Abstract
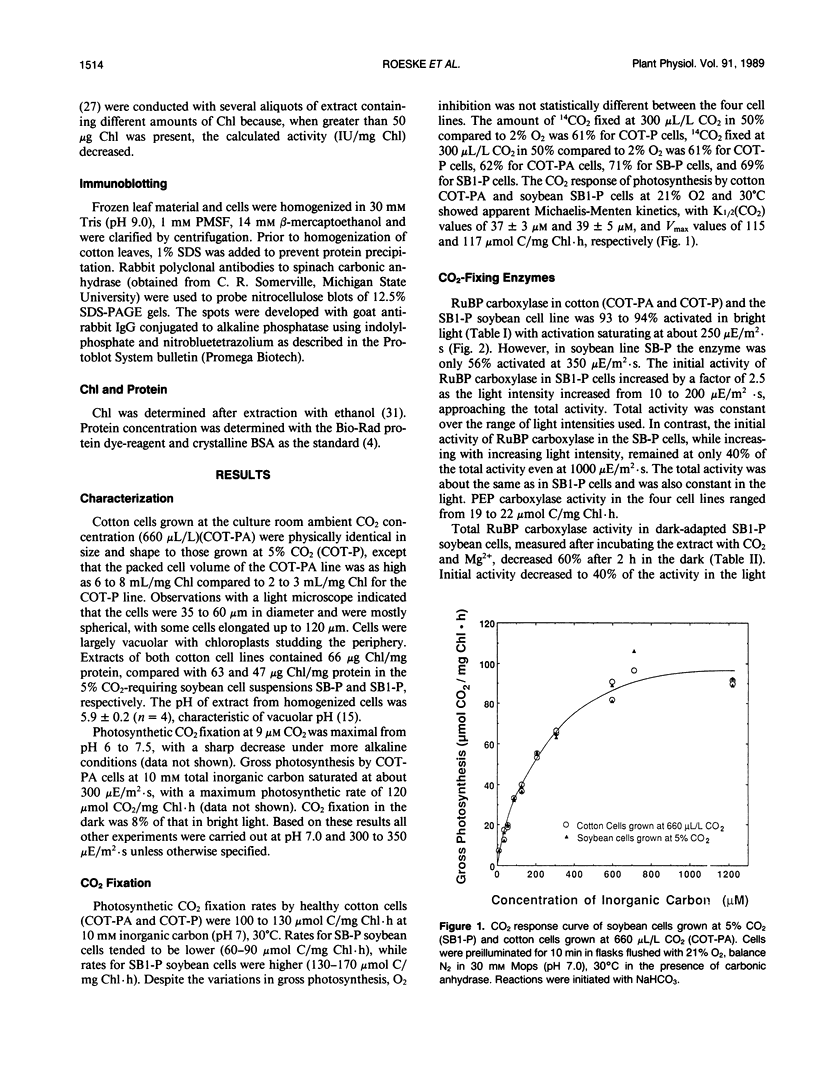
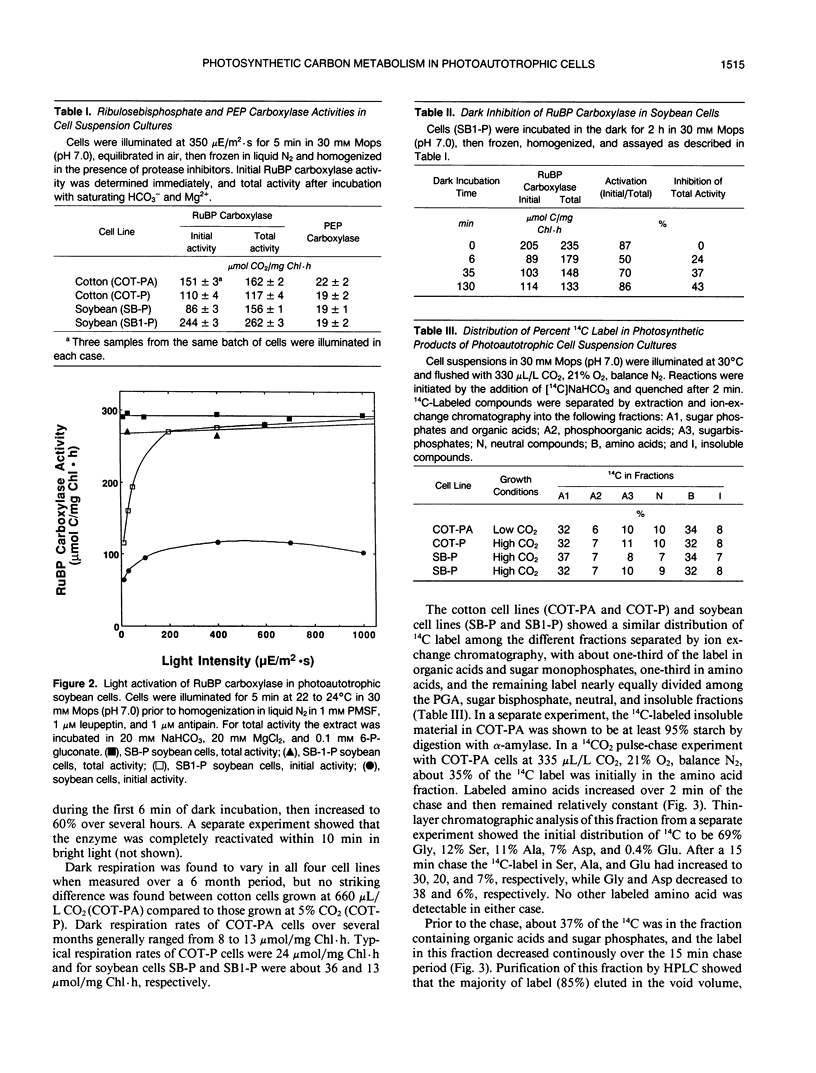
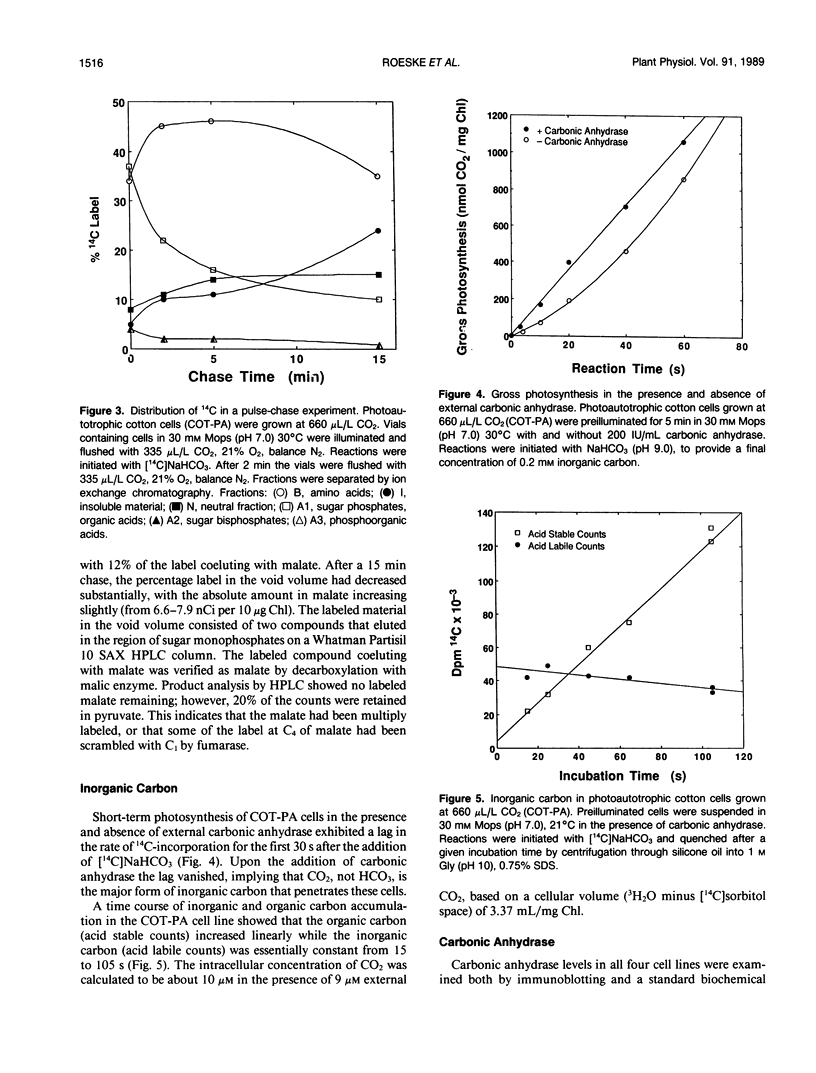
Photosynthetic carbon metabolism was characterized in four photoautotrophic cell suspension cultures. There was no apparent difference between two soybean (Glycine max) and one cotton (Gossypium hirsutum) cell line which required 5% CO2 for growth, and a unique cotton cell line that grows at ambient CO2 (660 microliters per liter). Photosynthetic characteristics in all four lines were more like C3 mesophyll leaf cells than the cell suspension cultures previously studied. The pattern of 14C-labeling reflected the high ratio of ribulosebisphosphate carboxylase to phosphoenolpyruvate carboxylase activity and showed that CO2 fixation occurred primarily by the C3 pathway. Photorespiration occurred at 330 microliters per liter CO2, 21% O2 as indicated by the synthesis of high levels of 14C-labeled glycine and serine in a pulse-chase experiment and by oxygen inhibition of CO2 fixation. Short-term CO2 fixation in the presence and absence of carbonic anhydrase showed CO2, not HCO3−, to be the main source of inorganic carbon taken up by the low CO2-requiring cotton cells. The cells did not have a CO2-concentrating mechanism as indicated by silicone oil centrifugation experiments. Carbonic anhydrase was absent in the low CO2-requiring cotton cells, present in the high CO2-requiring soybean cell lines, and absent in other high CO2 cell lines examined. Thus, the presence of carbonic anhydrase is not an essential requirement for photoautotrophy in cell suspension cultures which grow at either high or low CO2 concentrations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chastain C. J., Ogren W. L. Photorespiration-induced reduction of ribulose bisphosphate carboxylase activation level. Plant Physiol. 1985 Apr;77(4):851–856. doi: 10.1104/pp.77.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M. E., Sherrard J. H., Widholm J. M. Photoautotrophic growth of soybean cells in suspension culture: I. Establishment of photoautotrophic cultures. Plant Physiol. 1983 Jun;72(2):426–429. doi: 10.1104/pp.72.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. B., Bligny R., Rebeille F., Douce R., Leguay J. J., Mathieu Y., Guern J. A P Nuclear Magnetic Resonance Study of Intracellular pH of Plant Cells Cultivated in Liquid Medium. Plant Physiol. 1982 Oct;70(4):1156–1161. doi: 10.1104/pp.70.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B., Chollet R. Growth and Development of Soybean (Glycine max [L.] Merr.) Pods: CO(2) Exchange and Enzyme Studies. Plant Physiol. 1975 Apr;55(4):745–748. doi: 10.1104/pp.55.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. M., Ogren W. L., Widholm J. M. Photosynthetic characteristics of a photoautotrophic cell suspension culture of soybean. Plant Physiol. 1987 Aug;84(4):1451–1456. doi: 10.1104/pp.84.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Oxygen inhibition of photosynthesis and stimulation of photorespiration in soybean leaf cells. Plant Physiol. 1978 Jan;61(1):62–67. doi: 10.1104/pp.61.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Parry M. A., Gutteridge S., Keys A. J. Species variation in the predawn inhibition of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1986 Dec;82(4):1161–1163. doi: 10.1104/pp.82.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Carbonic Anhydrase-Deficient Mutant of Chlamydomonas reinhardii Requires Elevated Carbon Dioxide Concentration for Photoautotrophic Growth. Plant Physiol. 1983 Oct;73(2):268–272. doi: 10.1104/pp.73.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Xu C., Blair L. C., Rogers S. M., Govindjee, Widholm J. M. Characteristics of Five New Photoautotrophic Suspension Cultures Including Two Amaranthus Species and a Cotton Strain Growing on Ambient CO(2) Levels. Plant Physiol. 1988 Dec;88(4):1297–1302. doi: 10.1104/pp.88.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]


