Abstract
A new method, called the mixed culture recovery (MCR) method, has been developed to determine whether recovery of culturable bacterial cells from a population of largely nonculturable cells is due to resuscitation of the nonculturable cells from a viable but nonculturable state or simply to growth of residual culturable cells. The MCR method addresses this issue in that it involves the mixing of two easily distinguishable strains (e.g., lactose positive and negative) in such a way that large numbers of nonculturable cells of both strains are present together with a small number of culturable cells of only one strain, performing a nutrient addition resuscitation procedure, and then plating the cells to determine whether both cell types are recoverable. In repeated experiments with strains of Escherichia coli, Klebsiella pneumoniae, Enterococcus faecalis, Enterobacter aerogenes, and Salmonella choleraesuis, only cells of the culturable strain were recovered after application of various resuscitation techniques. These results suggest that the nonculturable cells were dead and that the apparent resuscitation was merely due to the growth of the remaining culturable cells.
Numerous studies have shown that bacteria which are normally culturable form large populations of nonculturable cells when subjected to prolonged incubation in sterile soil or water (4, 30). This phenomenon has been most intensively studied with cells of Escherichia coli in sterile freshwater or sterile seawater, where it has been observed that as the number of culturable cells declines (as determined by plate counts), the total number of cells present remains unchanged (as determined by a direct count method) (4, 6–8, 10, 13, 16–19, 21, 27, 29, 31, 35, 36). While one possible explanation for these results is that the nonculturable cells are dead (4), there has been advanced the alternative explanation that the nonculturable cells have entered a state in which they are still viable but cannot be cultured by standard microbiological methods (3, 9, 30); the cells are said to be in the viable but nonculturable (VBNC) state. The VBNC hypothesis has been the subject of much interest and debate, especially since it has formed the basis of questions about the potential threat of bacteria which cannot be detected by standard microbiological testing methods to public health (3, 4, 9, 30). Significantly, there has not been any indication that standard bacteriological methods are inadequate.
Recovery of culturable cells from a population of nonculturable cells would provide convincing support for the VBNC hypothesis. The appearance of large numbers of culturable cells after the addition of nutrients to populations of nonculturable cells has been reported to occur via a process termed resuscitation (23, 30). However, such recovery studies can be confounded by the presence of a low level of culturable cells, which can grow in response to the addition of nutrients and give the illusion of resuscitation. Carefully performed nutrient addition experiments with purely nonculturable populations of E. coli cells indicated that no culturable cells were recovered (4). One possible explanation for this result is that nonculturable cells of E. coli are dead. However, it has also been suggested that the presence of culturable cells is required for recovery of nonculturable cells, perhaps due to the production by culturable cells of a resuscitation-inducing factor that triggers resuscitation of nonculturable cells (25, 33, 38). This possibility could not be confirmed or ruled out by studies that employed pure cultures, since it was not possible to determine whether the additional culturable cells were new cells or resuscitated nonculturable cells.
This question has been addressed in the present study through the development of a simple technique termed the mixed culture recovery (MCR) method, in which mixtures of easily distinguishable culturable and nonculturable cells are used to determine whether only culturable cells or both culturable and nonculturable cells have responded to various resuscitation techniques.
MATERIALS AND METHODS
Bacterial strains and preparation of inocula.
The strains of enteric bacteria chosen for this study have all been reported to enter the VBNC state (30). Pairs of these strains that were easily distinguishable on carbohydrate indicator media were identified. For E. coli, the pair of strains consisted of the standard prototrophic wild-type E. coli K-12 strain W3110 (2), which is lactose positive, and a lactose-negative derivative of this strain (with the lac operon deleted) constructed for this work, designated LBB329. The other pairs of strains, obtained from the American Type Culture Collection (Rockville, Md.), consisted of Klebsiella pneumoniae ATCC 211 and ATCC 132 (dulcitol positive and negative, respectively), Enterococcus faecalis ATCC 12953 and ATCC 10741 (inositol positive and negative, respectively), Enterobacter aerogenes ATCC 49469 and ATCC 43175 (dulcitol positive and negative, respectively), and Salmonella choleraesuis subspecies choleraesuis serotype typhimurium ATCC 13311 and ATCC 25376 (cellobiose positive and negative, respectively). Fresh cultures of the strains that had been grown for 14 h at 37°C in Luria-Bertani (LB) medium were washed with sterile 0.9% saline, adjusted to the desired cell concentration, and added to the water microcosms in 10-ml inocula. Each microcosm had an initial population of 3 × 108 CFU per ml.
Media and chemicals.
Tryptone, yeast extract, brain heart infusion (BHI) medium, Bacto Peptone, Bacto Proteose Peptone, lactose, and Bacto Agar were obtained from Difco Laboratories (Detroit, Mich.). Nalidixic acid, inositol, dulcitol, cellobiose, acridine orange, 2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyltetrazolium chloride (INT), and neutral red were obtained from Sigma Chemical Co. (St. Louis, Mo.). LB medium (34) was used to grow the strains; LB agar is LB medium with 15 g of Bacto Agar per liter. Neutral red plates consisted of 17 g of Bacto Peptone, 3 g of Bacto Proteose Peptone, 5 g of sodium chloride, 0.03 g of neutral red, and 10 g of the desired carbohydrate per liter of distilled water; before autoclaving, the pH of the medium was adjusted to 7.1.
Water microcosms.
Artificial seawater was prepared with Instant Ocean (Aquarium Systems, Mentor, Ohio) at a concentration of 3.5% in distilled water. One-liter aliquots of water were placed into 2-liter Erlenmeyer flasks; the flasks were capped with foam plugs and paper covers and autoclaved for 45 min. After inoculation, the flasks were incubated at 20°C.
Colony and cell counting.
Samples were removed directly from the water microcosms and diluted in sterile 0.9% saline for subsequent cell counting. Plate counts were performed by plating 0.1-ml samples in duplicate on LB agar and incubating the plates at 37°C for 72 h prior to colony counting. All of the colonies on plates containing less than 300 colonies were added up, and the total was divided by the total volume plated to estimate the CFU per ml. Acridine orange direct counts (AODC) were achieved by the method of Hobbie et al. (22), using a 0.9% saline diluent and staining with 0.01% acridine orange at room temperature for 15 min; this indicated the total number of cells per milliliter, regardless of whether they were able to grow into visible colonies. Direct viable counts (DVC) were obtained by the method of Kogure et al. (26), with incubation of the samples in 0.025% yeast extract and 0.002% nalidixic acid at room temperature overnight prior to acridine orange staining. Cells which were elongated to at least twice the length of AODC controls were scored as viable. The diluent was Vogel-Bonner minimal medium (37). The INT reduction technique of Quinn (32) was also used as a viable-count method. As a third viable-count method, the Live/Dead kit of Molecular Probes, Inc. (Eugene, Oreg.) was employed in accordance with the instructions supplied by the company; this kit utilizes a mixture of the stains SYTO 9 and propidium iodide (PI) to evaluate cell membrane integrity. A Nikon Optiphot fluorescent microscope with an HBO-100 light source was used for the examination of the preparations at a magnification of ×1,000.
Preparation of the MCR inocula.
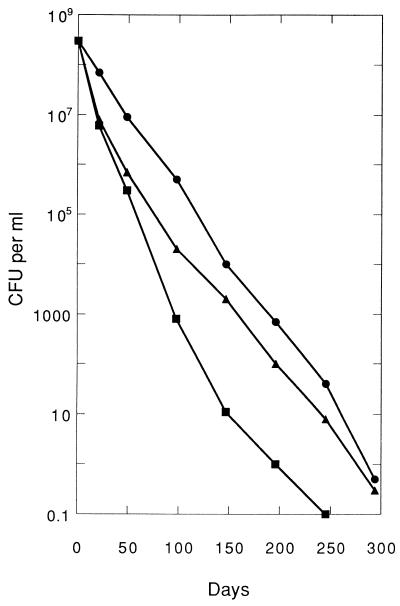
The plate count results were used to mix and dilute samples from each pair of microcosms in such a manner as to yield a sample with about one culturable cell of either strain per milliliter. Inocula of 0.3 ml from these diluted samples were added to each of 10 10-ml tubes of LB medium; thus, about 3 of the 10 tubes would have received one culturable cell of either strain, and the other tubes would have received zero culturable cells. When the number of culturable cells had fallen below 1 CFU per ml (Fig. 1), samples were prepared with about 1 CFU per 10 ml, and 3-ml inocula were added to each of 10 100-ml flasks of LB medium; thus, about 3 of the 10 flasks would have received one culturable cell.
FIG. 1.
Decline in numbers of enteric bacteria in sterile seawater at 20°C. Shown are counts of CFU per milliliter of seawater for S. choleraesuis (•), E. aerogenes (▪), and E. coli (▴). The plots for K. pneumoniae and E. faecalis were very similar to that for E. coli and therefore are not shown. Each point is the mean of values from duplicate microcosms. In each case, the standard error was approximately 12%.
When only culturable cells of either strain were present at the beginning of the study, they were the only cells added to the LB medium tubes. As the study progressed and populations of nonculturable cells developed to increasing degrees, the inocula contained increasingly larger numbers of such cells. The number of nonculturable cells added to each tube was calculated by dividing the total number of cells present by the number of culturable cells at that time point and then multiplying that ratio by the inoculum volume. For example, on day 147, the number of culturable E. coli cells had fallen to about 1,900 CFU per ml (Fig. 1). With the total number of cells per milliliter (nonculturable plus culturable), as determined by AODC, being 3 × 108 and the inoculum volume being 0.3 ml, the number of nonculturable cells added to each tube was calculated to be (3 × 108 total cells − 1,900 CFU per ml/1,900 CFU per ml) (0.3 ml), or 47,000 nonculturable cells. This value, calculated at each time point, is reported in Table 1.
TABLE 1.
Nutrient addition MCR results
| Day | Results for:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
E. coli
|
K. pneumoniae
|
E. faecalis
|
E. aerogenes
|
S. choleraesuis
|
||||||
| NC cells addeda | MCR resultb | NC cells addeda | MCR resultb | NC cells addeda | MCR resultb | NC cells addeda | MCR resultb | NC cells addeda | MCR resultb | |
| 0 | 0 | 2+ | 0 | 2+, 1− | 0 | 1+, 1 mixed | 0 | 1+, 1− | 0 | 1+, 2−, 1 mixed |
| 28 | 11 | 2−, 1 mixed | 19 | 1− | 10 | 2+ | 15 | 2+, 1− | 1.2 | 3− |
| 49 | 130 | 2+, 2− | 180 | 1+, 1− | 160 | 1+, 1 mixed | 200 | 1+, 2− | 10 | 2+, 1− |
| 98 | 5,000 | 1+, 3− | 3,600 | 2+, 1− | 5,000 | 1+ | 6.4 × 104 | 1+, 2− | 23 | 2+, 2− |
| 147 | 4.7 × 104 | 2− | 5.3 × 104 | 3− | 2.8 × 104 | 3+ | 5.0 × 106 | 2+ | 600 | 1+, 2−, 1 mixed |
| 196 | 9.0 × 105 | 3+ | 8.7 × 105 | 2+, 1−, 1 mixed | 5.0 × 105 | 1+, 2− | 6.9 × 107 | 2+ | 9.6 × 104 | 3− |
| 245 | 1.0 × 107 | 2+, 1− | 3.0 × 107 | 2− | 1.8 × 107 | 2− | 9.0 × 108 | 3− | 2.3 × 106 | 2+ |
| 294 | 2.3 × 108 | 2+, 2− | 3.3 × 108 | 1+, 2− | 1.1 × 108 | 2+ | NAc | NA | 1.5 × 108 | 2+, 2− |
The number of nonculturable (NC) cells added to each tube is listed.
The number of tubes which showed growth and the nature of the cultures which were obtained are listed. For example, “1+, 2−, 1 mixed” indicates that 4 of the 10 inoculated tubes showed growth, of which 1 was a pure culture of carbohydrate-positive cells, 2 were pure cultures of carbohydrate-negative cells, and 1 was a mixed culture of both carbohydrate-positive and -negative cells.
NA, not applicable.
For those time points at which both of the strains of each pair had essentially the same CFU per milliliter, equal volumes of each microcosm were mixed and thus about equal numbers of nonculturable cells of each strain were added to each tube. When the CFU-per-milliliter values were not the same, different volumes were mixed to yield a suspension with equal CFU per milliliter of both strains. This yielded mixtures for which the ratio of nonculturable cells was the inverse of the initial ratio of culturable cells. For example, on day 98, the lactose-positive E. coli had fallen to about 3.5 × 104 CFU per ml and the lactose-negative E. coli had fallen to about 1.2 × 104 CFU per ml, a ratio of about 3:1 (this was the largest difference exhibited in the culturable cell counts in any of the studies). The initial mixture was prepared with 1 ml from the lactose-positive microcosm and 3 ml from the lactose-negative microcosm. This mixture had about 1.8 × 104 CFU per ml, with equal CFU of lactose-positive cells and lactose-negative cells. It was diluted 1.8 × 104-fold to yield a suspension with about 1 CFU per ml, which was used to inoculate the LB medium tubes. Calculated in the same manner as above, each 0.3-ml inoculum contained about 5,000 nonculturable cells; since the initial mixture was made in a 1:3 ratio, these 5,000 nonculturable cells consisted of about 1,300 lactose-positive cells and about 3,700 lactose-negative cells.
RESULTS
Decline of enteric bacteria levels in sterile seawater.
In a previous study, it was shown that strains of E. coli gradually became nonculturable during prolonged incubation in sterile river water or sterile seawater at 37°C or in sterile seawater at 20°C but exhibited no such decline at lower temperatures or in sterile soil (4). These studies were extended to the other enteric bacteria used in this work, and the same pattern was observed. Seawater or river water at 37°C was felt to be a condition not likely to be encountered in real environments. Therefore, the microcosm chosen for the present study was sterile seawater at 20°C. The pairs of strains of E. coli, K. pneumoniae, E. faecalis, E. aerogenes, and S. choleraesuis were each incubated separately in the sterile seawater, with the initial cell concentration being about 3 × 108 CFU per ml in all cases.
Five different methods were used to monitor the bacterial cells in the seawater microcosms: plate counts on LB agar, AODC, DVC, INT reduction, and SYTO 9-PI staining. As measured by AODC, the number of bacterial cells remained constant at the initial level, but the plate counts gradually declined (Fig. 1). The DVC paralleled the plate counts. With the SYTO 9-PI staining technique, the cells stained fluorescent green over the entire course of the studies, indicative of cell membrane integrity (28). At the outset of the studies (up to day 28) with the E. coli and E. aerogenes cells, the INT counts were approximately the same as the plate counts and the DVC. However, at the later time points, the INT counts of these cells were lower than the plate counts or DVC. In contrast, for the E. faecalis, K. pneumoniae, and S. choleraesuis cells, the INT counts remained constant at the initial level (i.e., the same as the AODC).
Outline of the MCR method.
The objective of the MCR method is to determine whether recovery of culturable bacterial cells from a population of largely nonculturable cells is due to resuscitation of the nonculturable cells from a VBNC state or is simply due to the presence of residual culturable cells. Utilization of a pair of easily distinguishable bacterial strains is the central feature of the MCR method. For example, a mixed population consisting of large numbers of nonculturable lactose-negative and lactose-positive E. coli cells and also containing one or a few culturable lactose-negative E. coli cells but no culturable lactose-positive E. coli cells is obtained and subjected to a nutrient addition recovery protocol followed by plating on lactose indicator plates. If the only response of the mixture of cells is growth of the few culturable lactose-negative cells, then only lactose-negative colonies will be obtained. If some of the nonculturable cells (which include both lactose-positive and lactose-negative cells) have been resuscitated, then both lactose-positive and lactose-negative colonies will be obtained.
Response of the MCR inocula to nutrient addition.
The cultures were subjected to a nutrient addition MCR test at various time points during the course of the study by inoculation into tubes of LB medium (Table 1). The preparation of the MCR inocula is described in Materials and Methods. The sets of 10 inoculated tubes were incubated at 37°C for 48 h on a platform shaker at 300 rpm prior to being scored for growth. In every case throughout the study the result was unambiguous, with the tube contents showing either full-density growth or no growth at all. For the tube cultures which did show growth, the final densities ranged from about 6 × 108 cells per ml for the E. faecalis cultures to about 2 × 109 cells per ml for the other strains. To score the tube cultures showing growth, they were diluted and plated on neutral red plates supplemented with the appropriate carbohydrate. In addition, samples from two tube cultures that did not show growth were also plated to check for growth; in no case were colonies ever obtained from the contents of these tubes. For the tube cultures showing growth, the plating tests were also unambiguous, with most (102 of 108) of the cultures being composed of either pure carbohydrate-negative or pure carbohydrate-positive cell types and an occasional (6 of 108) culture being composed of roughly a 50:50 mixture of positive and negative cell types (Table 1). Toward the end of the study (beyond day 147), the LB medium tube cultures were somewhat turbid right after inoculation due to the large numbers of nonculturable cells that were added. There was still a clear difference between this initial turbidity and that of tube cultures which showed full-density growth; nevertheless, at these later time points, samples from all 10 tubes were plated to check for growth and examined by AODC to see if any increase in cell number had occurred. In every case for these tube cultures that showed no growth, no colonies were obtained and the number of cells initially inoculated was the same as that found after the 48-h incubation.
To test whether the LB medium was too dilute or too rich for resuscitation to occur, additional sets of tubes of other media were used on days 49 and 147 of studies. A richer medium (BHI) was employed, as well as a 4% LB medium and a 20% LB medium. The MCR results (Table 2) exhibited essentially the same pattern as those obtained with the LB medium.
TABLE 2.
Mixed culture recovery with various media
| Day | Medium used | MCR results fora:
|
||||
|---|---|---|---|---|---|---|
| E. coli | K. pneumoniae | E. faecalis | E. aerogenes | S. choleraesuis | ||
| 49 | 4% LB | 2− | 1+, 1− | 2+, 2− | 1+, 2− | 2+ |
| 20% LB | 1+, 1−, 1 mixed | 1+, 1− | 2+ | 1+, 2− | 2+, 1− | |
| BHI | 2+, 1− | 2−, 1 mixed | 1+, 2− | 2+, 2− | 2− | |
| 147 | 4% LB | 1+, 2− | 2+, 2− | 2+ | 1+, 2− | 1+, 2−, 1 mixed |
| 20% LB | 2+, 2− | 1+, 2− | 2+, 2−, 1 mixed | 1+, 2− | 2+, 1− | |
| BHI | 2+, 1− | 2+, 1− | 2+, 1−, 1 mixed | 1+, 1− | 2+, 1− | |
See footnote b of Table 1 for an explanation of the nomenclature.
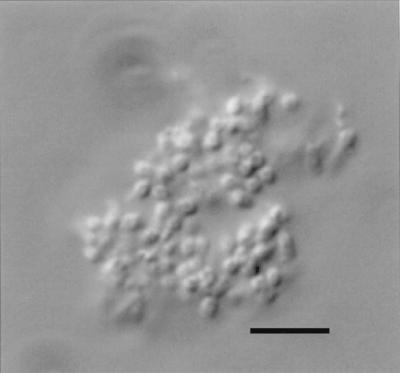
The effects of shifting the temperature prior to performing the MCR procedure were tested on days 98 and 196 of the studies. Samples from the microcosms were placed at 4 and 37°C for 24 h prior to the performance of the MCR test. For most of the samples, the temperature shifts had no effect on the MCR result (Table 3). However, after samples of the E. aerogenes microcosms had been held at 37°C, the contents of all 10 of the tubes in the subsequent MCR test exhibited growth. The resulting cultures all contained roughly a 50:50 mixture of dulcitol-positive and dulcitol-negative cells. It was observed microscopically that clumps of cells had formed in the E. aerogenes microcosms, each composed of several hundred cells (Fig. 2). These clumps were not observed after the 24-h incubation at 37°C, suggesting that clump dispersion was the cause of the increases in CFU counts. To test this idea, two additional tests were performed on samples from the E. aerogenes microcosms at day 98. First, microcosm samples were shifted to 37°C and monitored by hourly plate counts; the CFU-per-milliliter values were found to increase about 200-fold in a 4-h time span, starting about 8 h after the temperature shift. Complete dispersion of the cell clumps was also observed during this time period. After adjusting for this increased number of CFU, another MCR test was performed; this resulted in growth of unmixed cultures in 3 of the 10 tubes (one dulcitol positive and two dulcitol negative). Second, samples of the E. aerogenes microcosms were diluted (prior to the temperature shift) to about 1 CFU per ml, and 0.3-ml aliquots were placed in small capped tubes. These diluted samples were incubated at 37°C for 24 h, and then used as inocula in an MCR test; the result was unmixed growth in 4 of the 10 tube cultures (two dulcitol positive and two dulcitol negative).
TABLE 3.
Mixed culture recovery after temperature shifts
| Day | Temp shift (°C)a | MCR results forb:
|
||||
|---|---|---|---|---|---|---|
| E. coli | K. pneumoniae | E. faecalis | E. aerogenes | S. choleraesuis | ||
| 98 | 4 | 2+, 2− | 1+, 2− | 2+, 1− | 2+ | 1+, 1− |
| 37 | 2+, 1−, 1 mixed | 1+, 1− | 2+, 2− | 10 mixed | 1+, 2− | |
| 196 | 4 | 2+, 2− | 1+, 1− | 2− | 2+, 2− | 2+, 1− |
| 37 | 2+, 2− | 2+ | 2+, 1− | 10 mixed | 1+, 2−, 1 mixed | |
The temperature to which the cultures were shifted for 24 h prior to the performance of the MCR test. See the text for details.
See footnote b of Table 1 for an explanation of the nomenclature.
FIG. 2.
Bright-field light microscope image of a clump of E. aerogenes cells, formed after 98 days in seawater at 20°C. An Olympus AX-70 microscope was used in combination with a 60× oil objective lens. Bar, 5 μm.
Growth characteristics of the LB medium cultures.
The doubling times of the strains in LB medium were determined by hourly plate counts, both with fresh cultures and with microcosm samples, on days 49, 147, and 196. The age of the culture did not affect the doubling time, and both strains in each pair exhibited the same growth rate. The doubling times were 18 min for the E. coli strains, 38 min for the K. pneumoniae strains, 65 min for the E. faecalis strains, 36 min for the E. aerogenes strains, and 32 min for the S. choleraesuis strains. These measurements were repeated with 50:50 and 90:10 mixtures of each pair of strains, and in every case these ratios were maintained over the entire course of the growth curve.
Limit of detection on neutral red indicator medium.
The sensitivity of the indicator plate tests was addressed by performing spike-recovery plating experiments on the neutral red indicator medium. A small number of cells (5 to 10) of one carbohydrate phenotype were mixed with a wide range of much larger numbers of cells (from 104 to 108) of the other carbohydrate phenotype. When the carbohydrate-positive cells were the minority, it was very easy to distinguish the resulting positive colonies or papillae against a background lawn of negative colonies. Thus, the limit of detection was at least one positive cell in 108 negative cells. When the carbohydrate-negative cells were the minority, very large numbers of positive colonies tended to obscure the negative colonies, yielding a limit of detection of one negative cell in 105 positive cells. Additional platings performed with unspiked samples yielded pure colonies or lawns, indicating that the strains used in this study were genetically stable.
DISCUSSION
Strains of the enteric bacteria E. coli, K. pneumoniae, E. faecalis, E. aerogenes, and S. choleraesuis, inoculated at high levels into sterile seawater at 20°C and monitored for nearly 300 days, displayed declining plate counts and accumulation of large numbers of nonculturable cells. The total cell counts as determined by AODC remained constant at the initial level, while plate counts indicated that the number of viable and culturable cells dropped to less than 1 CFU per ml in about 300 days (Fig. 1). The DVC results were consistent with the plate counts when those cells which had elongated to at least twice the length of AODC controls were counted as positive. However, all of the other cells in the DVC samples exhibited a slight increase in size due to swelling. Viable-cell counts performed by the INT reduction technique were not consistent with the plate counts or DVC results; even lower viable-cell counts were obtained by INT reduction for the strains of E. coli and E. aerogenes, while those for the strains of E. faecalis, K. pneumoniae, and S. choleraesuis remained constant at their initial levels. Furthermore, with the SYTO 9-PI staining technique, all of the cells of every strain remained fluorescent green, indicating that the cells retained an intact, undamaged membrane (28). Positive INT reduction results, swollen cells in DVC samples, and positive AODC and SYTO 9-PI staining results have been interpreted in other studies as an indication that nonculturable cells were still viable and thus in the VBNC state (3, 30).
It has been reported by others that temperature shifts or nutrient addition can resuscitate nonculturable bacteria (23, 30). However, samples of microcosms in this study, prepared so as to contain only nonculturable cells, did not yield any culturable cells after either a temperature shift or a nutrient addition. This observation is consistent with earlier results obtained with strains of E. coli (4). It has also been suggested that the presence of culturable cells is required for the resuscitation of nonculturable cells (25, 33, 38). This possibility was addressed by the development of the MCR method.
The MCR nutrient addition tests were designed such that zero culturable cells would be placed into each of about 7 of 10 tubes of LB medium and one culturable cell would be placed into each of about 3 of those 10 tubes. The number of nonculturable cells added to each tube increased over the course of this study, so that a very broad range of ratios of nonculturable to culturable cells was tested (Table 1). However, there was a small chance that a tube could receive two culturable cells and an even smaller chance of it receiving more than two culturable cells. The predicted frequencies of these occurrences are given by Poisson’s law, which states that the probability of a tube receiving k cells (where k = 0, 1, 2, or >2) is equal to [(e−t)(tk)]/k!, where t equals the fraction of 1 ml used as the inoculum. Solving this equation for t = 0.3 (the volume used in this study), the probability of a tube receiving zero cells is 0.74, the probability of it receiving one cell is 0.22, the probability of it receiving two cells is 0.033, and the probability of the tube receiving more than two cells is 0.0036. It must also be kept in mind that half of the time a tube receiving two cells will receive two cells of the same type; thus, the probability of it receiving two different types of cells is 0.017. Setting aside for the moment the two E. aerogenes MCR tests that yielded only mixed cultures (discussed below), the data in Tables 1 to 3 represent the growth results for 870 tubes. It would be predicted that about 74% of these tubes would have received zero culturable cells; the actual result was very close to this value, with about 73% (637) of the tube cultures not showing any growth (Tables 1 to 3). It would also be predicted that almost 2% of the 870 tubes (about 17 tubes) would have received two or more cells of different types and yielded a mixed culture. Again, the actual result was very close to this value, with mixed cultures observed in 13 tubes (Tables 1 to 3).
One conclusion that can be drawn from the MCR results is that the tubes containing only nonculturable cells yielded no culturable cells and that the tubes containing both nonculturable cells and culturable cells yielded only culturable cells derived from those initial culturable cells; this interpretation would also suggest that the culturable cells were not capable of resuscitating the nonculturable cells. There were, however, 13 mixed cultures obtained during this study which could be interpreted as providing evidence of resuscitation of nonculturable cells. An alternative explanation for these 13 mixed cultures is that they were derived from an initial inoculum of two culturable cells of different types. This latter explanation is more consistent with all of the results, since these tubes arose at the frequency expected for such an event and were comprised of a 50:50 mixture of both cell types, and two had developed at the start of the study, when nonculturable cells were not present.
It was observed that the members of pairs of strains used in this study both grew at about the same rate in the LB medium, including in mixed culture, making it unlikely that one strain had overwhelmed the other during the MCR growth period. Furthermore, even if some of the MCR samples had experienced unbalanced growth, the spike-recovery tests indicated that the plating method was sensitive enough to detect small minority populations. Other media were tested as well and had no effect on the MCR results (Table 2).
Temperature shifts were also used in an attempt to resuscitate the nonculturable cells. With one exception, the temperature shifts had no effect on the cells (Table 3). The exception was observed during warming of the E. aerogenes microcosms, which resulted in a 200-fold increase in the population of culturable cells over a 4-h period. This rapid increase, equivalent to a doubling time of 31 min, occurred in seawater with no carbon source and with strains whose a doubling time in LB medium was 36 min; clearly, growth of culturable cells was not the cause of the increased numbers of culturable cells. This result is consistent with the VBNC hypothesis of resuscitation of nonculturable cells. It was also observed that these microcosms contained clumps of cells which were dispersed when warmed to 37°C. This observation suggested an alternative explanation: namely, that the dispersion of the clumps was responsible for the increase in number of culturable cells. Dispersion of many culturable cells from a clump of cells could give the illusion of resuscitation of nonculturable cells during an MCR procedure. Using CFU-per-milliliter values from the untreated microcosms to calculate the dilution factor to reach 1 CFU per ml for the MCR inocula without being aware of the increased culturable cell count in the warmed samples would actually result in MCR inocula containing hundreds of culturable cells of both strain types. Indeed, the MCR tubes inoculated from the warmed and diluted samples all exhibited growth of mixed cultures. Two additional experiments, however, provided support for the idea that dispersion of cells from clumps was responsible for the increase in culturable cell counts. Performing the MCR tests after taking into account the increased culturable cell count, or preparing the MCR inocula before the warming step, yielded growth of unmixed cultures in 3 or 4 of each set of 10 tubes. These results suggest that the clumps of E. aerogenes cells contained hundreds of culturable cells which were dispersed by warming, rapidly turning each clump from 1 CFU into hundreds of CFU. In other studies with E. coli, Micrococcus luteus, and Vibrio vulnificus, the appearance of additional culturable cells at rates exceeding the growth rate has been interpreted as indicating resuscitation of VBNC cells (12, 24, 39). The formation and dispersion of clumps of culturable cells should be considered in such studies.
There has not been a rigorous demonstration that the cell staining techniques used in this study, which play such a crucial role in VBNC experiments, can discriminate between viable and nonviable cells. Some cell staining results of the type obtained in the present work have been interpreted by others to indicate that nonculturable cells were still viable and thus in the VBNC state. However, the fact that nonculturable cells may stain the same as culturable cells does not mean that they are alive; what is needed is some kind of independent confirmation of cell viability which demonstrates that the cells meet a definition of cell viability such as the “ability of a single cell to attain a population discernible by the observer” (5). Workers in the VBNC field have addressed this key question by experimenting with various resuscitation techniques in an attempt to return nonculturable cells to a state in which they do exhibit discernible population increases. The techniques that have been reported to resuscitate nonculturable cells are nutrient addition, temperature shifts, and nutrient addition in the presence of culturable cells (23, 25, 30, 33, 38). As demonstrated by the results presented in this study, great care must be taken to avoid being misled by the illusion of resuscitation. For example, when performing a resuscitation experiment on nonculturable cells in the presence of culturable cells, one obvious issue which can confound the results is whether the discernible population increases are merely due to the response of the culturable cells to the resuscitation manipulation. In addition, in a report on a study with V. vulnificus, Whitesides and Oliver (39) suggested that nutrients in full-strength media may inhibit the resuscitation of nonculturable cells and that a resuscitation method such as temperature shifting or the use of dilute media may give better results. Finally, as reported here, the dispersion of clumps containing many culturable cells can give the illusion of resuscitation of nonculturable cells.
With these issues in mind, a variety of types of resuscitation methods were examined in the studies presented here, including nutrient addition with rich or dilute media, temperature shifts, and temperature shifts plus nutrient addition; these resuscitation techniques were performed on a wide range of numbers of nonculturable cells, both in the presence and in the absence of culturable cells. While the occasional mixed MCR culture tube and the response of the E. aerogenes cultures to a temperature upshift were suggestive of resuscitation of nonculturable cells, such an interpretation did not hold up under closer scrutiny, as discussed above. An alternative interpretation of the results presented here is that addition of nutrients to a mixture consisting of many nonculturable cells and a few culturable cells had no effect other than enabling the residual culturable cells to grow and that subjecting these cell mixtures to temperature shifts had no effect other than dispersion of clumps of cells. The VBNC hypothesis could be extended to argue that a means to resuscitate these nonculturable cells is not known, but such a position cannot be addressed by any finite experiment. A conclusion that is consistent with all of the experimental results presented here is that the nonculturable cells were dead.
For the enteric bacteria studied here for nearly 300 days in seawater, the AODC results indicated that no cells had been lost through lysis or by other means. The SYTO 9-PI results suggested that the cells had intact, undamaged membranes. The cellular swelling seen in the DVC samples, as well as the continued reduction of INT in some of the strains, suggested that the cells had residual metabolic activity. How could dead bacterial cells exhibit such staining results? Other studies of bacteria during prolonged starvation have indicated that processes such as ribosome degradation (11, 14), loss of chromosomal DNA (15), and programmed cell death (1, 40) cause a gradual decline in essential cellular capabilities, eventually leading to the death of the cell. Perhaps cells which die in such a gradual manner, in a laboratory microcosm free of predators, persist as “bags of enzymes” (20), stable for long periods under the study conditions employed and retaining some enzymatic activity. It is possible that this was the nature of the dead nonculturable cells observed in these studies.
ACKNOWLEDGMENTS
We thank Richard L. Ornberg for preparing the light microscope image; Shirley A. Landon and Michele VanBoxlaere of the American Type Culture Collection for identifying suitable pairs of enteric bacteria; Wesley E. Workman, Michael A. Heitkamp, and Gary M. Bond for critically reading the manuscript; and Rita R. Colwell and James D. Oliver for helpful discussions.
REFERENCES
- 1.Aizenman E, Engelberg-Kulka H, Glaser G. An Escherichia coli chromosomal “addiction module” regulated by 3′,5′-bispyrophosphate: a model for programmed bacterial cell death. Proc Natl Acad Sci USA. 1996;93:6059–6063. doi: 10.1073/pnas.93.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann B J. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1190–1219. [Google Scholar]
- 3.Barer M R, Gribbon L T, Harwood C R, Nwoguh C E. The viable but nonculturable hypothesis and medical bacteriology. Rev Med Microbiol. 1993;4:183–191. [Google Scholar]
- 4.Bogosian G, Sammons L E, Morris P J L, O’Neil J P, Heitkamp M A, Weber D B. Death of the Escherichia coli K-12 strain W3110 in soil and water. Appl Environ Microbiol. 1996;62:4114–4120. doi: 10.1128/aem.62.11.4114-4120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Button D K, Schut F, Quang P, Martin R, Robertson B R. Viability and isolation of marine bacteria by dilution culture: theory, procedures, and initial results. Appl Environ Microbiol. 1993;59:881–891. doi: 10.1128/aem.59.3.881-891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd J J, Colwell R R. Maintenance of plasmids pBR322 and pUC8 in nonculturable Escherichia coli in the marine environment. Appl Environ Microbiol. 1990;56:2104–2107. doi: 10.1128/aem.56.7.2104-2107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd J J, Colwell R R. Long-term survival and plasmid maintenance of Escherichia coli in marine microcosms. FEMS Microbiol Ecol. 1993;12:9–14. [Google Scholar]
- 8.Caldwell B A, Ye C, Griffiths R P, Moyer C L, Morita R Y. Plasmid expression and maintenance during long-term starvation-survival of bacteria in well water. Appl Environ Microbiol. 1989;55:1860–1864. doi: 10.1128/aem.55.8.1860-1864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colwell R R. Nonculturable but still viable and potentially pathogenic. Zentbl Bakteriol. 1993;279:154–156. doi: 10.1016/s0934-8840(11)80392-0. [DOI] [PubMed] [Google Scholar]
- 10.Davies C M, Long J A H, Donald M, Ashbolt N I. Survival of fecal microorganisms in marine and freshwater sediments. Appl Environ Microbiol. 1995;61:1888–1896. doi: 10.1128/aem.61.5.1888-1896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis B D, Luger S M, Tai P C. Role of ribosome degradation in the death of starved Escherichia coli cells. J Bacteriol. 1986;166:439–445. doi: 10.1128/jb.166.2.439-445.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dukan S, Lévi Y, Touati D. Recovery of culturability of an HOCl-stressed population of Escherichia coli after incubation in phosphate buffer: resuscitation or regrowth? Appl Environ Microbiol. 1997;63:4204–4209. doi: 10.1128/aem.63.11.4204-4209.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan S, Glover L A, Killham K, Prosser J I. Luminescence-based detection of activity of starved and viable but nonculturable bacteria. Appl Environ Microbiol. 1994;60:1308–1316. doi: 10.1128/aem.60.4.1308-1316.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberl L, Givskov M, Poulsen L K, Molin S. Use of bioluminescence for monitoring the viability of individual Pseudomonas putida KT2442 cells. FEMS Microbiol Lett. 1997;149:133–140. [Google Scholar]
- 15.Enroth, H., C. Pahlsson, and L. Engstrand. 1995. Do viable but non-culturable coccoid forms of Helicobacter pylori exist? Gastroenterology 108(Suppl. 4):A89.
- 16.Fish J T, Pettibone G W. Influence of freshwater sediment on the survival of Escherichia coli and Salmonella sp. as measured by three methods of enumeration. Lett Appl Microbiol. 1995;20:277–281. doi: 10.1111/j.1472-765x.1995.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 17.Flint K P. The long-term survival of Escherichia coli in river water. J Appl Bacteriol. 1987;63:261–270. doi: 10.1111/j.1365-2672.1987.tb04945.x. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier M J, Le Rudulier D. Survival in seawater of Escherichia coli cells grown in marine sediments containing glycine betaine. Appl Environ Microbiol. 1990;56:2915–2918. doi: 10.1128/aem.56.9.2915-2918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González J M, Iriberri J, Egea L, Barcina I. Characterization of culturability, protistan grazing, and death of enteric bacteria in aquatic ecosystems. Appl Environ Microbiol. 1992;58:998–1004. doi: 10.1128/aem.58.3.998-1004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gribbon L T, Barer M R. Oxidative metabolism in nonculturable Helicobacter pylori and Vibrio vulnificus cells studied by substrate-enhanced tetrazolium reduction and digital image processing. Appl Environ Microbiol. 1995;61:3379–3384. doi: 10.1128/aem.61.9.3379-3384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimes D J, Colwell R R. Viability and virulence of Escherichia coli suspended by membrane chamber in semitropical ocean water. FEMS Microbiol Lett. 1986;34:161–165. [Google Scholar]
- 22.Hobbie J E, Daley R J, Jasper S. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaprelyants A S, Gottschal J C, Kell D B. Dormancy in non-sporulating bacteria. FEMS Microbiol Rev. 1993;104:271–286. doi: 10.1111/j.1574-6968.1993.tb05871.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaprelyants A S, Kell D B. Dormancy in stationary-phase cultures of Micrococcus luteus: flow cytometric analysis of starvation and resuscitation. Appl Environ Microbiol. 1993;59:3187–3196. doi: 10.1128/aem.59.10.3187-3196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaprelyants A S, Mukamolova G V, Kell D B. Estimation of dormant Micrococcus luteus cells by penicillin lysis and by resuscitation in cell-free spent culture medium at high dilution. FEMS Microbiol Lett. 1994;115:347–352. [Google Scholar]
- 26.Kogure K, Simidu U, Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979;25:415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- 27.Linder K, Oliver J D. Membrane fatty acid and virulence changes in the viable but nonculturable state of Vibrio vulnificus. Appl Environ Microbiol. 1989;55:2837–2842. doi: 10.1128/aem.55.11.2837-2842.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloyd D, Hayes A J. Vigour, vitality and viability of microorganisms. FEMS Microbiol Lett. 1995;133:1–7. [Google Scholar]
- 29.McFeters G A, Terzieva S I. Survival of Escherichia coli and Yersinia enterocolitica in stream water: comparison of field and laboratory exposure. Microb Ecol. 1991;22:65–74. doi: 10.1007/BF02540213. [DOI] [PubMed] [Google Scholar]
- 30.Oliver J D. Formation of viable but nonculturable cells. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 239–272. [Google Scholar]
- 31.Porter J, Edwards C, Pickup R W. Rapid assessment of physiological status in Escherichia coli using fluorescent probes. J Appl Bacteriol. 1995;79:399–408. doi: 10.1111/j.1365-2672.1995.tb03154.x. [DOI] [PubMed] [Google Scholar]
- 32.Quinn J P. The modification and evaluation of some cytochemical techniques for the enumeration of metabolically active heterotrophic bacteria in the aquatic environment. J Appl Bacteriol. 1984;57:51–57. doi: 10.1111/j.1365-2672.1984.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 33.Ravel J, Knight I T, Monahan C E, Hill R T, Colwell R R. Temperature-induced recovery of Vibrio cholerae from the viable but nonculturable state: growth or resuscitation? Microbiology. 1995;141:377–383. doi: 10.1099/13500872-141-2-377. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Scheuerman P R, Schmidt J P, Alexander M. Factors affecting the survival and growth of bacteria introduced into lake water. Arch Microbiol. 1988;150:320–325. doi: 10.1007/BF00408301. [DOI] [PubMed] [Google Scholar]
- 36.Smith J J, Howington J P, McFeters G A. Survival, physiological response, and recovery of enteric bacteria exposed to a polar marine environment. Appl Environ Microbiol. 1994;60:2977–2984. doi: 10.1128/aem.60.8.2977-2984.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel H J, Bonner D M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 38.Votyakova T V, Kaprelyants A S, Kell D B. Influence of viable cells on the resuscitation of dormant cells in Micrococcus luteus cultures held in an extended stationary phase: the population effect. Appl Environ Microbiol. 1994;60:3284–3291. doi: 10.1128/aem.60.9.3284-3291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitesides M D, Oliver J D. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl Environ Microbiol. 1997;63:1002–1005. doi: 10.1128/aem.63.3.1002-1005.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yarmolinsky M B. Programmed cell death in bacterial populations. Science. 1995;267:836–837. doi: 10.1126/science.7846528. [DOI] [PubMed] [Google Scholar]