute, Berlin, Germany.
REFERENCES
- 1.Barrow G I, Feltham R K A, editors. Cowan and Steel’s manual for the identification of medical bacteria. Cambridge, United Kingdom: Cambridge University Press; 1993. [Google Scholar]
- 2.Brayton P R, West P A, Russek E, Colwell R R. New selective plating medium for isolation of Vibrio vulnificus biogroup 1. J Clin Microbiol. 1983;17:1039–1044. doi: 10.1128/jcm.17.6.1039-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant R G, Jarvis J, Janda J M. Use of sodium dodecyl sulfate-polymyxin B-sucrose medium for isolation of Vibrio vulnificus from shellfish. Appl Environ Microbiol. 1987;53:1556–1559. doi: 10.1128/aem.53.7.1556-1559.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalsgaard A, Dalsgaard I, Høi L, Larsen J L. Comparison of a commercial biochemical kit and an oligonucleotide probe for identification of environmental isolates of Vibrio vulnificus. Lett Appl Microbiol. 1996;22:184–188. doi: 10.1111/j.1472-765x.1996.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 5.Dalsgaard A, Frimodt-Møller N, Bruun B, Høi L, Larsen J L. Clinical manifestations and epidemiology of Vibrio vulnificus infections in Denmark. Eur J Clin Microbiol Infect Dis. 1996;15:227–231. doi: 10.1007/BF01591359. [DOI] [PubMed] [Google Scholar]
- 6.Dalsgaard A, Høi L. Prevalence and characterization of Vibrio vulnificus isolated from shrimp products imported into Denmark. J Food Prot. 1997;60:1132–1135. doi: 10.4315/0362-028X-60.9.1132. [DOI] [PubMed] [Google Scholar]
- 7.Fleiss J L. Statistical methods for rates and proportions. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1981. [Google Scholar]
- 8.Hlady W G, Klontz K C. The epidemiology of Vibrio infections in Florida, 1981–1993. J Infect Dis. 1996;173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 9.Høi L, Larsen J L, Dalsgaard I, Dalsgaard A. Occurrence of Vibrio vulnificus biotypes in Danish marine environments. Appl Environ Microbiol. 1998;64:7–13. doi: 10.1128/aem.64.1.7-13.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massad G, Oliver J D. New selective and differential medium for Vibrio cholerae and Vibrio vulnificus. Appl Environ Microbiol. 1987;53:2262–2264. doi: 10.1128/aem.53.9.2262-2264.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miceli G A, Watkins W D, Rippey S R. Direct plating procedure for enumerating Vibrio vulnificus in oysters (Crassostrea virginica) Appl Environ Microbiol. 1993;59:3519–3524. doi: 10.1128/aem.59.11.3519-3524.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris J G, Wright A C, Roberts D M, Wood P K, Simpson L M, Oliver J D. Identification of environmental Vibrio vulnificus isolates with a DNA probe for the cytotoxin-hemolysin gene. Appl Environ Microbiol. 1987;53:193–195. doi: 10.1128/aem.53.1.193-195.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliver J D. Vibrio vulnificus. In: Doyle M P, editor. Foodborne bacterial pathogens. New York, N.Y: Marcel Dekker, Inc.; 1989. pp. 569–600. [Google Scholar]
- 14.Oliver J D, Guthrie K, Preyer J, Wright A C, Simpson L M, Siebling R, Morris J G. Use of colistin-polymyxin B-cellobiose agar for isolation of Vibrio vulnificus from the environment. Appl Environ Microbiol. 1992;58:737–739. doi: 10.1128/aem.58.2.737-739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Søgaard H. The pharmacodynamics of polymyxin antibiotics with special reference to drug resistance liability. J Vet Pharmacol Ther. 1982;5:219–231. doi: 10.1111/j.1365-2885.1982.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Oliver J D. Value of cellobiose-polymyxin B-colistin agar for isolation of Vibrio vulnificus from oysters. J Food Prot. 1995;58:439–440. doi: 10.4315/0362-028X-58.4.439. [DOI] [PubMed] [Google Scholar]
- 17.Tamplin M L, Capers G M. Persistence of Vibrio vulnificus in tissues of Gulf Coast oysters, Crassostrea virginica, exposed to seawater disinfected with UV light. Appl Environ Microbiol. 1992;58:1506–1510. doi: 10.1128/aem.58.5.1506-1510.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamplin M L, Martin A L, Ruple A D, Cook D W, Kaspar C W. Enzyme immunoassay for identification of Vibrio vulnificus in seawater, sediment, and oysters. Appl Environ Microbiol. 1991;57:1235–1240. doi: 10.1128/aem.57.4.1235-1240.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods G L, Washington J A. Antibacterial susceptibility tests: dilution and disk diffusion methods. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 1327–1341. [Google Scholar]