Abstract
Objective
To evaluate a health system‐wide intervention distributing free home‐disposal bags to surgery patients prescribed opioids.
Data Sources and Study Setting
We collected patient surveys and electronic medical record data at an academic health system.
Study Design
We conducted a prospective observational study. The bags were primarily distributed at pharmacies, though pharmacists delivered bags to some patients. The primary outcome was disposal of leftover opioids (effectiveness). Secondary outcomes were patient willingness to dispose and factors associated with disposal (effectiveness), recalling receipt of the bag (reach), and recalling receipt of bags and disposal over time (maintenance). We used a modified Poisson regression to evaluate the relative risk of disposal. Inverse probability of treatment weighting, based on propensity scores, was used to account for differences between survey responders and non‐responders and reduce nonresponse bias.
Data Collection/Extraction Methods
From August 2020 to May 2021, we surveyed patients 2 weeks after discharge (allowing for home opioid use). Eligibility criteria were age ≥18, English being primary language, valid email address, hospitalization ≤30 days, discharge home, and an opioid prescription sent to a system pharmacy.
Principal Findings
We identified 5134 patients with 2174 completing the survey (response rate 42.3%). Among respondents, 1375 (63.8%) recalled receiving the disposal bag. Among 1075 respondents with leftover opioids, 284 (26.4%) disposed, 552 (51.3%) planned to dispose, 79 (7.4%) did not plan to dispose, 69 (6.4%) had undecided, and 91 (8.5%) had not considered disposal. Recalling receipt of the bag (incidence rate ratio [IRR] 1.25, 95% confidence interval [CI] 1.13–1.37) was positively associated with disposal. Patients who used opioids in the last year were less likely to dispose (IRR 0.82, 95% CI 0.73–0.93). Disposal rates remained stable over the study period while recalling receipt of bags trended up.
Conclusions
A pragmatic implementation of a disposal intervention resulted in lower disposal rates than prior trials.
Keywords: health system intervention, implementation, opioids, prevention of opioid‐related harms, surgery
What is known on this topic
In the United States, most patients will have leftover prescription opioids after surgery.
Opioid misuse continues to be a problem and risk assessment and mitigation interventions (e.g., disposal of unused opioids) are needed.
In small‐scale trials, distribution of a home‐disposal bag to surgery patients increased the likelihood of disposal of leftover prescription opioids.
What this study adds
A pragmatic implementation of a system‐wide intervention distributing home‐disposal bags resulted in 26.4% disposal rate, while 51.3% of surgery patients were planning to dispose but had not yet done so.
Recalling receipt of the disposal bag is an important factor in promoting disposal.
1. INTRODUCTION
More than 64 million operations are performed each year in the United States, and 56%–70% of these patients will be prescribed opioids. 1 , 2 , 3 , 4 Although opioid prescribing after surgery has decreased in recent years, between 50% and 92% of patients still have leftover opioids. 5 , 6 , 7 , 8 , 9 Eleven percent of individuals who died from prescription‐opioid overdoses received their prescription from a surgeon. 10 In 2020, 24% of the 68,630 opioid‐related overdose deaths involved prescription opioids. 11 Amidst a crisis in which 247,000 people died from prescription opioid overdoses between 1999 and 2019, the US government has advocated for the secure storage of opioids followed by immediate disposal when treatment is complete. 12 , 13 , 14 However, few patients store opioids securely, even in households with children. 15 , 16 , 17 Without interventions, only 4%–9% of patients will dispose of opioids after surgery. 7 , 8 , 18 In 2018, the Substance Use‐Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (SUPPORT Act) provided the United States Food and Drug Administration (FDA) with the authority to require drug manufacturers to provide a safe drug disposal system. The FDA has not exercised its authority yet, but in 2022 requested public comments regarding a mandated Risk Evaluation and Mitigation Strategy. 19 The Stanford‐Lancet Commission on the North American Opioid Crisis recently called for raising the quality of excess opioid disposal programs to foster healthier environments and reduce the incidence of addiction. 20
To date, the most common medication disposal interventions are public health campaigns asking patients to return medications to take‐back days or drop boxes. Although these campaigns tout impressive quantities of returned medications, most are not controlled substances like opioids. 18 , 21 , 22 , 23 , 24 One study from Kentucky estimated that only 0.3% of dispensed opioids were ultimately disposed of. 25 Interventions using only education to promote disposal (e.g., handouts and videos) have shown either null 26 , 27 , 28 or modest effects (i.e., absolute increases in disposal of 11%–22%). 29 , 30 The lone study using financial incentives to promote opioid disposal found that only 30% of veterans participated, despite being paid $5 per returned tablet (max $50). 31
Prior studies by our group and others suggest that providing postoperative home‐disposal bags resulted in 55%–95% of patients disposing of their leftover opioids. 32 , 33 , 34 , 35 , 36 However, a subsequent trial showed disappointing results, with only 14% of patients disposing of their opioids. 37 However, no study to date has reported on the feasibility of a pragmatic, low‐effort opioid disposal quality improvement intervention implemented outside of a research trial or across an entire health system.
In this prospective observational study, we examined the reach, effectiveness, and maintenance of an ongoing health system intervention providing home‐disposal bags to every patient filling an opioid prescription after surgery. 38 In addition, we sought to test whether a stage‐based behavior change framework we previously adapted from the Precaution Adoption Process Model (PAPM) could help us further promote safe and appropriate disposal. 39 , 40 Our primary outcome was patient‐reported disposal of leftover prescription opioids (effectiveness). Our secondary outcomes were patient willingness to dispose and factors associated with disposal (effectiveness based on PAPM stages), the percentage of eligible patients recalling receipt of the disposal bag (reach); factors associated with disposal (effectiveness); and recalling receipt of the bag and disposal rates over time (maintenance).
2. METHODS
2.1. Health system disposal intervention
After we established the effectiveness of disposal bags at a large, regional, tertiary care, academic health system in a pilot study, 32 our system used savings generated from the United States' federal 340B Drug Pricing Program to fund the distribution of free disposal bags to every patient filling an opioid prescription at our 16 pharmacies. The program was officially launched in August 2020. Each pharmacy unit was allowed to implement the intervention as they saw fit. Most units provided the bags at the point‐of‐care delivery window at the pharmacy. However, several motivated and engaged pharmacists, when available, provided bags and patient education at discharge to admitted inpatients.
2.2. Study setting, participants, and design
We conducted a prospective observational study of patients undergoing surgery from August 2020 to May 2021 at a large, regional, tertiary care, academic health system. Patients underwent surgery in nine different operating room suites (seven located at the main campus and two located at outpatient surgery centers). Patient‐level inclusion criteria were an operation with a provider in the University of Utah Department of Surgery, an opioid prescription sent at discharge to a health system pharmacy, age ≥18 years, primary language listed as English, an email address on file, and discharge to home or home health. Discharge‐level exclusion criteria were length of stay greater than 30 days, death during admission, operation and discharge in the prior 30 days, and discharge to a nursing facility, rehab facility, hospice, another hospital, or correctional facility. Patients undergoing multiple operations in a single hospitalization were classified by their primary procedure.
Two weeks after discharge, we invited eligible patients via email to complete a web‐based REDCap survey (Supplement S1). We selected this time point based on prior studies suggesting that up to 96.8% of patients cease using opioids by 2 weeks after discharge. 41 , 42 , 43 For outpatient surgery, the discharge date was the same as the surgery date. For inpatient surgery, we used the discharge date to ensure patients had an adequate amount of time to use their opioids at home. At the start of the survey, patients were presented with a consent letter describing the study, potential risks, and requesting authorization to link survey responses with electronic medical record data. The survey asked questions about patients' quantity of leftover opioids; recollection of receiving a disposal bag; receipt of, or intention to request, an opioid refill; pain management satisfaction; experiences with pain prior to surgery; current smoking, alcohol, or illicit drug use; prior use of opioids within the past year; completion and manner of disposal; and reasons for disposal (or not). To maximize response, patients received up to four emails requesting participation. We excluded survey responses that were incomplete. We merged survey responses with data on patient demographics, clinical characteristics, and discharge prescriptions from our electronic medical record system. We converted opioid dosing into morphine milligram equivalents (MME). For reference, one 5 mg tablet of oxycodone is equivalent to 7.5 MME. 44 We used the responses from the first survey completed for patients who completed more than one survey. The study was approved by the Institutional Review Board at the University of Utah and was conducted in compliance with Strengthening The Reporting of OBservational studies in Epidemiology (STROBE) guidelines. 45
2.3. Study outcomes
In this analysis, our primary effectiveness outcome was patient‐reported disposal of leftover prescription opioids (versus non‐disposal). We first asked patients to indicate their stage of readiness to dispose based on our prior work adapted from the Precaution Adoption Process Model (PAPM). 40 Understanding where patients are in their decision‐making process can inform future interventions that promote disposal. Our prior studies suggest that the choice to dispose results not from a single discrete decision but a progression through stages of readiness (Figure 1). We asked patients to report their willingness to dispose and categorized them into one of five stages: “I have not thought about disposal” (PAPM Stage 2), “I am undecided about whether I will dispose of them” (Stage 3), “I do not plan to dispose of them” (Stage 4), “I plan to dispose of them but have not yet” (Stage 5), and “I have disposed of them” (Stage 6). 40 We did not include Stage 1 as the survey created awareness. We defined our primary outcome as disposal (Stage 6) versus non‐disposal (Stages 2–5). Our secondary outcomes were patient willingness to dispose and factors associated with disposal (effectiveness), the percentage of eligible patients reporting receipt of the disposal bag (reach), and recalling receipt of the bag and disposal over time (maintenance). We also examined the patient‐, surgery‐, and patient‐reported factors associated with disposal.
FIGURE 1.
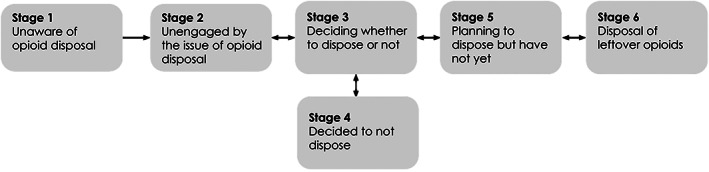
A theoretical framework of patient willingness to dispose based on the Precaution Adoption Process Model. 39
2.4. Analysis
We summarized patient characteristics and survey responses using the mean and standard deviation or median and interquartile range for continuous variables; for categorical variables, we reported frequency and percentages. The prespecified race and ethnicity terms were extracted from the electronic medical record. We stratified descriptive tables by survey response status—patients who completed at least one survey (“survey respondents”) are compared with patients who did not (“nonresponders”). We used responses from patients' first survey for those who underwent multiple discharges. We tested for differences using a nonparametric Wilcoxon rank sum test for continuous variables and chi‐squared or Fisher's exact test for categorical variables. In cases where the normal conditions of a chi‐squared test were not met, we calculated p‐values via Monte‐Carlo simulation.
We used modified Poisson regression to determine the relative risk of disposal following surgery according to patient demographics, surgery characteristics, patient experiences with pain, opioid prescription and usage, and recall of disposal bag being provided. 46 We chose a binary outcome of disposal versus non‐disposal rather than trying to fit the PAPM model for two reasons. First, an ordered model would have required dropping PAPM Stage 4 as a response level. Second, intentionality consists of a combination of concepts, awareness of opioid handling safety and willingness to dispose, that were not equally represented among the possible response levels. We constructed this model using the subset of patients who had leftover opioids and were exposed to the intervention (i.e., filled their prescription at a health system pharmacy). We included all variables in the adjusted model (rather than using a selection process) as our goal was identifying the relevant factors rather than creating a predictive model.
To reduce survey nonresponse bias, we used inverse probability of treatment weighting or “nonresponse weights”. 47 , 48 Incorporating nonresponse weights in our outcome model adjusted the respondent covariate distributions to be more similar to the original sample, thus reducing the potential for nonresponse bias. 49 The nonresponse weights were derived from a gradient‐boosted logistic regression where the outcome was response status and the predictors included surgery type, surgical specialty, and all available patient characteristics at baseline (age, sex, rurality, race, ethnicity, smoking, alcohol and drug use). 50
Our outcome model standard errors were adjusted via robust clustered sandwich estimators to account for clustering by surgeon. 51 We assessed multicollinearity using the variance inflation factor and all values were <2, indicating no collinearity issues. 52 We report relative risks or incident rate ratios (IRRs), where higher values indicate a greater probability of disposal, 95% confidence intervals (CIs), and p‐values. This same modeling framework was repeated for our secondary outcome of disposed or planning to dispose.
We examined maintenance of the intervention by plotting patient recall of receiving the disposal bag and patient‐reported disposal (aggregated by month) and fitting a simple regression line to visualize the trend. We assessed statistical significance at the 0.05 level using two‐tailed tests and conducted all analyses in R‐statistics v. 3.6.2 (R Core Team 2021).
3. RESULTS
We identified 5134 surgery patients with an opioid prescription sent at discharge to a health system pharmacy. Overall, 2174 patients completed at least one survey while 2960 patients did not (response rate 42.3%). Twenty‐nine patients opted out of the study. The characteristics of eligible patients and their operations are shown in Table 1. In comparison to nonresponders, survey respondents were more likely to be age ≥65 years (30.7% vs. 15.7%, p < 0.001) and identify as Caucasian/White (92.6% vs 87.5%, p < 0.001). Respondents were less likely than non‐respondents to identify as Hispanic/Latino (5.7% vs. 8.8%, p < 0.001), be current smokers (4.7% vs. 8.8%, p < 0.001), or use illicit drugs (5.2% vs. 8.6%, p < 0.001). Most of the operations were outpatient procedures (survey respondents n = 1563, 71.9%; nonresponders n = 2166, 73.2%) and done electively (survey respondents n = 1996, 91.9%; nonresponders n = 2628, 88.9%). The most common specialties performing operations were general surgery, urology, and otolaryngology. The mean MME prescribed to nonresponders was higher than responders (114 vs. 102, p < 0.001).
TABLE 1.
Characteristics of the surgery patients with prescribed opioids sent to health system pharmacies and their operations.
| Overall | Survey respondents | Nonresponders | p‐value a | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 5134 | 2174 | 42.3% | 2960 | 57.7% | |||
| Age group | <0.001 | ||||||
| 18–64 | 4004 | 78.0% | 1507 | 69.3% | 2497 | 84.3% | |
| ≥65 | 1130 | 22.0% | 667 | 30.7% | 463 | 15.7% | |
| Sex | 0.17 b | ||||||
| Female | 2615 | 50.93% | 1134 | 52.2% | 1481 | 50.04% | |
| Male | 2518 | 49.05% | 1040 | 47.8% | 1478 | 49.93% | |
| Nonbinary | 1 | 0.02% | 0 | 0.0% | 1 | 0.03% | |
| Home location | 0.10 | ||||||
| Nonrural | 3865 | 75.3% | 1612 | 74.1% | 2253 | 76.1 | |
| Rural | 1269 | 24.7% | 562 | 25.9% | 707 | 23.9% | |
| Race | <0.001 | ||||||
| Asian | 59 | 1.1% | 22 | 1.0% | 37 | 1.3% | |
| Black or African American | 70 | 1.4% | 22 | 1.0% | 48 | 1.6% | |
| Caucasian/White | 4534 | 88.3% | 1986 | 91.4% | 2548 | 86.1% | |
| Native Hawaiian/Pacific Islander | 50 | 1.0% | 11 | 0.5% | 39 | 1.3% | |
| Other/Unknown | 421 | 8.2% | 133 | 6.1% | 288 | 9.7% | |
| Ethnicity | <0.001 | ||||||
| Hispanic/Latino | 382 | 7.4% | 124 | 5.7% | 258 | 8.8% | |
| Non‐Hispanic/Latino | 4752 | 92.6% | 2050 | 94.3% | 2702 | 91.2% | |
| Current smoker | <0.001 | ||||||
| No | 4771 | 92.9% | 2071 | 95.3% | 2700 | 91.2% | |
| Yes | 363 | 7.1% | 103 | 4.7% | 260 | 8.8% | |
| Current alcohol use | 0.24 | ||||||
| No | 3325 | 64.8% | 1388 | 63.8% | 1937 | 65.4% | |
| Yes | 1809 | 35.2% | 786 | 36.2% | 1023 | 34.6% | |
| Current illicit drug use | <0.001 | ||||||
| No | 4769 | 92.9% | 2062 | 94.8% | 2707 | 91.4% | |
| Yes | 365 | 7.1% | 112 | 5.2% | 253 | 8.6% | |
| Surgery type | 0.31 | ||||||
| Outpatient surgery | 3729 | 72.6% | 1563 | 71.9% | 2166 | 73.2% | |
| Inpatient surgery | 1405 | 27.4% | 611 | 28.1% | 794 | 26.8% | |
| Urgency of surgery | 0.001 | ||||||
| Elective | 4624 | 90.1% | 1996 | 91.9% | 2628 | 88.9% | |
| Urgent | 478 | 9.3% | 170 | 7.8% | 308 | 10.4% | |
| Emergent | 28 | 0.6% | 7 | 0.3% | 21 | 0.8% | |
| Surgical specialty | <0.001 | ||||||
| Cardiothoracic | 307 | 6.0% | 144 | 6.6% | 163 | 5.5% | |
| General surgery | 2155 | 42.0% | 927 | 42.6% | 1228 | 41.5% | |
| Otolaryngology | 745 | 14.5% | 306 | 14.1% | 439 | 14.8% | |
| Plastics | 646 | 12.6% | 259 | 11.9% | 387 | 13.1% | |
| Transplant | 126 | 2.5% | 49 | 2.3% | 77 | 2.6% | |
| Urology | 1060 | 20.6% | 468 | 21.5% | 592 | 20.0% | |
| Vascular | 95 | 1.8% | 21 | 1.0% | 74 | 2.5% | |
| Mean MME prescribed at discharge (SD) | 108.7 | 123.7 | 102 | 101 | 114 | 138 | <0.001 c |
| Mean MME consumed (SD) | 69 | 109 | |||||
Abbreviations: MME, morphine milligram equivalent; SD, standard deviation.
p‐value for chi‐square test.
p‐value for Fisher's exact test.
p‐value for Wilcoxon rank‐sum test.
Patient‐reported outcomes are shown in Table 2. Most participants were very satisfied (n = 1637, 76.2%) or somewhat satisfied (n = 325, 15.1%) with their pain management after surgery. Two‐thirds of participants recalled receiving a disposal bag (n = 1375, 63.8%).
TABLE 2.
Patient‐reported outcomes of the patients with prescribed opioids sent to health system pharmacies.
| Survey respondents | ||
|---|---|---|
| n | % | |
| Patient used opioids in the last year | ||
| No | 1712 | 78.5% |
| Yes | 462 | 21.5% |
| Patient satisfaction with pain management | ||
| Very satisfied | 1637 | 76.2% |
| Somewhat satisfied | 325 | 15.1% |
| Neither satisfied nor dissatisfied | 74 | 3.5% |
| Somewhat dissatisfied | 80 | 3.7% |
| Very dissatisfied | 32 | 1.5% |
| Expectations of pain after surgery | ||
| “I experienced less pain than I expected” | 861 | 40.1% |
| “I experienced about as much pain as I expected” | 863 | 40.1% |
| “I experienced more pain than I expected” | 425 | 19.8% |
| Patient reported having pain issues with prior surgeries | ||
| No prior surgeries | 199 | 9.3% |
| No | 1531 | 71.4% |
| Yes | 414 | 19.3% |
| Patient recalled receiving disposal bag | ||
| No (or unsure) | 782 | 36.2% |
| Yes | 1375 | 63.8% |
| Precaution adoption process model stage | ||
| Stage 6‐“I have disposed of them” | 284 | 26.4% |
| Stage 5‐“I plan to dispose of them but have not yet” | 552 | 51.3% |
| Stage 3‐“I am undecided about whether I will dispose of them” | 69 | 6.4% |
| Stage 4‐“I do not plan to dispose of them” | 79 | 7.4% |
| Stage 2‐“I have not thought about disposal” | 91 | 8.5% |
Among the 1075 respondents with leftover opioids who reported their disposal plans, 284 (26.4%) had disposed 2 weeks after discharge (PAPM Stage 6). The remaining participants planned to dispose but had not yet (Stage 5, n = 552, 51.3%), did not plan to dispose (Stage 4, n = 79, 7.4%), were undecided about disposal (Stage 3, n = 69, 6.4%), or had not considered disposal (Stage 2, n = 91, 8.5%). We found an upward trend in monthly aggregated rates of patient recall of receiving the disposal bag but no significant change in disposal over the 10‐month study period (Figure 2). There may be a seasonal trend for both recall and disposal.
FIGURE 2.
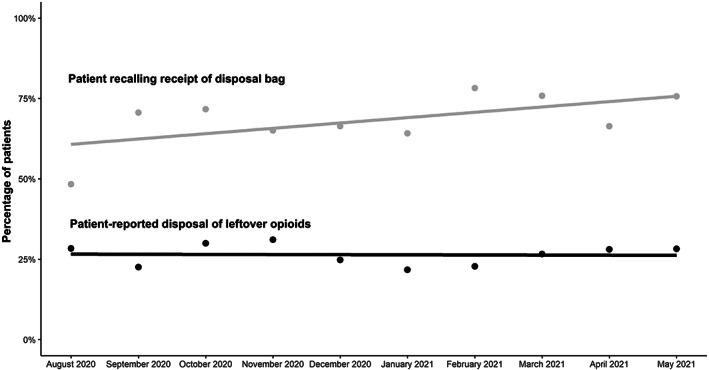
Disposal rates aggregated by month over the study period. The lower black dots and line indicate the monthly percentage of patient‐reported disposal and linear regression line, respectively. The upper light gray dots and line indicate monthly percentage of patients recalling receipt of the disposal bag and linear regression line, respectively.
On univariable analysis, disposal was positively associated with recalling receipt of a home‐disposal bag (incidence rate ratio [IRR] 1.24, 95% confidence interval [CI] 1.13–1.36) (Table 3). Disposal was negatively associated with patient use of opioids in the last year (IRR 0.81, 95% CI 0.72–0.91). On multivariable analysis, disposal was positively associated with recalling receipt of a home‐disposal bag (IRR 1.25, 95% CI 1.13–1.37). Patients who used opioids in the last year were less likely to dispose (IRR 0.82, 95% CI 0.73–0.93).
TABLE 3.
Univariable and multivariable modified Poisson regression analyses examining prescription opioid disposal among survey respondents with leftover prescription opioids that were filled at a health system pharmacy.
| Univariable a | Multivariable a | |||||
|---|---|---|---|---|---|---|
| Incidence rate ratio | 95% CI | p‐value | Incidence rate ratio | 95% CI | p‐value | |
| Patient recalled receiving disposal bag | ||||||
| No (or unsure) | Referent | Referent | ||||
| Yes | 1.24 | 1.13, 1.36 | <0.001 | 1.25 | 1.13, 1.37 | <0.001 |
| Patient used opioids in the last year | ||||||
| No | Referent | Referent | ||||
| Yes | 0.81 | 0.72, 0.91 | <0.001 | 0.82 | 0.73, 0.93 | 0.003 |
Abbreviation: CI, confidence interval.
The following variables were included in the multivariable model but are not shown due to non‐significance: age, race, ethnicity, sex, rural home location, current smoker, current alcohol use, urgency of surgery, surgery type (outpatient vs. inpatient), current illicit drug use, median morphine milligram equivalents prescribed at discharge, percentage of morphine milligram equivalents consumed, patient satisfaction, pain issues with prior surgeries, and expectations of pain after surgery. Models account for clustering by surgeon and differences between survey responders and nonresponders through inverse probability weighting.
4. DISCUSSION
In this study, we evaluated the reach, effectiveness, and maintenance of a health system‐wide intervention distributing home‐disposal bags to all patients prescribed opioids after surgery. Based on patients' recall of receiving the bag, we reached 69.5% of the eligible patients who were prescribed opioids. Among patients to whom the bag was distributed, 26.4% disposed of their leftover opioids. On multivariable analysis, disposal was positively associated with recalling receipt of the bag and negatively associated with those who used opioids in the last year.
Our disposal rate of 26.4% was lower than most prior disposal bag studies which reported rates of 55%–95%. 32 , 33 , 34 , 35 , 36 Several factors likely contribute to this lower rate of disposal. First, the prior studies were conducted as time‐limited trials which allowed for close follow‐up and monitoring by research personnel. Second, we allowed each of the 16 pharmacy units to decide to how they wanted to distribute disposal bags and education. We selected this implementation approach based on feedback from pharmacy stakeholders suggesting it would reduce the complexity and labor costs of implementation and allow for tailoring to local resources and conditions (which were constrained during the COVID‐19 pandemic). However, our findings suggest more active patient engagement and intensive implementation strategies are needed. Passive distribution was also cited by one trial as the likely explanation for their low disposal rate of 14%. 37 We are currently conducting a post‐formative mixed methods evaluation to identify and address the barriers to implementations and to guide the design of future interventions and implementation efforts.
Nonetheless, our study has several important findings. First, recalling receipt of the disposal bag was strongly associated with disposal which suggests that the bags can positively influence patient behavior. Second, despite our pragmatic low‐effort implementation approach, disposal rates were maintained over the study period. Third, the stages‐based Precaution Adoption Process Model provides important insights into patient willingness to dispose. Two weeks after discharge, 51.3% of eligible patients planned to dispose but had not yet (Stage 5). Prior studies suggest that most surgery patients are no longer using opioids less than a week after surgery. Our prior study on the barriers and facilitators to disposal suggests that these patients have not disposed because there is a low perceived risk associated with non‐disposal or are having difficulty converting their decision into action. 40 Interventions targeting this large population of well‐intentioned patients could increase the effectiveness of disposal interventions. Surprisingly, only 7.4% of patients did not plan to dispose (Stage 4). This suggests that most patients are amenable to disposing of their leftover opioids. A prospective cohort study patients undergoing inpatient surgery combined patient education, reminder phone calls 1–3 days prior to in‐person clinic follow‐ups, and a clinic drop box to achieve an 83% disposal rate. 9 While promising, the study was labor‐intensive and reliant on study coordinators to provide education and the reminder calls and patients to attend in‐person follow‐up visits. Whether these results can be replicated on a larger scale and in an era in which follow‐up appointments are increasingly virtual remains to be seen. Automated reminders tailored to patients' stage of readiness to dispose and reasons for (non‐)disposal may provide many of the benefits while allowing for better sustainability and scalability. Finally, there may be a seasonal trend in disposal though more data is needed. Prior studies have found seasonal trends in opioid prescriptions and prescription opioid‐related overdoses and suicides. 53 , 54 , 55
Our study has several limitations. First, we relied on participants to report their disposal behaviors and accurately self‐report their stage of readiness to dispose. While we had a 42% response rate, our findings may still be subject to nonresponder error as our respondents were likely to be older adults, living in rural locations, and identify as Caucasian/White. We have mitigated the possibility of survey nonresponse bias by incorporating nonresponse weights in our outcome model. This approach adjusts for differences between responders and nonresponders using the covariates available among both groups, that is, the baseline characteristics. However, it is possible that there are additional covariates (outside of the covariates that we measured) that could be related to the tendency for a person to respond. Second, our response rate was above what would be expected for an email survey despite recent research showing a decrease in survey response rates over the past few decades. 56 , 57 Studies have also shown that response rates are not good proxies for validity, and response rates as low as 18% can still provide reliable estimates of exposure‐outcome relationships. 58 Additional modes of survey distribution, such as text messaging or paper surveys, could not only increase the response rate but also reach nonresponders and otherwise ineligible participants. Third, social desirability bias may lead to over‐reporting of disposal. However, in our prior qualitative study, participants openly shared why they did not dispose. Future studies may use indirect‐questioning and non‐randomized‐response techniques to evaluate social desirability bias in opioid‐disposal behaviors. Fourth, we were unable to establish a baseline disposal rate within this study or conduct a randomized trial comparing distribution of disposal bags with a minimal intervention arm of the follow‐up survey alone. The lack of a comparison group means that external factors might affect our primary outcome. For example, a local health system announced a contemporaneous opioid‐free surgery program. 59 National pharmacy chains have expanded the availability of drop boxes and one company had previously announced they would distribute a free home‐disposal kit with every opioid prescription (though whether the program was active during our study period is not known). 60 , 61 However, almost all of our surgery patients fill their opioids at one of our pharmacies. Several states (not in our catchment area) have enacted laws requiring manufacturers to fund drug disposal. In 2020, the state of Washington launched their state‐wide drug take‐back program (which is funded by drug manufacturers) though no opioid‐specific disposal outcomes have been reported. 62 Finally, news media and the federal government have increased awareness of the opioid crisis and its associated risks. 63 , 64
5. CONCLUSION
Prescription opioids are a critical tool for managing pain after surgery, but their over‐supply likely contributes to the opioid crisis in the United States. Our pragmatic low‐effort implementation of a system‐wide disposal intervention resulted in a lower disposal rate than previous controlled trials though we were able to reach two‐thirds of patients and maintain the intervention over time. Our study suggests that motivating patients who are planning to dispose but have not yet done so will be critical to increasing the effectiveness of health‐system disposal interventions. Further studies translating the interventions used in small‐scale research studies into health‐system interventions and testing implementation strategies in a variety of health systems are also needed.
FUNDING INFORMATION
The research reported in this publication was supported by the University of Utah Study Design and Biostatistics Center, funded partly by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (Award Numbers UL1TR002538 and KL2TR002539), and a Veterans Affairs Health Services Research & Development Research Career Scientist Award (RCS 14–232). Infrastructure support for author AJG was provided, in part, by the Greater Intermountain Node (GIN; NIH/NIDA 1UG1DA049444) of the National Institute on Drug Abuse Clinical Trials Network and the Department of Veterans Affairs Health Services Research and Development Service Informatics, Decision‐Enhancement, and Analytic Sciences (IDEAS; CIN 13–414) Center of Innovation. The content is the responsibility solely of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veteran Affairs.
Supporting information
Data S1. Supporting information.
ACKNOWLEDGMENTS
The authors would like to thank Erin Fox, Kavish Choudhary, and Nathan Hagen for their assistance in implementing the intervention, Adam McDougal for his assistance in cleaning and merging the survey data with the electronic medical records, and Megan Reynolds for copy‐editing.
Huang LC, Bleicher J, Torre M, et al. Evaluating a health system‐wide opioid disposal intervention distributing home‐disposal bags. Health Serv Res. 2023;58(6):1256‐1265. doi: 10.1111/1475-6773.14227
REFERENCES
- 1. Hall MJ. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Rep. 2017;102:15. [PubMed] [Google Scholar]
- 2. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. doi: 10.1136/bmj.j5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wunsch H, Wijeysundera DN, Passarella MA, Neuman MD. Opioids prescribed after low‐risk surgical procedures in the United States, 2004–2012. JAMA. 2016;315(15):1654‐1657. doi: 10.1001/jama.2016.0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McDermott KW, Liang L. Overview of operating room procedures during inpatient stays in U.S. hospitals, 2018. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); Published online 2021. Accessed April 23, 2023. https://hcup‐us.ahrq.gov/reports/statbriefs/sb281‐Operating‐Room‐Procedures‐During‐Hospitalization‐2018.pdf [Google Scholar]
- 5. Vu JV, Howard RA, Gunaseelan V, Brummett CM, Waljee JF, Englesbe MJ. Statewide implementation of postoperative opioid prescribing guidelines. N Engl J Med. 2019;381(7):680‐682. doi: 10.1056/NEJMc1905045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maughan BC, Hersh EV, Shofer FS, et al. Unused opioid analgesics and drug disposal following outpatient dental surgery: a randomized controlled trial. Drug Alcohol Depend. 2016;168:328‐334. doi: 10.1016/j.drugalcdep.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 7. Thiels CA, Ubl DS, Yost KJ, et al. Results of a prospective, multicenter initiative aimed at developing opioid‐prescribing guidelines after surgery. Ann Surg. 2018;268(3):457‐468. doi: 10.1097/SLA.0000000000002919 [DOI] [PubMed] [Google Scholar]
- 8. Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066‐1071. doi: 10.1001/jamasurg.2017.0831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Porter ED, Bessen SY, Molloy IB, et al. Guidelines for patient‐centered opioid prescribing and optimal FDA‐compliant disposal of excess pills after inpatient operation: prospective clinical trial. J Am Coll Surg. 2021;232(6):823‐835.e2. doi: 10.1016/j.jamcollsurg.2020.12.057 [DOI] [PubMed] [Google Scholar]
- 10. Lev R, Lee O, Petro S, et al. Who is prescribing controlled medications to patients who die of prescription drug abuse? Am J Emerg Med. 2016;34(1):30‐35. doi: 10.1016/j.ajem.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 11. Opioid Overdose Rates . National Institute on Drug Abuse. 2022. Accessed March 20, 2022 https://www.drugabuse.gov/drug-topics/trends-statistics/overdose-death-rates
- 12. CDC . Prevent opioid misuse. Published November 20, 2020. Accessed July 18, 2021 https://www.fda.gov/drugs/safe-disposal-medicines/disposal-unused-medicines-what-you-should-know
- 13. FDA . Disposal of unused medicines: what you should know. Published October 1, 2020. Accessed July 18, 2021 https://www.fda.gov/drugs/safe-disposal-medicines/disposal-unused-medicines-what-you-should-know
- 14. CDC . Drug overdose deaths ‐ prescription opioids. Published March 17, 2021. Accessed July 18, 2021 https://www.cdc.gov/drugoverdose/deaths/prescription/overview.html
- 15. McDonald EM, Kennedy‐Hendricks A, McGinty EE, Shields WC, Barry CL, Gielen AC. Safe storage of opioid pain relievers among adults living in households with children. Pediatrics. 2017;139(3):e20162161. doi: 10.1542/peds.2016-2161 [DOI] [PubMed] [Google Scholar]
- 16. Bartels K, Mayes LM, Dingmann C, Bullard KJ, Hopfer CJ, Binswanger IA. Opioid use and storage patterns by patients after hospital discharge following surgery. Costigan M, ed. PLoS One. 2016;11(1):e0147972. doi: 10.1371/journal.pone.0147972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaither JR, Shabanova V, Leventhal JM. US National Trends in pediatric deaths from prescription and illicit opioids, 1999–2016. JAMA Netw Open. 2018;1(8):e186558. doi: 10.1001/jamanetworkopen.2018.6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma CS, Batz F, Juarez DT, Mscja LCL. Drug take Back in Hawai‘i: partnership between the University of Hawai‘i Hilo College of Pharmacy and the narcotics enforcement division. Hawaii J Med Public Health. 2014;73(1):6. [PMC free article] [PubMed] [Google Scholar]
- 19. Providing Mail‐Back Envelopes and Education on Safe Disposal With Opioid Analgesics Dispensed in an Outpatient Setting; Establishment of a Public Docket; Request for Comments. United States Food and Drug Administration; 2022. Accessed April 23, 2023. https://www.federalregister.gov/documents/2022/04/21/2022-08372/providing-mail-back-envelopes-and-education-on-safe-disposal-with-opioid-analgesics-dispensed-in-an [Google Scholar]
- 20. Stanford‐Lancet Commission on the North American Opioid Crisis . Recommendation 5a: policymakers in the USA should implement more effective procedures to reduce the supply of excess opioid pills. Accessed February 4, 2022 https://opioids.stanford.edu/recommendation-5a
- 21. Stewart H, Malinowski A, Ochs L, Jaramillo J, McCall K, Sullivan M. Inside Maine's medicine cabinet: findings from the drug enforcement administration's medication take‐back events. Am J Public Health. 2015;105(1):e65‐e71. doi: 10.2105/AJPH.2014.302207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shealy KM, Ritter MS, Wyatt AS, Eagerton DH. Trends in potentially abused medications returned during medication take‐back days. J Am Pharm Assoc. 2019;59(4):575‐578. doi: 10.1016/j.japh.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 23. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community‐based medication take‐back program. J Am Pharm Assoc. 2014;54(3):275‐279. doi: 10.1331/JAPhA.2014.13143 [DOI] [PubMed] [Google Scholar]
- 24. Gray J, Hagemeier N, Brooks B, Alamian A. Prescription disposal practices: a 2‐year ecological study of drug drop box donations in Appalachia. Am J Public Health. 2015;105(9):e89‐e94. doi: 10.2105/AJPH.2015.302689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egan KL, Gregory E, Sparks M, Wolfson M. From dispensed to disposed: evaluating the effectiveness of disposal programs through a comparison with prescription drug monitoring program data. Am J Drug Alcohol Abuse. 2017;43(1):69‐77. doi: 10.1080/00952990.2016.1240801 [DOI] [PubMed] [Google Scholar]
- 26. Cabo J, Hsi RS, Scarpato KR. Postoperative opiate use in urological patients: a quality improvement study aimed at improving opiate disposal practices. J Urol. 2019;201(2):371‐376. doi: 10.1016/j.juro.2018.09.052 [DOI] [PubMed] [Google Scholar]
- 27. Lewis J, Crawford S, Sullivan‐Boylai S, Poza R. A clinical trial of a video intervention targeting opioid disposal after general surgery: a feasibility study. J Surg Res. 2021;262:6‐13. doi: 10.1016/j.jss.2020.12.010 [DOI] [PubMed] [Google Scholar]
- 28. Rose P, Sakai J, Argue R, Froehlich K, Tang R. Opioid information pamphlet increases postoperative opioid disposal rates: a before versus after quality improvement study. Can J Anaesth. 2016;63(1):31‐37. doi: 10.1007/s12630-015-0502-0 [DOI] [PubMed] [Google Scholar]
- 29. Hasak JM, Roth Bettlach CL, Santosa KB, Larson EL, Stroud J, Mackinnon SE. Empowering post‐surgical patients to improve opioid disposal: a before and after quality improvement study. J Am Coll Surg. 2018;226(3):235‐240.e3. doi: 10.1016/j.jamcollsurg.2017.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de la Cruz M, Reddy A, Balankari V, et al. The impact of an educational program on patient practices for safe use, storage, and disposal of opioids at a comprehensive cancer center. Oncologist. 2017;22(1):115‐121. doi: 10.1634/theoncologist.2016-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu JY, Franklin JS, Gesek FA, Anderson JC. Buyback program of unused prescription opioids in US rural communities, 2017–2018. Am J Public Health. 2020;110(9):1318‐1324. doi: 10.2105/AJPH.2020.305730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stokes SM, Kim RY, Jacobs A, et al. Home disposal kits for leftover opioid medications after surgery: do they work? J Surg Res. 2020;245:396‐402. [DOI] [PubMed] [Google Scholar]
- 33. Brummett CM, Steiger R, Englesbe M, et al. Effect of an activated charcoal bag on disposal of unused opioids after an outpatient surgical procedure: a randomized clinical trial. JAMA Surg. 2019;154(6):558‐561. doi: 10.1001/jamasurg.2019.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lawrence AE, Carsel AJ, Leonhart KL, et al. Effect of drug disposal bag provision on proper disposal of unused opioids by families of pediatric surgical patients: a randomized clinical trial. JAMA Pediatr. Published online June 24. 2019;173(8):e191695. doi: 10.1001/jamapediatrics.2019.1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agarwal AK, Lee D, Ali Z, et al. Effect of mailing an at‐home disposal kit on unused opioid disposal after surgery: a randomized clinical trial. JAMA Netw Open. 2022;5(5):e2210724. doi: 10.1001/jamanetworkopen.2022.10724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang DA, Luong M, Barragan E, Bushnell F, Cho RH, Poon S. Disposal of unused opioids using an at‐home disposal method. J Pediatr Orthop Soc North Am Preventing opioid diversion and abuse by using an at‐home opioid disposal method: an improvement project in a pediatric outpatient surgical center. 2021;3(1):5. [Google Scholar]
- 37. Bicket MC, Fu D, Swarthout MD, White E, Nesbit SA, Monitto CL. Effect of drug disposal kits and fact sheets on elimination of leftover prescription opioids: the DISPOSE multi‐arm randomized controlled trial. Pain Med. 2021;22(4):961‐969. doi: 10.1093/pm/pnaa431 [DOI] [PubMed] [Google Scholar]
- 38. Glasgow RE, Harden SM, Gaglio B, et al. RE‐AIM planning and evaluation framework: adapting to new science and practice with a 20‐year review. Front Public Health. 2019;7:64. doi: 10.3389/fpubh.2019.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weinstein ND, Sandman PM. A model of the precaution adoption process: evidence from home radon testing. Health Psychol. 1992;11(3):170‐180. [DOI] [PubMed] [Google Scholar]
- 40. Huang LC, Johnson JE, Bleicher J, et al. Promoting disposal of left‐over opioids after surgery in rural communities: a qualitative description study. Health Educ Behav. Published online December 28. 2021;50(2):109019812110575. doi: 10.1177/10901981211057540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bleicher J, Fender Z, Johnson JE, et al. Use of post‐discharge opioid consumption patterns as a tool for evaluating opioid prescribing guidelines. Am J Surg Published online December. 2021:S0002961021007480;224:58‐63. doi: 10.1016/j.amjsurg.2021.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Boerum MS, Mann SL, Veith JP, et al. Patient‐reported opioid use for tissue expander–based breast reconstruction. J Plast Reconstr Aesthet Surg. 2021;74(11):2899‐2905. doi: 10.1016/j.bjps.2021.03.114 [DOI] [PubMed] [Google Scholar]
- 43. Hassan MM, Rahman OF, Hussain ZB, Burgess SL, Yen YM, Kocher MS. Opioid overprescription in adolescents and young adults undergoing hip arthroscopy. J Hip Preserv Surg. 2021;8(1):75‐82. doi: 10.1093/jhps/hnab048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. MDCalc Morphine Milligram Equivalents (MME) Calculator. Accessed June 1, 2022 https://www.mdcalc.com/calc/10170/morphine-milligram-equivalents-mme-calculator
- 45. The EQUATOR Network . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Accessed February 17, 2022 https://www.equator-network.org/reporting-guidelines/strobe/
- 46. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702‐706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 47. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1‐21. doi: 10.1214/09-STS313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leite W. Practical Propensity Score Methods Using R. SAGE Publications; 2016. [Google Scholar]
- 49. Bishop CD, Leite WL, Snyder PA. Using propensity score weighting to reduce selection bias in large‐scale data sets. J Early Interv. 2018;40(4):347‐362. doi: 10.1177/1053815118793430 [DOI] [Google Scholar]
- 50. McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403‐425. doi: 10.1037/1082-989X.9.4.403 [DOI] [PubMed] [Google Scholar]
- 51. Zeileis A. Object‐oriented computation of Sandwich estimators. J Stat Softw. 2006;16(9):1‐16. doi: 10.18637/jss.v016.i09 [DOI] [Google Scholar]
- 52. Cribari‐Neto F, Bernardino da Silva W. A new heteroskedasticity‐consistent covariance matrix estimator for the linear regression model. AStA Adv Stat Anal. 2011;95:129‐146. [Google Scholar]
- 53. Davis JM, Searles VB, Severtson SG, Dart RC, Bucher‐Bartelson B. Seasonal variation in suicidal behavior with prescription opioid medication. J Affect Disord. 2014;158:30‐36. doi: 10.1016/j.jad.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 54. Horvat CM, Martin B, Wu L, et al. Opioid e‐prescribing trends at discharge in a large pediatric health system. J Opioid Manag. 2019;15(2):119‐127. doi: 10.5055/jom.2019.0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abraham O, Rosenberger C, Tierney K, Birstler J. Investigating the use of a serious game to improve opioid safety awareness among adolescents: quantitative study. JMIR Serious Games. 2021;9(4):e33975. doi: 10.2196/33975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morton SMB, Bandara DK, Robinson EM, Carr PEA. In the 21st century, what is an acceptable response rate? Aust N Z J Public Health. 2012;36(2):106‐108. doi: 10.1111/j.1753-6405.2012.00854.x [DOI] [PubMed] [Google Scholar]
- 57. Guo Y, Kopec JA, Cibere J, Li LC, Goldsmith CH. Population survey features and response rates: a randomized experiment. Am J Public Health. 2016;106(8):1422‐1426. doi: 10.2105/AJPH.2016.303198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mealing NM, Banks E, Jorm LR, Steel DG, Clements MS, Rogers KD. Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Med Res Methodol. 2010;10(1):26. doi: 10.1186/1471-2288-10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Intermountain Healthcare . Intermountain healthcare first health system in utah to offer formal opioid‐free surgery program for patients wanting alternative pain control options. Accessed April 30, 2023 https://intermountainhealthcare.org/news/2020/02/opioid‐free‐surgery/
- 60. Exploring options for safe and effective in‐home opioid disposal. In: Duke Margolis Center for Health Policy; 2021. Accessed April 23, 2023 https://healthpolicy.duke.edu/sites/default/files/2022-06/Exploring%20Options%20for%20Safe%20and%20Effective%20In-Home%20Opioid%20Disposal_Meeting%20Summary.pdf
- 61. Walmart launches groundbreaking disposal solution to aid in fight against opioid abuse and misuse. Published January 17, 2018. Accessed April 23, 2023 https://corporate.walmart.com/newsroom/2018/01/17/walmart-launches-groundbreaking-disposal-solution-to-aid-in-fight-against-opioid-abuse-and-misuse
- 62. Stubbings J, Crawford SY, Menighan TE. A safe in‐home disposal system with every opioid prescription? Food and Drug Administration is considering a potential new risk evaluation and mitigation strategy that could impact pharmacists. J Am Pharm Assoc. 2022;62(2):413‐418. doi: 10.1016/j.japh.2021.11.009 [DOI] [PubMed] [Google Scholar]
- 63. Katz J, Sanger‐Katz M. ‘It's huge, It's historic, It's unheard‐of’: drug overdose deaths spike. The New York Times. Accessed April 20, 2023 https://www.nytimes.com/interactive/2021/07/14/upshot/drug-overdose-deaths.html
- 64. Actions the Biden‐Harris Administration has Taken to Address Addiction and the Overdose Epidemic. The White House. Published November 17, 2021 https://www.whitehouse.gov/ondcp/briefing‐room/2021/11/17/fact‐sheet‐actions‐the‐biden‐harris‐administration‐has‐taken‐to‐address‐addiction‐and‐the‐overdose‐epidemic/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.


