Abstract
Polyphenoloxidase from grape berries is extracted only by nonionic detergents with a hydrophilic-lipophilic balance between 12.4 and 13.5. The enzyme was partially purified in latent form, free of phenolics and chlorophylls, by using temperature phase partitioning in a solution of Triton X-114. This method permits the purification of the enzyme with the same fold purification as the commonly used method, but with a yield three times higher and a 90% reduction in time needed. The latent enzyme can be activated by different treatments, including trypsin and cationic and anionic detergents. Cetyltrimethylamonium bromide was found to be the most effective detergent activator, followed by sodium dodecyl sulfate. Polyphenoloxidase in grape berries, in spite of being an integral membrane protein, had an anomalous interaction with Triton X-114, remaining in the detergent-poor phase after phase separation. This could be explained by its having a short hydrophobic tail that anchors it to the membrane.
Full text
PDF



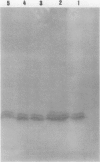
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cabanes J., García-Cánovas F., Lozano J. A., García-Carmona F. A kinetic study of the melanization pathway between L-tyrosine and dopachrome. Biochim Biophys Acta. 1987 Feb 20;923(2):187–195. doi: 10.1016/0304-4165(87)90003-1. [DOI] [PubMed] [Google Scholar]
- Egan R. W. Hydrophile-lipophile balance and critical micelle concentration as key factors influencing surfactant disruption of mitochondrial membranes. J Biol Chem. 1976 Jul 25;251(14):4442–4447. [PubMed] [Google Scholar]
- Flurkey W. H. In Vitro Biosynthesis of Vicia faba Polyphenoloxidase. Plant Physiol. 1985 Oct;79(2):564–567. doi: 10.1104/pp.79.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Carmona F., García Cánovas F., Lozano J. A. Epidermis tyrosinase is a hysteretic enzyme. Int J Biochem. 1980;11(3-4):325–327. doi: 10.1016/0020-711x(80)90237-2. [DOI] [PubMed] [Google Scholar]
- García-Carmona F., Cabanes J., García-Cánovas F. Enzymatic oxidation by frog epidermis tyrosinase of 4-methylcatechol and p-cresol. Influence of L-serine. Biochim Biophys Acta. 1987 Aug 5;914(2):198–204. doi: 10.1016/0167-4838(87)90064-1. [DOI] [PubMed] [Google Scholar]
- Golbeck J. H., Cammarata K. V. Spinach Thylakoid Polyphenol Oxidase : ISOLATION, ACTIVATION, AND PROPERTIES OF THE NATIVE CHLOROPLAST ENZYME. Plant Physiol. 1981 May;67(5):977–984. doi: 10.1104/pp.67.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes H. D., Breidenbach R. W. Plant plasma membrane proteins : immunological characterization of a major 75 kilodalton protein group. Plant Physiol. 1987 Dec;85(4):1048–1054. doi: 10.1104/pp.85.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- KENTEN R. H. Latent phenolase in extracts of broad-bean (Vicia fabaL.) leaves. 2. Activation by anionic wetting agents. Biochem J. 1958 Feb;68(2):244–251. doi: 10.1042/bj0680244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loomis W. D. Overcoming problems of phenolics and quinones in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31:528–544. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- Maher P. A., Singer S. J. Anomalous interaction of the acetylcholine receptor protein with the nonionic detergent Triton X-114. Proc Natl Acad Sci U S A. 1985 Feb;82(4):958–962. doi: 10.1073/pnas.82.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryde J. G., Phillips J. H. Fractionation of membrane proteins by temperature-induced phase separation in Triton X-114. Application to subcellular fractions of the adrenal medulla. Biochem J. 1986 Jan 15;233(2):525–533. doi: 10.1042/bj2330525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderhäll K., Carlberg I., Eriksson T. Isolation and Partial Purification of Prophenoloxidase from Daucus carota L. Cell Cultures. Plant Physiol. 1985 Aug;78(4):730–733. doi: 10.1104/pp.78.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Activation of polyphenol oxidase of chloroplasts. Plant Physiol. 1973 Feb;51(2):234–244. doi: 10.1104/pp.51.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack M. D., Kendall D. A., MacDonald R. C. Detergent effects on enzyme activity and solubilization of lipid bilayer membranes. Biochim Biophys Acta. 1983 Sep 7;733(2):210–215. doi: 10.1016/0005-2736(83)90524-2. [DOI] [PubMed] [Google Scholar]