Abstract
Mine tailings contain multiple toxic metal(loid)s that pose a threat to human health via inhalation and ingestion. The goals of this research include understanding the speciation and molecular environment of these toxic metal(loid)s (arsenic and lead) as well as the impacts particle size and residence time have on their bioaccessibilty in simulated gastric and lung fluid. Additionally, future work will include smaller size fractions (PM10 and PM2.5) of surface mine tailings, with the goal of increasing our understanding of multi-metal release from contaminated geo-dusts in simulated bio-fluids. This research is important to environmental human health risk assessment as it increases the accuracy of exposure estimations to toxic metal(loid)s.
Keywords: arsenic, geo-dust, human health, lead, mine tailings, simulated gastric fluid (SGF), simulated lung fluid (SLF)
Introduction
Arizona has approximately 60,000–100,000 abandoned or inactive mining sites. Mine tailing disposal sites in arid areas like Arizona are susceptible to wind erosion and become sources of airborne particulate matter or geo-dusts. These particles comprise contaminants like arsenic and lead, that are listed in the Agency for Toxic Substances and Disease Registry’s Priority List of Hazardous Substances and are detrimental to human health (1–4). Additionally, climate models predict that Southwestern US will become increasingly warmer and drier, thus potentially increasing the harmful effects of these airborne metal(loid) contaminants (5, 6).
In the current study, we employed an in vitro bioaccessibility method for the simulated gastric fluid (SGF) based on a standard operating procedure developed by Solubility/Bioavailability Research Consortium (SBRC) for arsenic and lead. The simulated lung fluid (SLF) method used a modified Gambles’ solution developed by Takayka (7–13). Bioaccessibility is determined based on metal release normalized to total metals determined from Aqua Regia extraction. Bioaccessibility studies provide valuable information regarding chemical reactions that occur between particles and bioassay fluids and can measure the solubility of metal(loid)s in SGF and SLF (14).
X-ray absorption fine structure spectroscopy (XAFS) is one of few methods that can provide structural and compositional information on most types of cations and anions sorbed at solid-solution interfaces. XAFS can provide information on the speciation of selected cations and anions in complex mixtures of phases, including sorbed species like metal(loid)-contaminated soils (15). Sample solutions were isolated by allowing the suspensions to settle, and then filtering them at 0.45 μm. The residual solid was lyophilized and analyzed at the Stanford Synchrotron Radiation Light Source (SSRL) to determine changes in the oxidation states of As and Fe and determine their local bonding environment. Additionally, scanning electron microscopy (SEM) imaging was conducted to elucidate changes in particle size and shape in unreacted and post-extraction solid samples.
Materials and methods
This research utilized a homogenized surface mine tailings sample from Iron King Mine to achieve the following: i) identify the particle size impact on arsenic and lead bioaccessibility, ii) determine the relative bioaccessibility of arsenic in SLF and SGF, and iii) determine the molecular speciation via post-extraction analysis of the remaining sample solid using synchrotron-based XAFS and X-ray absorption near edge structure (XANES) as well as X-ray fluorescence (XRF). Bioaccessibility was determined based on the total metals determined from a lithium metaborate/tetraborate fusion with inductively coupled plasma mass spectrometry detection (ActLab, Ontario, Canada). Several NIST quality control samples were analyzed to confirm precision.
Mine tailing surface samples were collected and sieved to obtain size fractions relevant to ingestion (150 μm) and then eventually includes inhalation relevant mine tailing size fractions (≤10 μm, PM10 and PM2.5). The 150 μm mine tailings were separated via metal sieving and then treated with SGF and SLF to determine the bioaccessibility of arsenic and lead. The bulk mine tailings surface sample (top 25 cm) were sieved to 150 μm and homogenized for the University of Arizona Superfund Research Program (SRP).
Samples were prepared in triplicate, covered to minimize light exposure, agitated at 60 rpm, and incubated at 37°C to simulate particle residence in the human gastric or lung system. Kinetic studies of the SRP group sample included ten resident times ranging from short (30 s time steps) to long (7 day) exposure time steps. These residence times were selected to a) develop the times relevant to particle resident times in the gastric (1 h) and lung (7 day) systems and b) gain an understanding of the release rates of metal(loid)s in these systems.
We hypothesized that (i) the smaller the particle size (i.e., the greater the surface area) the more accelerated the kinetics of arsenic and lead release would be, that (ii) bioaccessibility is a predictable function of the local contaminant-bonding environment as revealed from spectroscopy, and that (iii) an increase in pH and the composition of the SLF (i.e., the presence of phosphorus in SLF) can reduce the amount of arsenic present in the solution.
Results
Total bioaccessible metals are greater in the SGF than in SLF; moreover, release is maximized at 100 h in the SGF and at 15 min in the SLF (Figure 1). SEM images show an unreacted bulk mine tailing sample as well as a sample reacted in SGF at 1 h and in SLF at 48 h. The SGF reacted image illustrates aggressive dissolution of particles at 10 μm. The SLF image, also at 10 μm, illustrates small crystal particles that have likely reprecipitated from solution into the analyzed solid (Figure 2). The XRF microprobe analysis shows the physiochemical change from the unreacted tailings to the SGF reacted tailings, where As(V) is initially mostly associated with ferrihydrite, a hydrous ferric oxyhydroxide (as an adsorbed species determined by XAS, data not shown), and after reaction in SGF As(V) is associated with jarosite, a hydrous iron sulfate acting either as a surface complex or a stoichiometric component (Figure 3).
Figure 1.
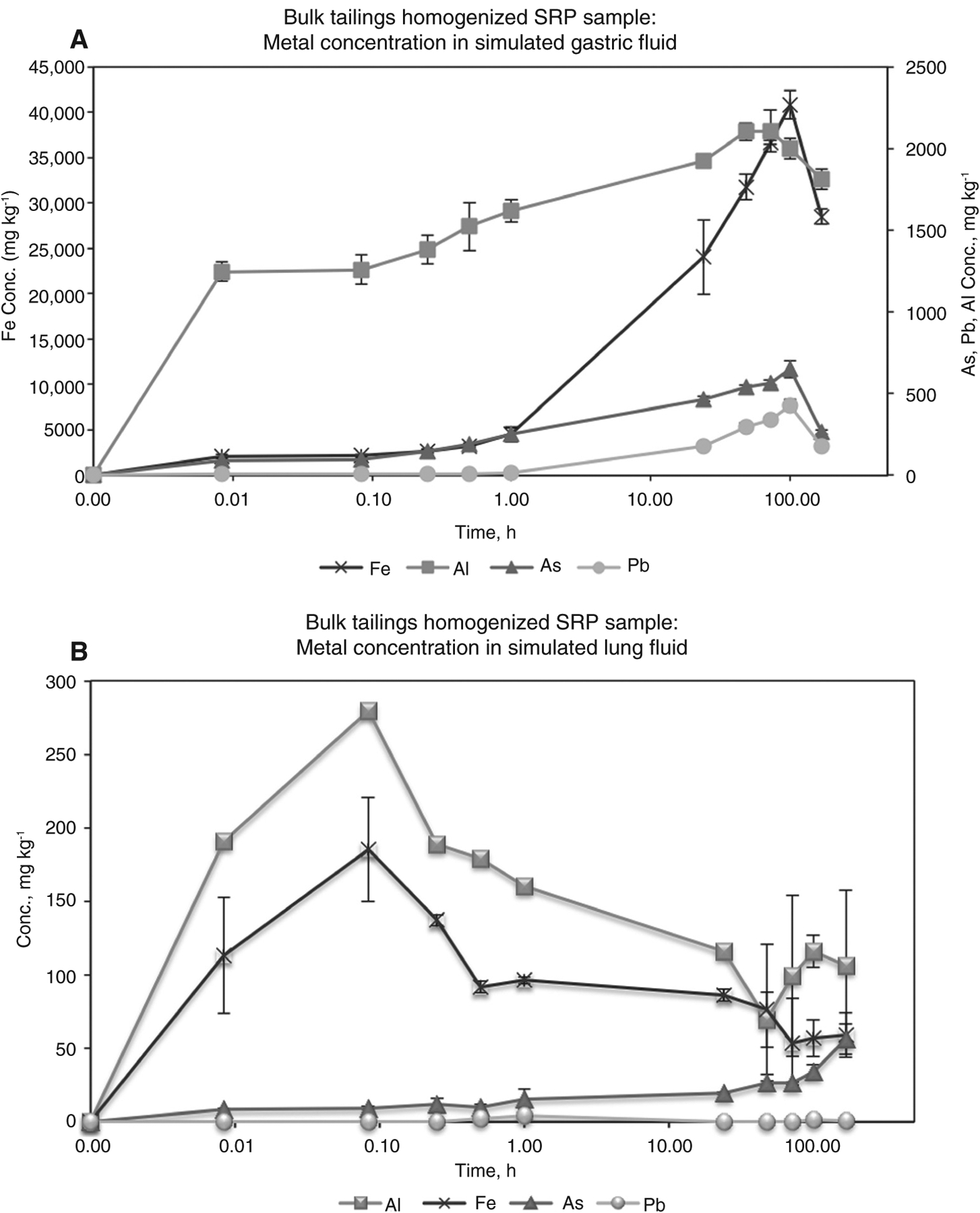
Bulk tailings homogenized SRP sample: metal concentration in SGF.Bulk tailings homogenized SRP sample: metal concentration in SLF.
Figure 2.
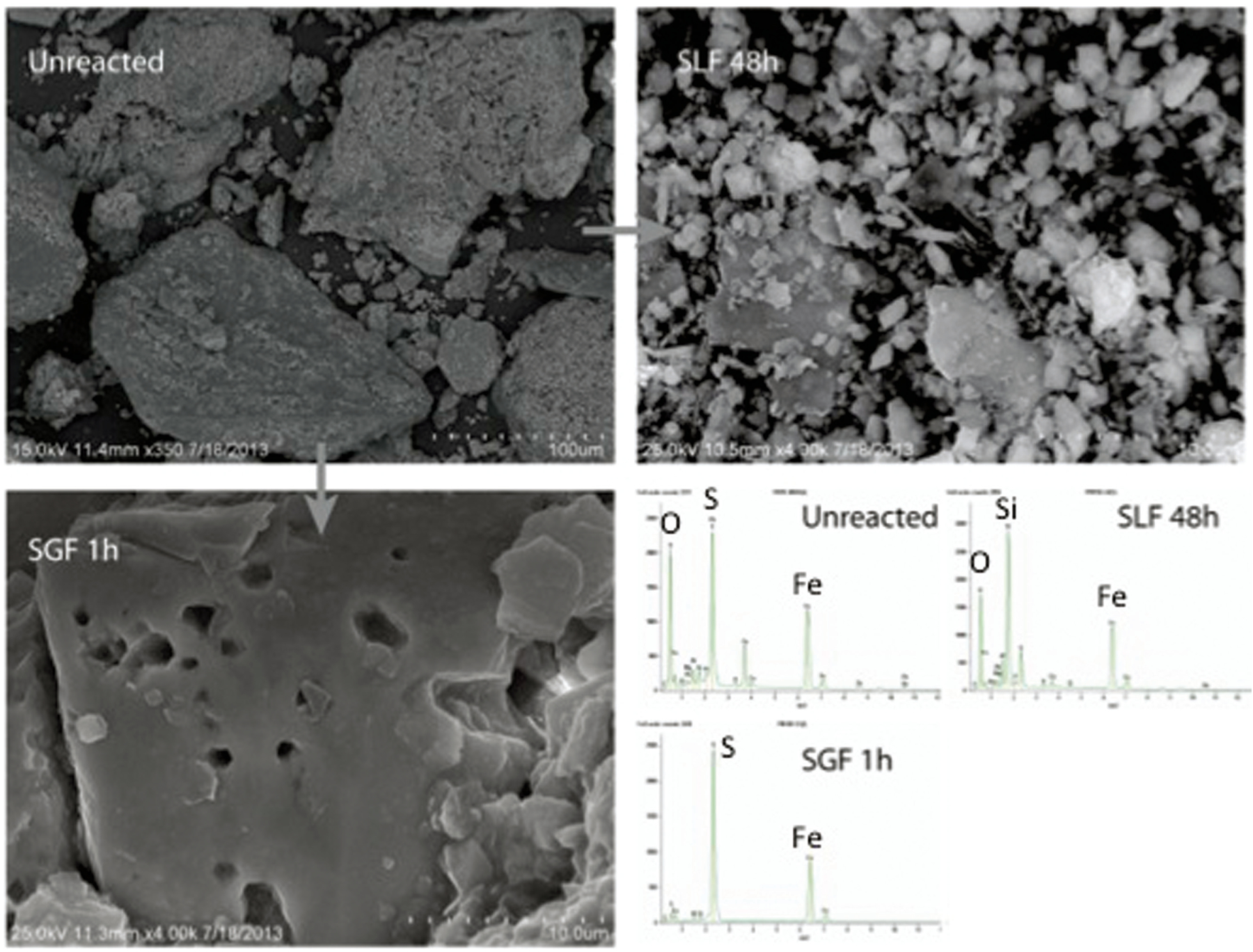
SEM imaging of unreacted mine tailings sample, SLF for 48 h (top right), SGF for 1 h (bottom left), and corresponding EDS data.
Figure 3.
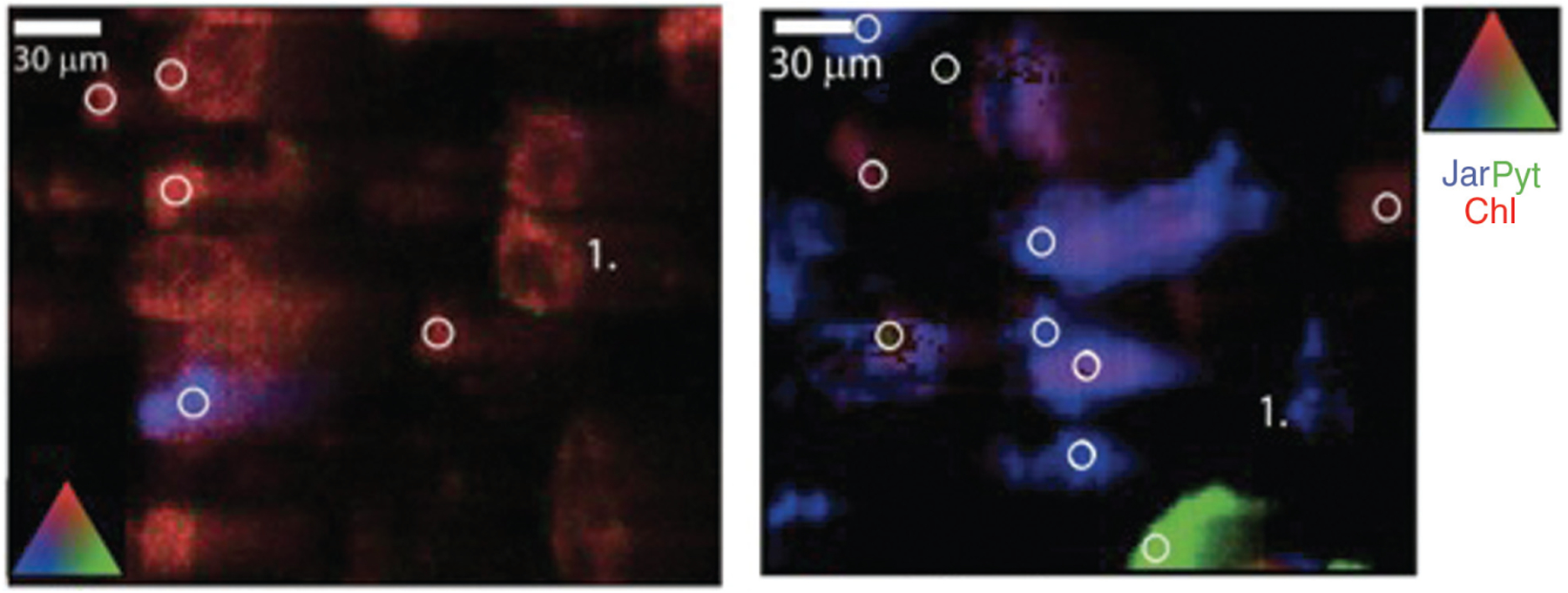
Fe XRF images of unreacted mine tailings (left), SGF 24-h extracted samples (a phase shift from from ferrihydrite to jarosite occurred after 24 h).
Discussion
The larger release of arsenic and lead ions into the SGF solution is consistent with the much more acidic pH (1.5) of the SGF, relative to SLF, condition. The SLF (pH 7.4) allows for a lower fraction of metal(loids) to be released. The release of metal(loid)s in the SGF peaks at 100 h, which is much later than the 1 h extraction time recommended in the EPA Standard Operation Procedure for an In Vitro Bioaccessibility Assay for Lead in Soil. The residence or extraction times for an SLF bioaccessibility assay have yet to be recommended by the EPA because particle sizes greatly influence residence time in the lung (16, 17). The current study found that the metal(loids) maximized well before a 1 h extraction time at 15 min (SLF) and well after at 100 h (SGF). Therefore, if a standard EPA bioaccessibility methodology is utilized for either an SGF or SLF bioassay, a 1-h residence time would grossly underestimate the amount of bioaccessible arsenic or lead one would be exposed to (16).
Conclusions
ICP-MS data indicate that resident extraction times, pH, and bioassay mineral composition are all important indicators of bioaccessibility. In addition, iron, arsenic, and lead concentrations in the SGF bioassay display greater concentrations than those in the SLF bioassay, which is likely due to the decreased pH (3, 5, 18). It is generally agreed that iron oxides are the dominant soil constituents responsible for arsenic sorption (31). However, arsenic sorption has also been shown to be correlated to aluminum oxides and soil or clay content. In this regard, further study is needed to understand the lack of As-Fe correlation in the kinetic release data (3, 19, 20). The SLF bioassay that contains salts and phosphates illustrates an interesting kinetic release curve with an initial metal(loid) release (15 min), a re-precipitation or sorption phase, and another smaller release (post 48 h) (Figure 1). This may be due to the formation of new phases that precipitate iron or aluminum hydroxyl sulfates, or the arsenic desorption that may have been driven by phosphates present in the solution (3, 21). XAFS data indicated that no change in oxidation state of As(V), the predominant form in soil, occurred. This is important because As(V) is the less toxic form of environment from soil to human health.. arsenic, given that As(III) is more soluble and, therefore, bioavailable (3, 18–20, 22–26, 30). The XRF data also indicated that iron is undergoing a change from ferrihydrite to jarosite in the gastric bioassay. Additionally, arsenate has been known to substitute for sulfate in jarosite and could be the reason there is a similar loss of arsenic and iron in the SGF after 100 h (29).
Tailings in arid and semi-arid climates may present a greater human health risk associated with direct particulate exposure from fugitive dusts. It has been observed that the bioavailability of metals in tailings is controlled by metal speciation, not total mass concentration. The bioaccessibility of these dusts varies with temporary and simulated target organ exposure. Finally, secondary mineral precipitation, observed with SEM (and XRD, not shown), may play an important role in the availability of surface sites for re-adsorption of released contaminants. Additionally, given that particle size is a driving factor in bioaccessibility inhalation, relevant mine tailing particulate matter (PM10 and PM2.5) are currently being conducted and will be published at a later date (17, 27, 28).
Contributor Information
Nazune Menka, Department of Soil, Water and Environmental Science, University of Arizona, PO Box 210038, Tucson AZ, 85721-0038, USA.
Rob Root, Department of Soil, Water and Environmental Science, University of Arizona, Tucson, AZ, USA.
Jon Chorover, Department of Soil, Water and Environmental Science, University of Arizona, Tucson, AZ, USA.
References
- 1.Agency for Toxic Substances and Disease Registry. Piority list of hazardous substances. 2011. Available at: http://www.atsdr.cdc.gov/spl/.Accessed on September 18, 2013.
- 2.Bradham KD, Laird B, Rasmussen PE, Schoof RA, Serda SM, et al. Assessing the bioavailability and risk from metal contaminated soils and dusts. Hum Ecol Risk Assess: An International Journal 2013. DOI: 10.1080/10807039.2013.802633. [DOI] [Google Scholar]
- 3.Naidu R, Smith E, Owens G, Bhattacharya P, Nadebaum P, editors. Managing arsenic in the environment from soil to human health. Victoria, Australia: CSIRO Publishing, 2006:56pp [Google Scholar]
- 4.Lantz RC. Role of oxidative stress in arsenic-induced toxicity. Drug Metab Rev 2006;38:791–804. [DOI] [PubMed] [Google Scholar]
- 5.Whitacre S Soil controls on arsenic bioaccessibility: arsenic fractions and soil properties. (Electronic Thesis or Dissertation). Retrieved from https://etd.ohiolink.edu/. Accessed on September 18, 2013.
- 6.Csavina J, Landazuri A, Wonaschutz A, Rine K, Rheinheimer P, et al. Metal and metalloid contaminants in atmospheric aerosols from mining operations. Water Air Soil Poll 2011;221:145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drysdale ME. Application of simulated lung fluid analysis to characterize the influence of smelter activity on the respiratory bioaccessibility of nickel-bearing soils in Kalgoorlie, Western australia. Library and Archives Canada, Bibliothèque et Archives Canada. 2009. [Google Scholar]
- 8.Harrington AD, Hylton S, Schoonen MA. Pyrite-driven reactive oxygen species formation in simulated lung fluid: implications for coal workers’ pneumoconiosis. Environ Geochem Health 2012;34:527–38. [DOI] [PubMed] [Google Scholar]
- 9.Juhasz AL, Weber J, Smith E, Naidu R, Marschner B, et al. Evaluation of SBRC-gastric and SBRC-intestinal methods for the prediction of in vivo relative lead bioavailability in contaminated soils. Environ Sci Technol 2009;43:4503–9. [DOI] [PubMed] [Google Scholar]
- 10.Kelley ME, Brauning SE, Schoof RA, Ruby MV. Assessing oral bioavailability of metals in soil. Columbus, OH: Battelle Press, 2002. [Google Scholar]
- 11.Makris KC, Quazi S, Nagar R, Sarkar D, Datta R, et al. In vitro model improves the prediction of soil arsenic bioavailability: worst-case scenario. Environ Sci Technol 2008;42:6278–84. [DOI] [PubMed] [Google Scholar]
- 12.Ruby MV, Davis A, Schoof R, Eberle S, Sellstone CM. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ Sci Technol 1996;30:422–30. [Google Scholar]
- 13.Takaya M, Shinohara Y, Serita F, Ono-Ogasawara M, Otaki N, et al. Dissolution of functional materials and rare earth oxides into pseudo alveolar fluid. Ind Health 2006;44:639–44. [DOI] [PubMed] [Google Scholar]
- 14.Plumlee G, Ziegler T. Soils and other earth materials. Environ Geochem 2005;9:263. [Google Scholar]
- 15.Brown GE, Calas G. Mineral-aqueous solution interfaces and their impact on the environment. Geochem Perspect 2011;1: 483–742. [Google Scholar]
- 16.Environmental Protection Agency. Standard operating procedures for an in vitro bioaccessibilility for lead in soil No. EPA9200.1–86. 2008.0.
- 17.World Health Organization. Hazard prevention and control in the work environment: airborne dust. Prevention and Control Exchange (PACE).Geneva: WHO, Department of Protection of the Human Environment, Occupational Environmental Health, WHO/SDE/OEH/99.14. 1999. [Google Scholar]
- 18.Csavina J, Field J, Taylor MP, Gao S, Landázuri A, et al. A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations. Sci Total Environ 2012;433:58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fendorf SE, Sparks DL, Page A, Helmke P, Loeppert R, et al. X-ray absorption fine structure spectroscopy. Met Soil Anal. Part 3. Chem Met 1996;377–416. [Google Scholar]
- 20.Fendorf S, Eick MJ, Grossl P, Sparks DL. Arsenate and chromate retention mechanisms on goethite. 1. surface structure. Environ Sci Technol 1997;31:315–20. [Google Scholar]
- 21.O’Reilly SE, Strawn DG, Sparks DL. Residence time effects on arsenate adsorption/desorption mechanisms on goethite. Soil Sci Soc Am J 2001;65:67–77. [Google Scholar]
- 22.Campbell KM, Hering JG. Biogeochemical mechanisms of arsenic mobilization. In: Ahuja S, editor. Arsenic contamination of groundwater: mechanism, analysis, and remediation. Hoboken, NJ: J.S. Wiley & Sons, 2008:95pp. [Google Scholar]
- 23.Kumaresan M, Riyazuddin P. Overview of speciation chemistry of arsenic. Curr Sci 2001;80:837–46. [Google Scholar]
- 24.Smith E, Naidu R, Alston AM. Arsenic in the soil environment: a review. Adv Agron 1998;64:149–95. [Google Scholar]
- 25.Walker SR, Jamieson HE, Lanzirotti A, Andrade CF, Hall GE. The speciation of arsenic in iron oxides in mine wastes from the giant gold mine, n.w.t.: application of synchrotron micro-xrd and micro-xanes at the grain scale. Can Mineral 2005;43: 1205–24. [Google Scholar]
- 26.Yokel RA, Lasley SM, Dorman DC. The speciation of metals in mammals influences their toxicokinetics and toxicodynamics and therefore human health risk assessment. J Toxicol Environ Health 2006;9:63–85. [DOI] [PubMed] [Google Scholar]
- 27.Bright DA, Richardson GM, Dodd M. Do current standards of practice in canada measure what is relevant to human exposure at contaminated sites? I: A discussion of soil particle size and contaminant partitioning in soil. Human ecol Risk Assess 2006;12:591–605. [Google Scholar]
- 28.Davidson CI, Phalen RF, Solomon PA. Airborne particulate matter and human health: a review. Aerosol Sci Technol 2005;39:737–49. [Google Scholar]
- 29.Campbell KM, Root R, O’Day PA, Hering JG. A gel probe equilibrium sampler for measuring arsenic porewater profiles and sorption gradients in sediments: II. field application to haiwee reservoir sediment. Environ Sci Technol 2008;42:504–10. [DOI] [PubMed] [Google Scholar]
- 30.Appleton JD, Fuge R, McCall GJH. Joint Association of Geoscientists for International Development, Society for Environmental Geochemistry and Health, Geological Society of London. Environmental geochemistry and health: with special reference to developing countries. London: Geological Society. 1996. [Google Scholar]
- 31.Bradham KD, Scheckel KG, Nelson CM, Seales PE, Lee GE, et al. Relative bioavailability and bioaccessibility and speciation of arsenic in contaminated soils. Environ Health Perspect 2011;119:1629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


