Abstract
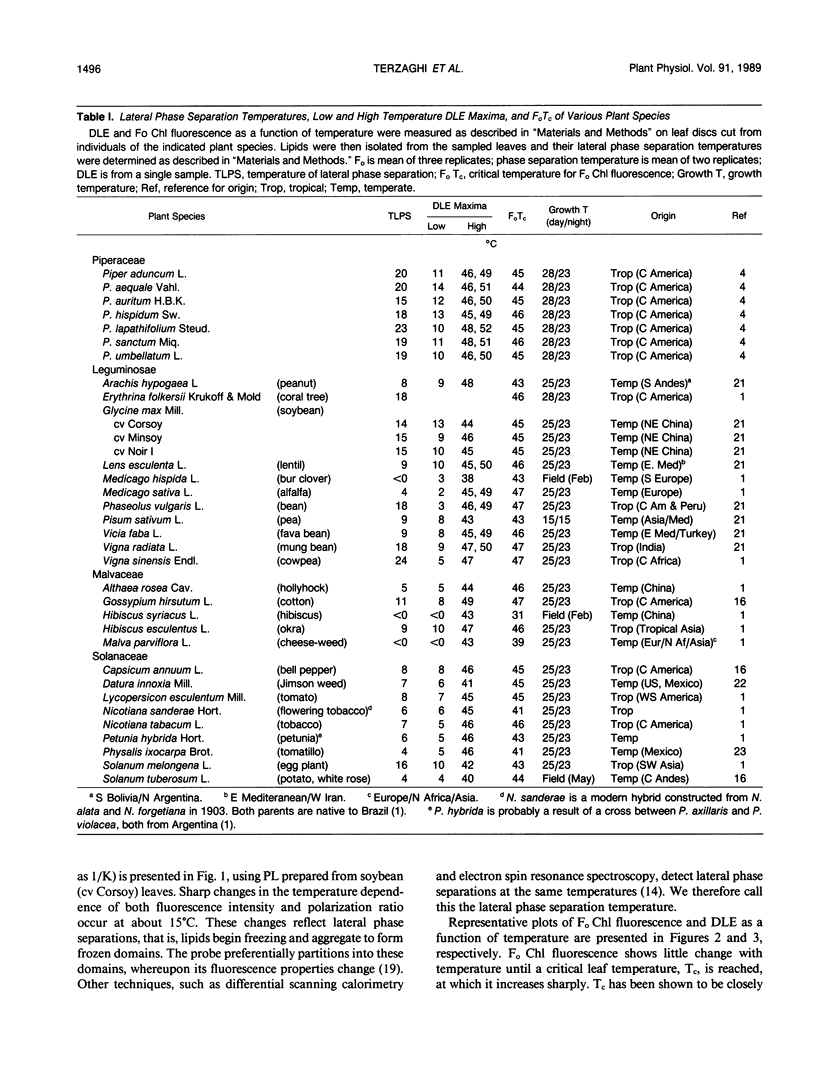
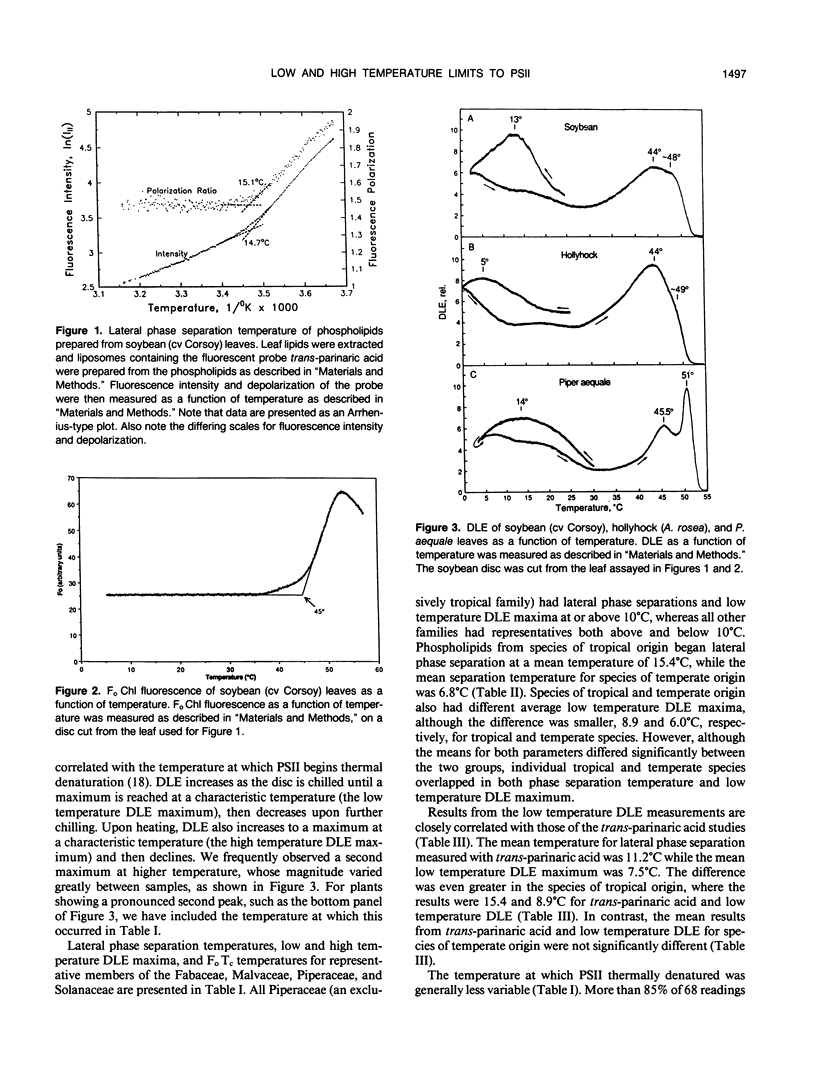
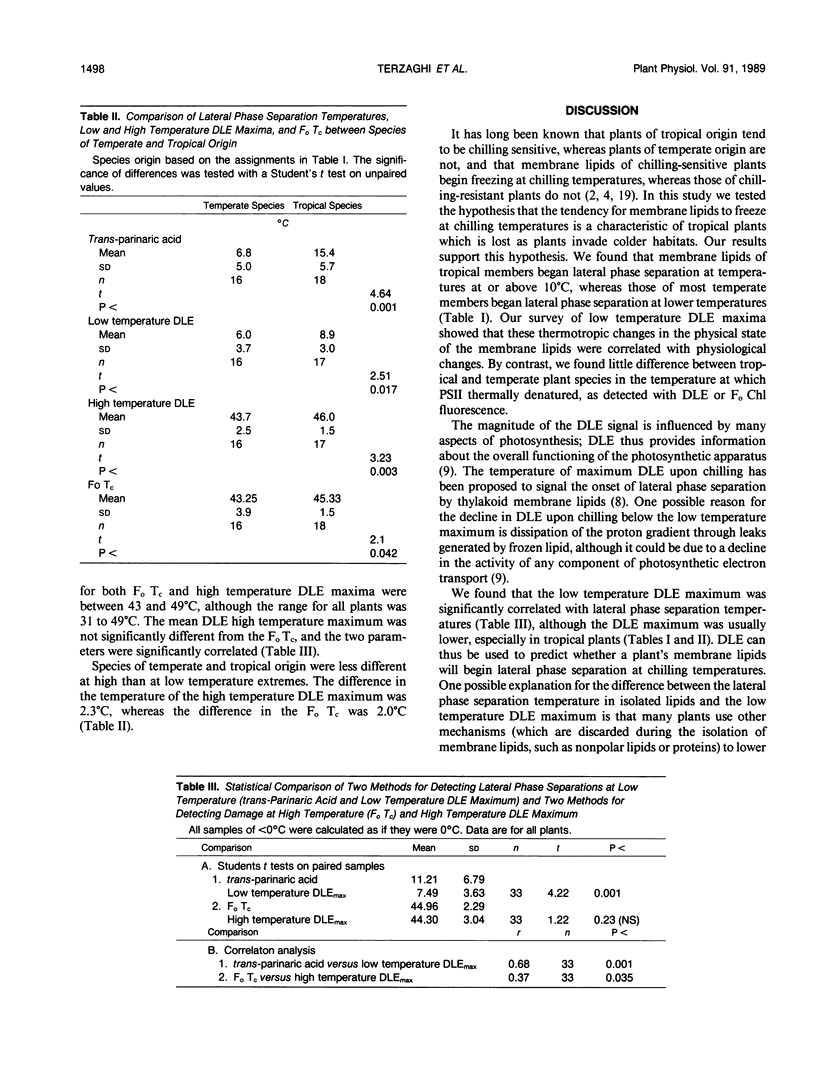
Many studies have shown that membrane lipids of chilling-sensitive plants begin lateral phase separation (i.e. a minor component begins freezing) at chilling temperatures and that chilling-sensitive plants are often of tropical origin. We tested the hypothesis that membranes of tropical plants begin lateral phase separation at chilling temperatures, and that plants lower the temperature of lateral phase separation as they invade cooler habitats. To do so we studied plant species in one family confined to the tropics (Piperaceae) and in three families with both tropical and temperate representatives (Fabaceae [Leguminosae], Malvaceae, and Solanaceae). We determined lateral phase separation temperatures by measuring the temperature dependence of fluorescence from trans-parinaric acid inserted into liposomes prepared from isolated membrane phospholipids. In all families we detected lateral phase separations at significantly higher temperatures, on average, in species of tropical origin. To test for associated physiological effects we measured the temperature dependence of delayed light emission (DLE) by discs cut from the same leaves used for lipid analysis. We found that the temperature of maximum DLE upon chilling was strongly correlated with lateral phase separation temperatures, but was on average approximately 4°C lower. We also tested the hypothesis that photosystem II (PSII) (the most thermolabile component of photosynthesis) of tropical plants tolerates higher temperatures than PSII of temperate plants, using DLE and Fo chlorophyll fluorescence upon heating to measure the temperature at which PSII thermally denatured. We found little difference between the two groups in PSII denaturation temperature. We also found that the temperature of maximum DLA upon heating was not significantly different from the critical temperature for Fo fluorescence. Our results indicate that plants lowered their membrane freezing temperatures as they radiated from their tropical origins. One interpretation is that the tendency for membranes to begin freezing at chilling temperatures is the primitive condition, which plants corrected as they invaded colder habitats. An alternative is that membranes which freeze at temperatures only slightly lower than the minimum growth temperature confer an advantage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Downton W. J., Berry J. A., Seemann J. R. Tolerance of photosynthesis to high temperature in desert plants. Plant Physiol. 1984 Apr;74(4):786–790. doi: 10.1104/pp.74.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J. M., Raison J. K. Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol. 1970 Apr;45(4):386–389. doi: 10.1104/pp.45.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. S., Berry J. A. Membrane phospholipid phase separations in plants adapted to or acclimated to different thermal regimes. Plant Physiol. 1980 Aug;66(2):238–241. doi: 10.1104/pp.66.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin N. S. Extraction of tissue lipids with a solvent of low toxicity. Methods Enzymol. 1981;72:5–7. doi: 10.1016/s0076-6879(81)72003-2. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Orr G. R. Phase transitions in thylakoid polar lipids of chilling-sensitive plants: a comparison of detection methods. Plant Physiol. 1986 Mar;80(3):638–645. doi: 10.1104/pp.80.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U., Armond P. A. Heat-induced changes of chlorophyll fluorescence in isolated chloroplasts and related heat-damage at the pigment level. Biochim Biophys Acta. 1978 Apr 11;502(1):138–151. doi: 10.1016/0005-2728(78)90138-x. [DOI] [PubMed] [Google Scholar]
- Seemann J. R., Berry J. A., Downton W. J. Photosynthetic response and adaptation to high temperature in desert plants : a comparison of gas exchange and fluorescence methods for studies of thermal tolerance. Plant Physiol. 1984 Jun;75(2):364–368. doi: 10.1104/pp.75.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Miljanich G. P., Dratz E. A. Phospholipid lateral phase separation and the partition of cis-parinaric acid and trans-parinaric acid among aqueous, solid lipid, and fluid lipid phases. Biochemistry. 1979 May 1;18(9):1707–1716. doi: 10.1021/bi00576a012. [DOI] [PubMed] [Google Scholar]


