Abstract
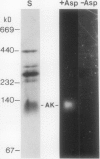
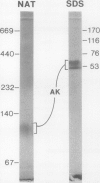
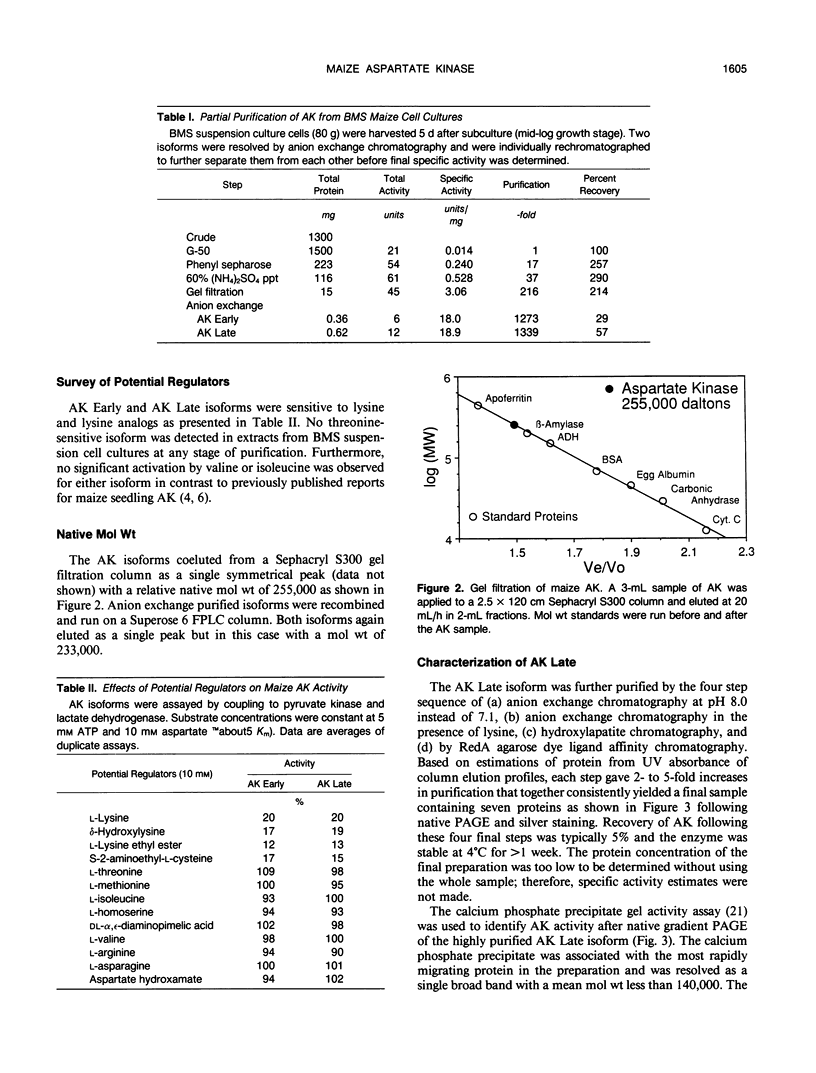
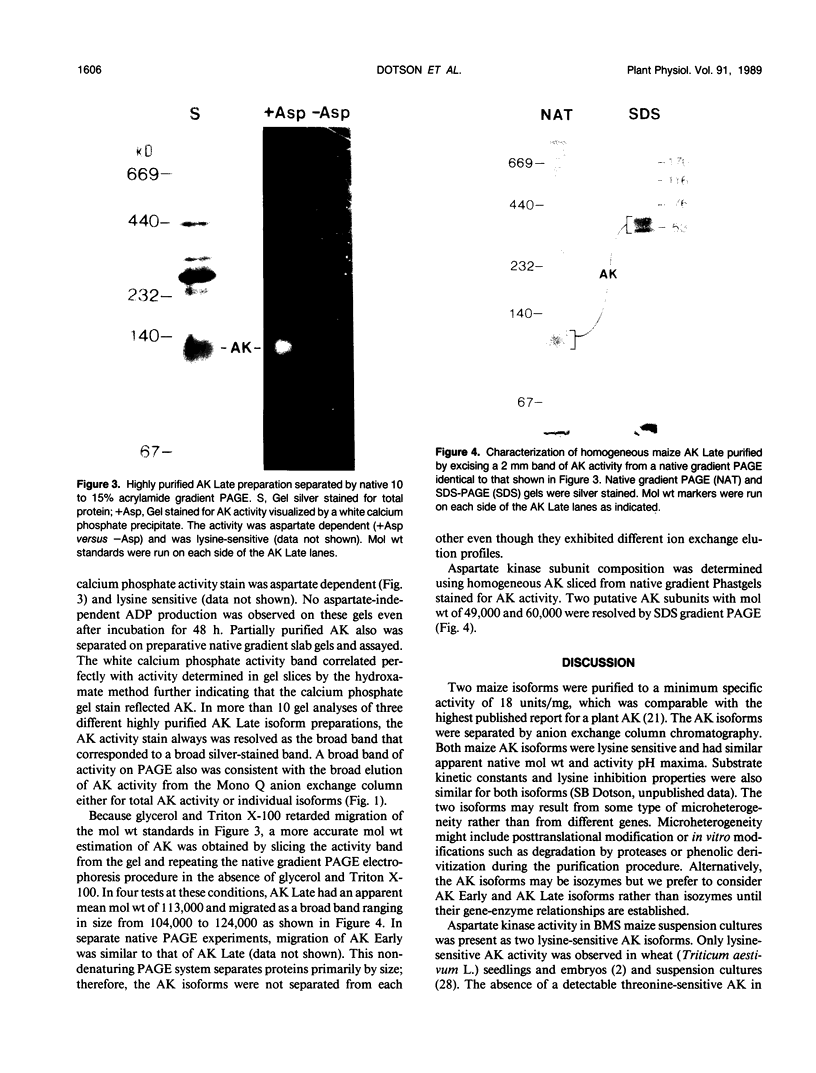
Aspartate kinase is a feedback-regulated enzyme that controls the first step common to the biosynthesis of lysine, threonine, isoleucine, and methionine in plants. Aspartate kinase was purified from Black Mexican Sweet maize (Zea mays L.) cell suspension cultures for physical and kinetic characterization studies. Partial purification and elution from an anion exchange column resolved two lysine-sensitive aspartate kinase isoforms. Both isoforms were purified >1,200-fold to a minimum specific activity of 18 units/milligram of protein. Both isoforms were sensitive to the lysine analogues S-2-aminoethyl-l-cysteine, l-lysine ethyl ester, and δ-hydroxylysine. No threonine-sensitive form of aspartate kinase was detected at any stage during the purification. Additional purification steps were combined with preparative gel electrophoresis to obtain apparently homogeneous lysine-sensitive aspartate kinase. Aspartate kinase appeared to be a tetramer with a holoenzyme molecular weight of 254,000 and to be composed of 49,000 and 60,000 subunits. The tetramer appeared to disassociate during native gel electrophoresis to 113,000 dalton species that retained aspartate kinase activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bryan P. A., Cawley R. D., Brunner C. E., Bryan J. K. Isolation and characterization of a lysine-sensitive aspartokinase from a multicellular plant. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1211–1217. doi: 10.1016/0006-291x(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Cassan M., Parsot C., Cohen G. N., Patte J. C. Nucleotide sequence of lysC gene encoding the lysine-sensitive aspartokinase III of Escherichia coli K12. Evolutionary pathway leading to three isofunctional enzymes. J Biol Chem. 1986 Jan 25;261(3):1052–1057. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Henke R. R., Wahnbaeck R. beta-Aspartokinase from developing endosperm of maize. Biochem Biophys Res Commun. 1977 Nov 7;79(1):38–45. doi: 10.1016/0006-291x(77)90057-2. [DOI] [PubMed] [Google Scholar]
- Heukeshoven J., Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988 Jan;9(1):28–32. doi: 10.1002/elps.1150090106. [DOI] [PubMed] [Google Scholar]
- McClure W. R. A kinetic analysis of coupled enzyme assays. Biochemistry. 1969 Jul;8(7):2782–2786. doi: 10.1021/bi00835a014. [DOI] [PubMed] [Google Scholar]
- Mills W. R. Photosynthetic formation of the aspartate family of amino acids in isolated chloroplasts. Plant Physiol. 1980 Jun;65(6):1166–1172. doi: 10.1104/pp.65.6.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir D., Paulus H. Properties and subunit structure of aspartokinase II from Bacillus subtilis VB217. J Biol Chem. 1977 Jul 10;252(13):4648–4651. [PubMed] [Google Scholar]
- Pechère J. F., Capony J. P. On the colorimetric determination of acyl phosphates. Anal Biochem. 1968 Mar;22(3):536–539. doi: 10.1016/0003-2697(68)90297-2. [DOI] [PubMed] [Google Scholar]
- Rafalski J. A., Falco S. C. Structure of the yeast HOM3 gene which encodes aspartokinase. J Biol Chem. 1988 Feb 15;263(5):2146–2151. [PubMed] [Google Scholar]
- Rognes S. E., Lea P. J., Miflin B. J. S-adenosylmethionine--a novel regulator of aspartate kinase. Nature. 1980 Sep 25;287(5780):357–359. doi: 10.1038/287357a0. [DOI] [PubMed] [Google Scholar]
- Veron M., Guillou Y., Fazel A., Cohen G. N. Reversible dissociation of aspartokinase I/homoserine dehydrogenase I from Escherichia coli K 12. The active species is the tetramer. Eur J Biochem. 1985 Sep 16;151(3):521–524. doi: 10.1111/j.1432-1033.1985.tb09133.x. [DOI] [PubMed] [Google Scholar]
- Wampler D. E., Takahashi M., Westhead E. W. Active subunits of the aspartokinase-homoserine dehydrogenase I complex from Escherichia coli. Biochemistry. 1970 Oct 13;9(21):4210–4216. doi: 10.1021/bi00823a024. [DOI] [PubMed] [Google Scholar]
- Wampler D. E., Westhead E. W. Two aspartokinases from Escherichia coli. Nature of the inhibition and molecular changes accompanying reversible inactivation. Biochemistry. 1968 May;7(5):1661–1671. doi: 10.1021/bi00845a007. [DOI] [PubMed] [Google Scholar]