Abstract
Objective
In numerous observational studies, there has been an indication that educational attainment (EA) can impact the intensity of pain and disability resulting from chronic musculoskeletal disorders. Nonetheless, the association observed in these studies is not entirely conclusive. The aim of this study was to investigate the genetic causal relationship between educational attainment and 12 musculoskeletal disorders using Mendelian randomization (MR).
Methods
The meta‐analysis of genome‐wide association studies (GWAS) identified 3952 single‐nucleotide polymorphisms (SNPs) associated with educational attainment (EA) from the Social Science Genetic Association Consortium (SSGAC). Genetic data for 12 musculoskeletal disorders, including osteonecrosis, osteoporosis, osteomyelitis, low back pain, gout, spinal stenosis, rheumatoid arthritis, meniscus derangement, rotator cuff syndrome, ankylosing spondylitis, cervicobrachial syndrome, and lateral epicondylitis, were obtained from the FinnGen consortium. We conducted a two‐sample Mendelian randomization analysis to examine the causal effect of EA on the risk of these musculoskeletal disorders using the TwoSampleMR package in R.
Results
Based on the inverse‐variance weighted (IVW) method, we found that a genetically predicted per standard deviation (SD) increase in EA was inversely associated with low back pain [odds ratio (OR) 0.46, 95% confidence interval (Cl) 0.51–0.61, p < 0.001], spinal stenosis (OR 0.62, 95% Cl 0.54–0.71, p < 0.001), rheumatoid arthritis (OR 0.65, 95% Cl 0.55–0.76, p < 0.001), meniscus derangement (OR 0.73, 95% Cl 0.65–0.82, p < 0.001), rotator cuff syndrome (OR 0.55, 95% Cl 0.49–0.61, p < 0.001), cervicobrachial syndrome (OR 0.50, 95% Cl 0.42–0.60, p < 0.001), and lateral epicondylitis (OR 0.30, 95% Cl 0.24–0.37, p < 0.001). There was no causal association between EA and osteonecrosis (OR 1.11, 95% CI 0.76–1.72, p = 0.60), osteoporosis (OR 0.91, 95% CI 0.65–1.27, p = 0.59), or osteomyelitis (OR 0.90, 95% CI 0.75–1.01, p = 0.22). Genetic predisposition to EA had a suggestive causal association with gout (OR 0.80, 95% CI 0.68–0.95, p = 0.01) and ankylosing spondylitis (OR 0.64, 95% CI 0.45–0.91, p = 0.01) after Bonferroni correction. None of the analyses revealed any horizontal pleiotropy or heterogeneity.
Conclusion
In our investigation, we have uncovered evidence supporting a causal relationship between low level of EA and the incidence of certain musculoskeletal disorders. In the future, it is imperative to ascertain risk factors such as lifestyle patterns linked with EA to uncover the underlying causal relationship and offer informed interventions for individuals.
Keywords: Causal Association, Education Attainment, Mendelian Randomization, Musculoskeletal Disorders
The overview of mendelian randomization design in the study. SSGAC, Social Science Genetic Association Consortium; GWAS, Genome‐wide association meta‐analysis; IVs, instrumental variables; LD, Linkage disequilibrium; SNP, Single Nucleotide Polymorphism.
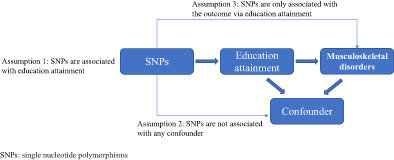
Introduction
Musculoskeletal disorders represent a significant contributor to persistent chronic pain and disability, affecting a wide range of individuals from working‐age adults to the elderly population. 1 These disorders have a lifetime prevalence of 75% or more, can have a significant negative impact on quality of life due to pain, functional limitations, and social disabilities. Additionally, they can impose a significant socioeconomic burden. 2
Educational attainment (EA) is a widely recognized social determinant of health, influencing health literacy and health‐related behaviors. 3 , 4 The generally low level of EA was found to be closely associated with a lower economic status, which in turn often coexists with unhealthy lifestyle behaviors such as alcohol abuse, heavy cigarette smoking, unhealthy diet, and being overweight. 4 Recent research has indicated that the unhealthy lifestyle behaviors mentioned above can accelerate the loss of musculoskeletal mass and function during the natural aging process, ultimately resulting in musculoskeletal pain. 5 Some individuals with long‐term musculoskeletal pain develop chronic musculoskeletal disorders such as lateral epicondylitis, cervicobrachial syndrome, and rotator cuff syndrome.
Furthermore, the level of EA is closely linked to both the frequency and intensity of leisure‐time physical activity as well as work‐related physical activity. 6 A Finnish national cohort study showed that long‐term exposure to physically heavy work was associated with an increased risk of musculoskeletal disorders. 7 The relationship between lower EA and the severity of chronic pain resulting from musculoskeletal disorders has been established in previous studies. Various factors, directly or indirectly linked to multiple musculoskeletal disorders, have been identified. Recent years have seen significant progress in the understanding of the genetic component in the development of these disorders, which is supported by clinical and epidemiological evidence. 8 , 9
Mendelian randomization (MR) is an innovative epidemiological analysis technique that uses genetic variables, specifically single nucleotide polymorphisms (SNPs), as instrumental variables (IVs) to draw conclusions about causality and assess the relationship between risk factors and disease outcomes. 10 The core idea behind MR is that genetic variations should be causally associated with disease risk. The issue with reverse causality can also be avoided by MR because genetic variation occurs prior to disease and the order of the two cannot be reversed. 11 In addition, due to the unpredictable separation of alleles during meiosis, MR can lessen the bias brought on by confounding variables. 11 However, few studies have focused on the association between EA and musculoskeletal disorders by using MR analysis.
In this study, we have utilized the MR design with two‐sample MR analysis to investigate the potential causal relationship between EA and 12 prevalent musculoskeletal disorders. This approach can provide a solid theoretical foundation for future research and clinical interventions.
Methods
Genetic Variants Associated with Educational Attainment
It is assumed that the IVs are valid if they meet all of the following criteria: (1) strongly associated with the EA; (2) not associated with confounding factors; (3) only associated with 12 common musculoskeletal disorders via EA.
We selected large‐scale genome‐wide association meta‐analysis (GWAS) summary statistics of single SNPs associated with EA from the Social Science Genetic Association Consortium (SSGAC). Okbay et al. reported the most recent meta‐analysis of GWAS for EA that enrolled 3,037,499 individuals of European ancestry by combining three sets of summary statistics: public results from our previous meta‐analysis of 69 cohorts (N = 324,162), new association results from 23 and Me cohort (N = 2,272,216), and new association results from a GWAS we conducted in UK Biobank (UKB) with an improved coding of the EA measure (N = 441,121). EA is evaluated by the number of years of schooling completed (Education Years) based on the International Standard Classification of Education 1997 classification scale. They identified 3952 significant SNPs associated with EA when p value of SNPs<5 × 10−8. After excluding the SNPs with potential linkage disequilibrium (LD) between multiple IVs (pairwise r2 > 0.001, clump window<10,000 kb), a total of 602 independent SNPs associated with EA were left.
To assess the potential bias caused by weak IVs, we calculated the F statistics to quantify the strength of the remaining instruments via related formula. 12 However, we identified that the SNP (rs28617748) did not meet the threshold of the rule‐of‐thumb value of 10 for the F statistics and was subsequently excluded from the analysis. Further information can be found in the supplementary Table S1 accompanying this study. Subsequently, we conducted a meticulous screening process using the PhenoScanner database (http://www.phenoscanner.medschl.cam.ac.uk/) to identify and exclude 19 SNPs associated with potential confounders and 12 common musculoskeletal disorders shown in Supplementary Table S2. In cases where the SNP data were unavailable from GWAS results, we employed the LDlink online platform (https://ldlink.nci.nih.gov/) to identify suitable proxy SNPs. Finally, we identified 582 independent SNPs as the IVs for assessing the causal relationship with EA. The flowchart of the detailed study design is shown in Figure 1.
Fig. 1.
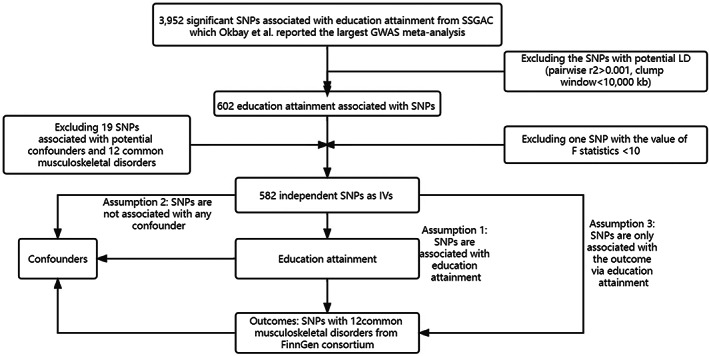
Flowchart of the detailed study design. SSGAC, Social Science Genetic Association Consortium; GWAS, genome‐wide association meta‐analysis; IVs, instrumental variables; LD, linkage disequilibrium; SNP, single nucleotide polymorphism.
GWAS Summary Data on Musculoskeletal Disorders
We retrieved GWAS summary data on musculoskeletal disorders such as osteonecrosis (1206 cases and 325,717 controls), osteoporosis (6303 cases and 325,717 controls), osteomyelitis (1532 cases and 325,717 controls), low back pain (25,163 cases and 248,831 controls), gout (7461 cases and 221,323 controls), spinal stenosis (16,698 cases and 248,831 controls), rheumatoid arthritis (11,178 cases and 221,323 controls), meniscus derangement (21,757 cases and 221,323 controls), rotator cuff syndrome (21,198 cases and 18,698 controls), lateral epicondylitis (3765 cases and 253,312 controls), cervicobrachial syndrome (6326 cases and 248,831 controls), and ankylosing spondylitis (2563 cases and 248,831 controls) from the latest eighth release of the FinnGen consortium. The ongoing FinnGen consortium has already reached 224,737 genotyped and phenotyped participants and combined genotyping data from Finns with digital health record data, creating a high‐quality database for academics to examine genetic variance in many diseases. 13 Detailed information on the participants, genotyping, and sample sizes, and definitions of the 12 musculoskeletal disorders can be found on the FinnGen website (https://www.fifinngen.fifi/en).
Statistical Analysis
We performed a two‐sample MR analysis to analyze the potential causal association between EA and 12 musculoskeletal disorders using three methods: inverse‐variance weighted (IVW), MR–Egger, and weighted median. 14 The MR analysis results were mainly based on IVW methods, which adopt a meta‐analysis of the Wald ratio for individual SNPs. IVW offers a reliable estimation of the causal estimates of exposure on the result if each genetic variant can be employed as an efficient IV. The heterogeneity between SNPs was evaluated by calculating Cochrane's Q statistic. If Cochrane Q‐derived p < 0.05, heterogeneity was considered to exist. The pleiotropy was detected using MR–Egger intercept analysis, which could unbiase estimates that are achieved in the presence of pleiotropic instruments with the assumption, with an intercept p value >0.05 indicating pleiotropy deficiency. 15 In addition, we also generated funnel plots to visually assess pleiotropy. There is no potential directional pleiotropy when the funnel plot is symmetric. 16 After removing the outliers, the MR‐PRESSO approach may detect outliers and produce estimates. In addition, we performed MR‐PRESSO and leave‐one‐SNP‐out analysis to identify potential violations. A leave‐one‐out analysis was conducted to evaluate whether the MR estimate was driven by a single SNP. The MR‐PRESSO method identified outliers and generated estimates after the outliers were removed. Moreover, the weighted median was used to further identify the potential causal association between exposures and outcomes.
All analyses were performed in R (version 4.1.0; Lucent Technologies; New Jersey, USA) using the “TwoSampleMR” package (version 0.4.26). 17 The Bonferroni correction was used in our analyses since 12 musculoskeletal disorders were used as outcomes. Associations with p < 0.00417 (0.05/12) were used to define significance, while p ≥ 0.00417 and <0.05 were regarded as suggestive causal associations.
Results
EA and Bone Disorders
For the three bone disorders, as shown using MR analysis of IVW in Figure 2, there was no potential genetic causal association between EA and osteonecrosis [odds ratio (OR) 1.11, 95% confidence interval (CI) 0.76–1.72, p = 0.60], osteoporosis (OR 0.91, 95% CI 0.65–1.27, p = 0.59), or osteomyelitis (OR 0.90, 95% CI 0.75–1.01, p = 0.22). The associations exhibited consistency in sensitivity analyses utilizing MR–Egger and weighted median methods, albeit yielding less precise estimations than the IVW method (Figures 3 and 4).
Fig. 2.
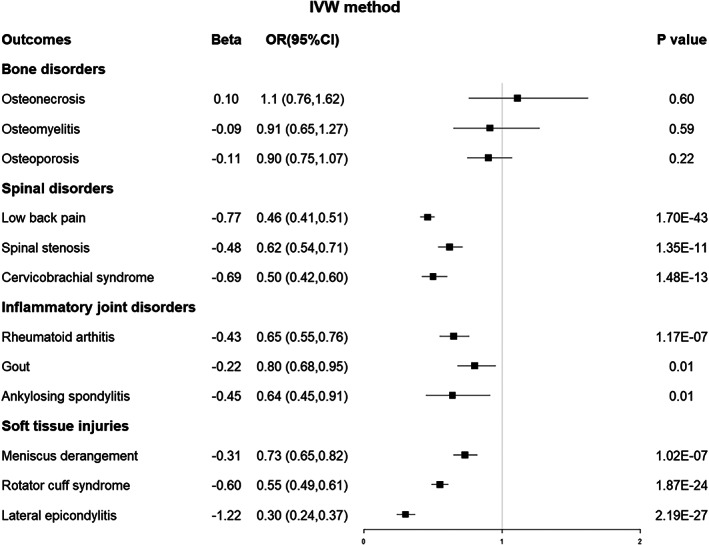
Forest plot of the MR results between EA and 12 musculoskeletal disorders based on the IVW method. MR, Mendelian randomization; EA, educational attainment; IVW, inverse‐variance weighted; OR, odds ratio; CI, confidence interval.
Fig. 3.
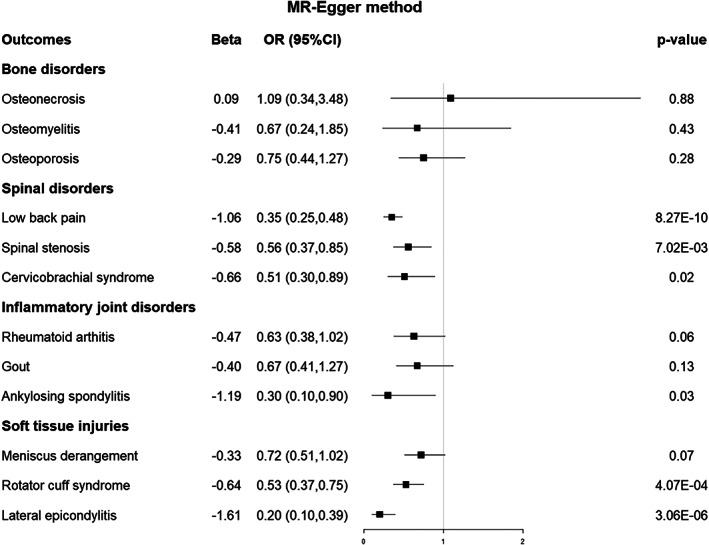
Forest plot of the MR results between EA and 12 musculoskeletal disorders based on the MR‐Egger method. MR, Mendelian randomization; EA, educational attainment; OR, odds ratio; CI, confidence interval.
Fig. 4.
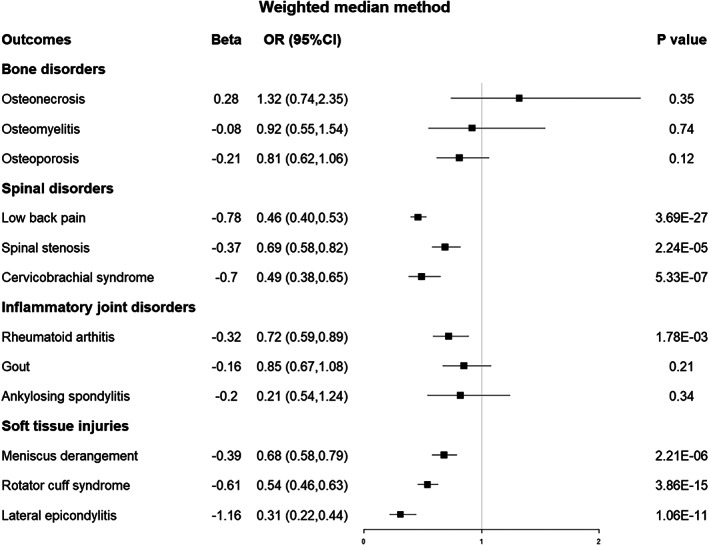
Forest plot of the MR results between EA and 12 musculoskeletal disorders based on the weighted median method. MR, Mendelian randomization; EA, educational attainment; OR, odds ratio; CI, confidence interval.
EA and Spinal Disorders
The primary analysis using IVW indicated that a genetically predicted one SD increase in EA was causally associated with an elevated risk of experiencing low back pain (OR 0.46, 0.51–0.61, p < 0.001), spinal stenosis (OR 0.62, 95% Cl 0.54–0.71, p < 0.001), and cervicobrachial syndrome (OR 0.50, 95% Cl 0.42–0.60, p < 0.001). (Figure 2) The outcomes obtained through the weighted median method, which closely resembled those of the IVW method, corroborated the causal association between EA and the three spinal disorders (Figure 4). However, the sensitivity analyses of MR–Egger only provided supportive evidence for the causal relationship between EA and low back pain (Figure 3).
EA and Inflammatory Joint Disorders
Regarding the three inflammatory joint disorders, the IVW results demonstrated that a genetically predicted one standard deviation increase in EA was associated with a reduced risk of rheumatoid arthritis (OR 0.65, 95% CI 0.55–0.76, p < 0.001) and provided suggestive evidence for the causal relationship between EA and gout (OR 0.80, 95% CI 0.68–0.95, p = 0.01) and ankylosing spondylitis (OR 0.64, 95% CI 0.45–0.91, p = 0.01) after Bonferroni correction (Figure 2). The sensitivity analyses of MR–Egger and weighted median methods are shown in Figures 3 and 4.
EA and Soft Tissue Injuries
IVW analysis suggested the presence of a causal effect between EA and meniscus derangement (OR 0.73, 95% CI 0.65–0.82, p < 0.001), rotator cuff syndrome (OR 0.55, 95% CI 0.49–0.61, p < 0.001), and lateral epicondylitis (OR 0.30, 95% CI 0.24–0.37, p < 0.001). Figures 2, 3, 4 displayed the sensitivity analyses conducted using MR–Egger and weighted median methods, respectively.
Heterogeneity or Pleiotropy
The presence of heterogeneity in the MR–Egger and IVW analyses is shown in Supplementary Table S3. Detailed scatter plots are shown in Supplementary Figures S1–S6. The MR–Egger regression analysis indicated no evidence of horizontal pleiotropy, as evidenced by intercept p values greater than 0.05 for all outcomes. The scatter plots and funnel plots and of SNPs associated with EA and their risk of musculoskeletal system diseases was demonstrated in Supplementary Figure S1–S6.
Discussion
In the present study, we investigated the causal impact of EA on 12 common musculoskeletal disorders. Our analysis revealed that education had protective effects against low back pain, spinal stenosis, rheumatoid arthritis, meniscus derangement, rotator cuff syndrome, cervicobrachial syndrome, and lateral epicondylitis. EA had suggestive genetic causality with gout and ankylosing spondylitis. There was no significant causal relationship, either positive or negative, between EA and osteoporosis, osteomyelitis, or osteonecrosis.
EA and Bone Disorders
Osteonecrosis is avascular necrosis, while osteomyelitis is a bone infection, and both are common in the childhood population. 18 , 19 A prospective study of bone and joint sepsis found no significant associations in socioeconomic and EA levels when the group with complete return of function was compared to the group with evidence of one or more complications, such as the chronicity of infection and osteonecrosis. 20 Our analysis yielded no evidence of a positive or negative causal association between EA and both of bone disorders, indicating that EA did not significantly impact the risk of both diseases. Recent studies suggested that elderly female adults with lower levels of education had a higher prevalence of osteoporosis. 8 , 21 However, these results were inconsistent with our study. The influence on the relationship between EA and osteoporosis may be mediated by additional socioeconomic status factors, such as family income and dietary habits. 22 , 23
EA and Spinal Disorders
Previous observational studies have identified a strong close association between EA and the risk of low back pain, spinal stenosis, and cervicobrachial syndrome. 9 , 24 , 25 Low EA was a clear independent contributor to poorer outcomes for low back pain following lumbar spine surgery. However, given the limited available evidence, it is challenging to draw definitive conclusions about this association. Using MR analysis, our findings suggested that a genetically determined increase in EA was associated with an increased risk of the above three spinal disorders. The mechanisms underlying these associations may involve variations in behavioral and environmental risk factors, such as occupation, access to and utilization of healthcare services, and adaptation to stress, which are influenced by educational status. 26 , 27
EA and Inflammatory Joint Disorders
In terms of inflammatory joint disorders, there was a possibility of an inverse causal relationship between EA and the risk of rheumatoid arthritis. We only observed a suggestive genetic causal association between EA and gout. This result was consistent with the observational study of Bowen‐Davies et al; although EA may serve as a risk factor for gout, it may not directly influence the presence or absence of gout. 28 In a large prospective cohort, Lukas et al found that lower educational level was associated with early inflammatory back pain in ankylosing spondylitis. 29 Our MR study provided suggestive evidence for a causal relationship between EA and the risk of ankylosing spondylitis. One possible explanation is that the effect of education on the risk of ankylosing spondylitis may be mediated by nongenetic factors, such as lifestyle and occupational exposures.
EA and Soft Tissue Injuries
Our findings suggested that there is a genetic causal association between EA and the risk of joint and soft tissue injuries such as rotator cuff syndrome, lateral epicondylitis, and meniscus derangement, resulting from repetitive use or overuse of the shoulder, elbow, and knee joints, respectively. However, the mechanisms underlying the genetic causal association are not clear and warrant further investigation. Lower education level was a significant predictor of future activity for patients with meniscus derangement. 30 Our results suggested that improved EA had a beneficial effect on reducing the risk of joint and soft tissue injuries.
Strengths and Limitations
To the best of our knowledge, this is the first study to employ MR analysis to investigate the causal association between EA and common musculoskeletal disorders. Then, we classified 12 musculoskeletal disorders into four main categories based on their primary features, which is comprehensive and well‐organized for respective discussion. Third, we only included individuals of European descent in the GWAS analysis, which helped to lower the potential bias from population stratification, as it reduced the genetic heterogeneity of the study population.
This study has several limitations. First, the summary GWAS data were restricted to individuals of European descent, and because ethnicity may affect causality, our results limited the generalizability of the results to other populations with different genetic backgrounds. Additionally, it is possible that heterogeneity in part of the results can lead to potential biases in the causal effect estimates. We assessed heterogeneity to perform leave‐one‐out analysis, which demonstrated that the overall risk estimation was not considerably affected. Third, our analysis could not be stratified by gender and age; thus, the analysis that was not adjusted for gender or age may limit the generalizability of the findings and the ability to draw firm conclusions. Education has been found to be strongly associated with income, occupation, and health behaviors in numerous studies. 31 Some factors, such as socioeconomic status, parental education level, access to educational resources, and cultural and societal factors, all contribute to an individual's level of education. However, whether these factors play a mediating role between EA and musculoskeletal disorders needs to be confirmed in further studies. Education is predominantly influenced by social and environmental factors, with genetic factors estimated to account for only about 20% of the variation. Thus, it would be erroneous to conclude that genetic effects are entirely unrelated to environmental factors.
Conclusion
In conclusion, the low level of education plays a causal role in the development of some musculoskeletal disorders. Our findings provide support for the causal relationship between lower EA and a heightened susceptibility to musculoskeletal disorders. In order to unveil the potential deeply causal relationship between musculoskeletal disorders and EA, it is imperative to identify risk factors such as lifestyle and dietary habits, and provide decision‐making interventions for individuals accordingly in the future.
Author Contributions
Conception and design: KS and YM. Administrative support: BS. Provision of study materials: YW, YZ, and JX. Collection and assembly of data: ML, LW. Data analysis and interpretation: KS, YM, YW, and YZ. Manuscript writing: KS and YM. All authors contributed to the article and approved the submitted version.
Competing of Interests
The authors declare that they have no competing interests.
Funding Information
This study was funded by National Natural Science Foundation of China (No. 81974347), China Postdoctoral Science Foundation (No.2021M702351), Medical Science and Technology Project of Health Commission of Sichuan Provincial (No.21PJ040), and Science and Technology Department of Sichuan Province (No.2023YFS0096).
Authorship Declaration
All authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors. All authors are in agreement with the manuscript.
Conflict of Interest
All authors declared no financial support or relationships that may pose a conflict of interest.
Ethics Statement
The data utilized for this study were derived from databases that are publicly accessible and do not contain any personally identifiable information. Consequently, the typical ethical parameters pertaining to the use of human subjects in research do not apply.
Supporting information
Fig. S1. The scatter plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Osteonecrosis. (B) Osteomyelitis. (C) Osteoporosis. (D) Gout.
Fig. S2. The scatter plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Low back pain. (B) Spinal stenosis. (C) Rheumatoid arthritis. (D) Meniscus derangement.
Fig. S3. The scatter plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Rotator cuff syndrome. (B) Ankylosing spondylitis. (C) Cervicobrachial syndrome. (D) Lateral epicondylitis.
Fig. S4. The funnel plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Osteonecrosis. (B) Osteomyelitis. (C) Osteoporosis. (D) Gout.
Fig. S5. The funnel plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Low back pain. (B) Spinal stenosis. (C) Rheumatoid arthritis. (D) Meniscus derangement.
Fig. S6. The funnel plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Rotator cuff syndrome. (B) Ankylosing spondylitis. (C) Cervicobrachial syndrome. (D) Lateral epicondylitis.
Table S1. The 602 independent SNPs left as instrumental variables associated with EA with F statistics.
Table S2. The excluding 19 SNPs associated with potential confounders and 12 common musculoskeletal disorders.
Table S3. The heterogeneity and horizontal pleiotropy tests in the MR analysis.
Kaibo Sun and Yue Ming contributed equally to this paper as first authors.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
References
- 1. Madan I, Grime PR. The management of musculoskeletal disorders in the workplace. Best Pract Res Clin Rheumatol. 2015;29(3):345–55. [DOI] [PubMed] [Google Scholar]
- 2. Burton K, Kendall N. Musculoskeletal disorders. BMJ. 2014;348:g1076. [DOI] [PubMed] [Google Scholar]
- 3. Massar K, Kopplin N, Schelleman‐Offermans K. Childhood socioeconomic position, adult educational attainment and health behaviors: the role of psychological capital and health literacy. Int J Environ Res Public Health. 2021;18(17):9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen AK, Syme SL. Education: a missed opportunity for public health intervention. Am J Public Health. 2013;103(6):997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nedergaard A, Henriksen K, Karsdal MA, Christiansen C. Musculoskeletal ageing and primary prevention. Best Pract Res Clin Obstet Gynaecol. 2013;27(5):673–88. [DOI] [PubMed] [Google Scholar]
- 6. He XZ, Baker DW. Differences in leisure‐time, household, and work‐related physical activity by race, ethnicity, and education. J Gen Intern Med. 2005;20(3):259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halonen JI, Shiri R, Mänty M, Sumanen H, Solovieva S, Viikari‐Juntura E, et al. Exposure to heavy physical work from early to later adulthood and primary healthcare visits due to musculoskeletal diseases in midlife: a register linked study. BMJ Open. 2019;9(8):e031564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai AJ. Disparities in osteoporosis by race/ethnicity, education, work status, immigrant status, and economic status in the United States. Eur J Intern Med. 2019;64:85–9. [DOI] [PubMed] [Google Scholar]
- 9. Kim HJ, Kim SC, Kang KT, Chang BS, Lee CK, Yeom JS. Influence of educational attainment on pain intensity and disability in patients with lumbar spinal stenosis: mediation effect of pain catastrophizing. Spine (Phila Pa 1976). 2014;39(10):E637–44. [DOI] [PubMed] [Google Scholar]
- 10. Holmes MV, Ala‐Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. 2017;14(10):577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng J, Baird D, Borges M‐C, Bowden J, Hemani G, Haycock P, et al. Recent developments in mendelian randomization studies. Curr Epidemiol Rep. 2017;4(4):330–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hao Y, Xiao J, Liang Y, Wu X, Zhang H, Xiao C, et al. Reassessing the causal role of obesity in breast cancer susceptibility: a comprehensive multivariable mendelian randomization investigating the distribution and timing of exposure. Int J Epidemiol. 2023;52(1):58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, et al. FinnGen: unique genetic insights from combining isolated population and national health register data. medRxiv. 2022; 2022.03.03.22271360. [Google Scholar]
- 14. Evans DM, Davey SG. Mendelian randomization: new applications in the coming age of hypothesis‐free causality. Annu Rev Genomics Hum Genet. 2015;16:327–50. [DOI] [PubMed] [Google Scholar]
- 15. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. 2015;44(2):512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology. 2017;28(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR‐base platform supports systematic causal inference across the human phenome. Elife. 2018;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coluzzi F, Rocco M, Green Gladden R, Persiani P, Thur LA, Milano F. Pain Management in Childhood Leukemia: diagnosis and available analgesic treatments. Cancers (Basel). 2020;12(12):3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Congedi S, Minotti C, Giaquinto C, Da Dalt L, Donà D. Acute infectious osteomyelitis in children: new treatment strategies for an old enemy. World J Pediatr. 2020;16(5):446–55. [DOI] [PubMed] [Google Scholar]
- 20. Nunn T, Rollinson P. Haematogenous pyogenic bone and joint sepsis – reducing avoidable morbidity. S Afr Med J. 2007;97(6):456–60. [PubMed] [Google Scholar]
- 21. Wu Q, Xu Y, Lin G. Trends and disparities in self‐reported and measured osteoporosis among US adults, 2007‐2014. J Clin Med. 2019;8(12):2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geiker NRW, Mølgaard C, Iuliano S, Rizzoli R, Manios Y, van Loon LJC, et al. Impact of whole dairy matrix on musculoskeletal health and aging‐current knowledge and research gaps. Osteoporos Int. 2020;31(4):601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Babatunde OT, Marquez S, Taylor A. Osteoporosis knowledge and health beliefs among men in midlife years. J Nutr Educ Behav. 2017;49(9):759–63.e1. [DOI] [PubMed] [Google Scholar]
- 24. Karran EL, Grant AR, Moseley GL. Low back pain and the social determinants of health: a systematic review and narrative synthesis. Pain. 2020;161(11):2476–93. [DOI] [PubMed] [Google Scholar]
- 25. Lekpa FK, Ndongo S, Ka O, Zeba D, Compaoré C, Pouye A, et al. Socio‐demographic and clinical profile of chronic pain with neuropathic characteristics in sub‐Saharan African elderly. Eur J Pain. 2013;17(6):939–43. [DOI] [PubMed] [Google Scholar]
- 26. Dionne CE, Von Korff M, Koepsell TD, Deyo RA, Barlow WE, Checkoway H. Formal education and back pain: a review. J Epidemiol Community Health. 2001;55(7):455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yap ZL, Summers SJ, Grant AR, Moseley GL, Karran EL. The role of the social determinants of health in outcomes of surgery for low back pain: a systematic review and narrative synthesis. Spine J. 2022;22(5):793–809. [DOI] [PubMed] [Google Scholar]
- 28. Bowen‐Davies Z, Muller S, Mallen CD, Hayward RA, Roddy E. Gout severity, socioeconomic status, and work absence: a cross‐sectional study in primary care. Arthritis Care Res (Hoboken). 2018;70(12):1822–8. [DOI] [PubMed] [Google Scholar]
- 29. Lukas C, Dougados M, Combe B. Factors associated with a bad functional prognosis in early inflammatory back pain: results from the DESIR cohort. RMD Open. 2016;2(1):e000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cox CL, Huston LJ, Dunn WR, Reinke EK, Nwosu SK, Parker RD, et al. Are articular cartilage lesions and meniscus tears predictive of IKDC, KOOS, and Marx activity level outcomes after anterior cruciate ligament reconstruction? A 6‐year multicenter cohort study. Am J Sports Med. 2014;42(5):1058–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davies NM, Hill WD, Anderson EL, Sanderson E, Deary IJ, Davey SG. Multivariable two‐sample mendelian randomization estimates of the effects of intelligence and education on health. Elife. 2019;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The scatter plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Osteonecrosis. (B) Osteomyelitis. (C) Osteoporosis. (D) Gout.
Fig. S2. The scatter plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Low back pain. (B) Spinal stenosis. (C) Rheumatoid arthritis. (D) Meniscus derangement.
Fig. S3. The scatter plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Rotator cuff syndrome. (B) Ankylosing spondylitis. (C) Cervicobrachial syndrome. (D) Lateral epicondylitis.
Fig. S4. The funnel plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Osteonecrosis. (B) Osteomyelitis. (C) Osteoporosis. (D) Gout.
Fig. S5. The funnel plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Low back pain. (B) Spinal stenosis. (C) Rheumatoid arthritis. (D) Meniscus derangement.
Fig. S6. The funnel plot of SNPs associated with education attainment and their risk of musculoskeletal system diseases. (A) Rotator cuff syndrome. (B) Ankylosing spondylitis. (C) Cervicobrachial syndrome. (D) Lateral epicondylitis.
Table S1. The 602 independent SNPs left as instrumental variables associated with EA with F statistics.
Table S2. The excluding 19 SNPs associated with potential confounders and 12 common musculoskeletal disorders.
Table S3. The heterogeneity and horizontal pleiotropy tests in the MR analysis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.


