ABSTRACT.
The prevalence of cannabis usage is increasing worldwide, including among both Indigenous and non-Indigenous Australians. The long-term effects of cannabis use on the lungs are well-known. However, the acute adverse effects on the lungs are sparsely reported. There are different ways in which cannabis can be inhaled, such as smoking or through a water vaporizing method known as a “bong.” An improvised innovative bong device that is commonly used in Northern Australia, called a “bucket bong,” uses water and air pressure to assist in cannabis inhalation. In this report, we describe three patients from remote and rural Northern Australian communities presenting with near–life-threatening events (acute pneumonitis and massive pneumothorax) immediately after the use of cannabis via bucket bong.
INTRODUCTION
According to a United Nations’ report, cannabis is one of the most widely used illicit drugs worldwide, and it is reported that 4% of the global population aged 15 to 64 years has used cannabis during their life course.1 The prevalence of cannabis use in Australia is reported to be significantly higher than the global average.1,2 Moreover, consumption of cannabis is quite prevalent among those residing in remote and rural communities of Australia.3 There are different ways in which cannabis can be inhaled, such as smoking or through a water vaporizing method known as a “bong.” A “bucket bong” is an innovative bong device that uses water and air pressure to assist in cannabis inhalation. The long-term effects of cannabis on the lungs are well-known.4,5 However, there is little evidence in the literature regarding acute lung injury after inhalation of cannabis, more specifically via a bucket bong. In this report, we describe three patients who presented with near catastrophic acute lung injury after the use of cannabis through a bucket bong, a common mode of bong use among the remote and rural communities of Northern Australia.
CASE REPORT
Case 1.
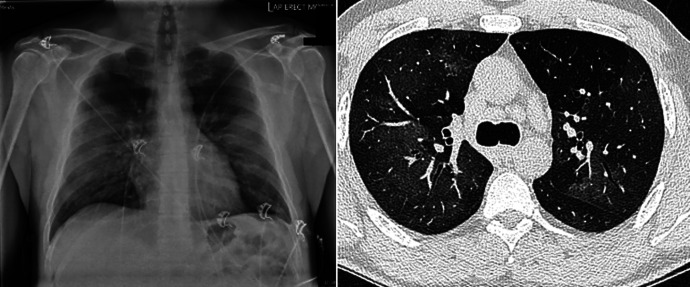
A 40-year-old male from a remote community in the Northern Territory (NT) of Australia initially presented to a local community health center with sudden onset of cough, shortness of breath, and fever. He was noted to be febrile with a temperature of 39.5°C and an oxygen saturation value down to 83% on room air. His symptoms started approximately 1 hour after smoking cannabis through a bucket bong. He was transferred (airlifted) to the Royal Darwin Hospital (RDH), a tertiary care university-affiliated teaching hospital in the capital city, Darwin, NT of Australia. Upon presentation, a chest x-ray and high-resolution computed tomography (CT) of the patient’s chest showed bilateral mid-zone alveolar opacity, particularly in the perihilar distribution, alongside ground glass opacities (Figure 1). He had raised inflammatory markers with a C-reactive protein level of 222 mg/L and a white cell count of 31.5 × 109/L with neutrophil predominance. The patient required admission to the intensive care unit because of persistent hypoxemia and required supplemental oxygen therapy. He was treated with a course of empirical antibiotics in view of being febrile at presentation and blood tests demonstrating raised inflammatory markers and high white cell count. No pathogenic organisms were cultured, and atypical pneumonia serology was subsequently negative. Otherwise, his connective tissue disease screen was unremarkable. The patient clinically improved, and after he recovered from his acute illness, pulmonary function tests revealed normal spirometry, normal total lung capacity, and mildly reduced diffusing capacity for carbon monoxide. It was concluded that this patient’s presentation was likely secondary to acute pneumonitis in the context of cannabis use through a bucket bong.
Figure 1.
Chest x-ray and high-resolution CT of the chest showing bilateral pulmonary opacity. CT = computed tomography.
Case 2.
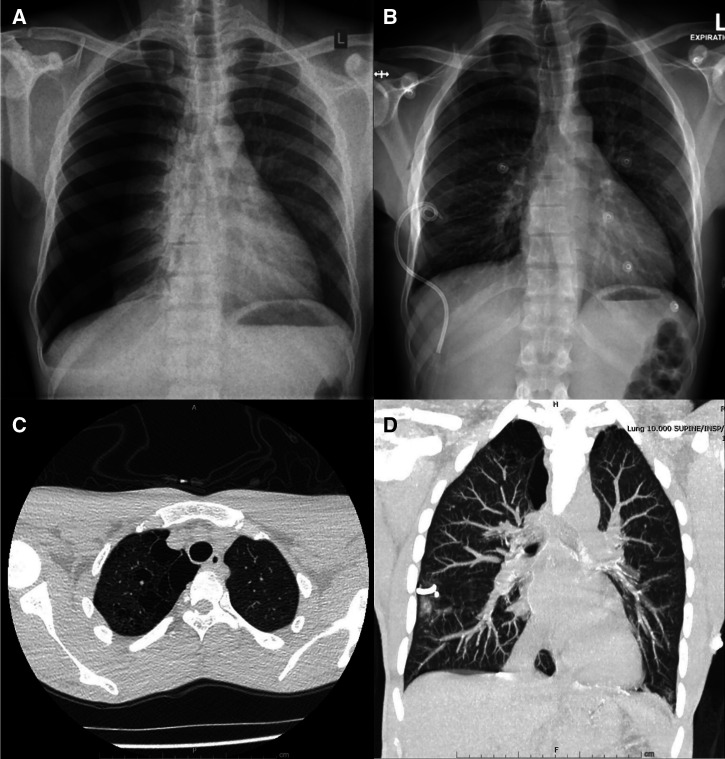
A 31-year-old male, again from a remote community in the NT of Australia, was transferred to RDH with a spontaneous right-sided pneumothorax requiring intercostal catheter (ICC) insertion (Figure 2A and B). The patient had no significant documented past medical comorbidities. However, he had a notable significant smoking history and cannabis use. It was noted that the current presentation occurred almost immediately after the consumption of cannabis through a bucket bong. A chest CT scan revealed background emphysematous changes with large bullous disease in the right lung apex (Figure 2C and D). The majority of the blood investigations were within normal ranges, including serum alpha-1 antitrypsin level. The patient had recurrence of his pneumothorax shortly after his ICC removal. Hence, he subsequently proceeded on to video-assisted thoracic surgery and talc pleurodesis and bullectomy. The cause of his pneumothorax was determined to be secondary to apical bullous lung disease, likely in the context of smoking cannabis via bucket bong.
Figure 2.
(A) Right-sided pneumothorax. (B) Intercostal catheter on the right side. (C and D) Chest CT showing emphysematous changes with bullae in the right lung apex. CT = computed tomography.
Case 3.
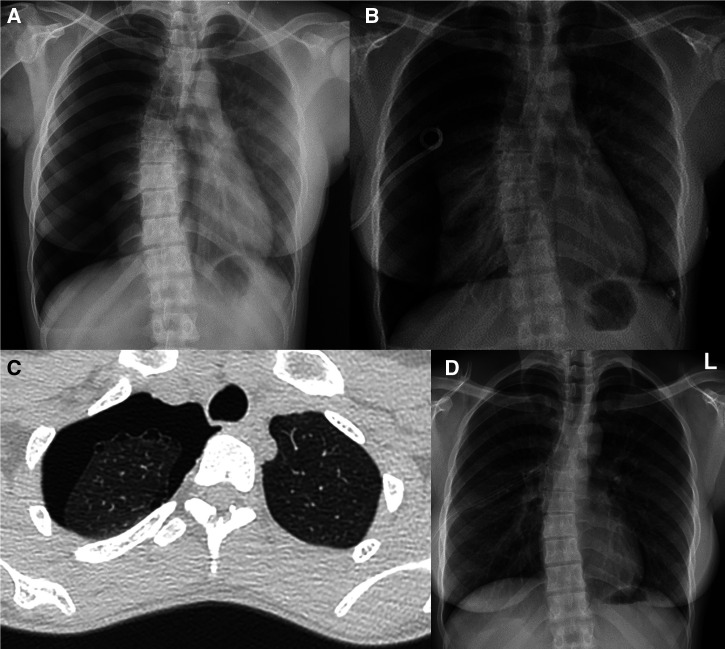
A 22-year-old female patient originally presented to a remote community health clinic with complaints of pleuritic chest pain and shortness of breath immediately after smoking cannabis via a bucket bong. She was initially misdiagnosed as having an exacerbation of airway disease and was treated with oral antibiotics. However, the astute reporting radiologist at the RDH alerted the community clinic about the presence of a large pneumothorax (Figure 3A), and she was eventually transferred to RDH by air after an ICC was inserted in the remote community health clinic by the medical retrieval team (Figure 3B). A chest CT scan subsequently demonstrated a moderate to large right-sided pneumothorax. Several right apical, tiny, subpleural bullae were also noted (Figure 3C). The ICC was subsequently replaced with a large-bore chest drain owing to minimal improvement (Figure 3D). The pneumothorax gradually improved, and the chest drain was removed 6 days later. She was discharged from the hospital with a plan for ongoing close follow-up and advice/education provided on the ill effects of using cannabis through a bucket bong. The patient’s spontaneous pneumothorax was deemed secondary to apical bullous lung disease in the setting of smoking cannabis via bucket bong.
Figure 3.
(A) Chest x-ray shows right-sided pneumothorax. (B) Intercostal catheter on the right side. (C) Chest CT shows large right-sided pneumothorax and bullae in the right lung apex. (D) Chest x-ray shows large-bore chest drain. CT = computed tomography.
DISCUSSION
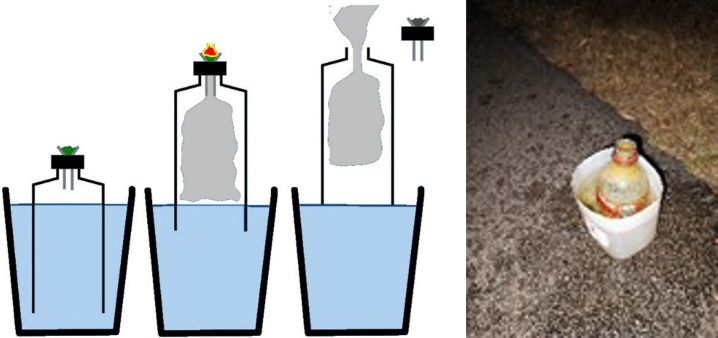
To the best of the authors’ knowledge, this is the first report of near-acute life-threatening lung conditions after consumption of cannabis via bucket bong, more specifically among people residing in the remote and rural communities of the Top End, NT region of Australia. There are different ways in which cannabis can be consumed.6 The most common method of consumption is via smoking cannabis mixed with tobacco. However, it may also be inhaled through a vaporizing device or added to food and eaten or drunk.6,7 Another typical method of use is through a bong, which uses water to assist in inhalation of the cannabis.6 There are different types of bongs, including the standard and most common bong that draws cannabis smoke from a metal cone through water before being inhaled into the lungs.6,7 Conversely, a bucket bong is an innovative method that uses water to create a suction force. The assembly of a bucket bong is indeed very simple. Conventionally, it is created by assembling a plastic bottle and bucket filled with water together as shown in Figure 4 (left image).8 The mechanism behind its use is that the water pressure draws air through the bottle outlet by altering the water and air pressure inside the bottle. As the water rises inside the bottle, it forces smoke out of the bottle outlet, which can be inhaled into the lungs in high concentration/volume and pressure.8,9 Anecdotally, this method allows users to get the maximum effects of cannabis instantly.
Figure 4.
Diagram showing bucket bong in operation and picture of an actual bucket bong abandoned after use.
There is comprehensive literature describing the effects of cannabis on lungs among recreational inhaled illicit drug users,10–12 including reports of patients diagnosed as having hypersensitive pneumonitis,13,14 pneumothorax, and pneumomediastinum.15,16 Moreover, lung function parameters among cannabis users in particular are noted to display higher forced vital capacity (FVC) and hyperinflation.17–20 The reason for this increase in FVC is unclear, but it is theorized that it is due to the training of respiratory muscles by the characteristic inhalation techniques used by cannabis users. Furthermore, cannabis is proposed to have an acute bronchodilator effect. Because bronchodilatation of the small airways can increase FVC, this could also be the reason for the increased FVC in cannabis users.21 Chest radiology details published in previous reports indicate that cannabis users could demonstrate the presence of emphysema and bullous disease.22–24 Although it is postulated that this could be related to prolonged cannabis use, the evidence surrounding this linkage is less convincing, as it is confounded by a significant proportion of cannabis users also having a history of smoking tobacco.
In the context of the patients presented in this report, alongside the current evidence mentioned above, we hypothesize that for the patient with acute pneumonitis, rapid inhalation of cannabis mixed with smoke in high concentrations and volume, in addition to the possible inhalation of toxic plastic chemical substances in a potentially unhygienic condition (Figure 4, right image) through the bucket bong likely contributed to acute pneumonitis. For the other two patients with pneumothorax, the same phenomena could be responsible. Moreover, the Valsalva maneuver associated with the inhalational technique of cannabis could have led to overdistension of the lungs, in turn dramatically increasing the total lung volume. For these patients, the presence of prior bullous disease would further increase the risk of pneumothorax.
In addition, the altered physiological mechanism of pulmonary gas flow and volume could explain the occurrence of pneumothorax among bucket bong users. Consider Bernoulli’s field equation for calculating pressure and flow rates:
where P is pressure, ρ is fluid density, v is fluid velocity, g is gravity, and h is the height of the fluid column. Subsets a and b are two points along the flow path, namely the oral cavity (a) and the end alveolus (b).
By making several calculation assumptions, such as 1) velocity at the end alveolus is zero—zeroing term vb, 2) the depth is constant (i.e., the weight of a column of air is negligible)—cancelling terms ha and hb on each side, 3) the pressure of each system at the start of inhalation (oral intake) is atmospheric—turning Pa into Patm, then Bernoulli’s equation could be simplified to . Setting up two equations 1) for normal inspiration and 2) for the bucket bong and dividing the equations gives .
We can simplify this once more by assuming that the densities of air and marijuana smoke are comparable (a generous assumption given there is evidence that marijuana smoke is denser than air, albeit variably dependent on factors such as packing density and relative humidity)—removing the ρ in each equation and recognizing that the geometry (or airways topology) of both circumstances is identical—allowing “velocity” to be substituted with “flow rate,” Q, giving , or . This could be interpreted as indicating that the relative pressure produced by a bucket bong compared to deep inspiration at the end alveolus is directly related to the relative squares of the initial volumetric flow rates. Volumetric flow rate, Q1, is measured directly during spirometry as the inspiratory flow rate, and Q2 depends on the build of the bucket bong; for example, a 2-L bong could be inhaled in approximately 1/4 of a second, giving a flow rate of 8 L/second.
The flow rate of a bucket bong (using 8 L/second), being significantly higher than deep inspiration (using 5 L/second), leads to a relative pressure of .
Considering that the bucket bong continues to force air inward, this becomes a plateau inspiratory pressure 3.5× higher than that achieved by maximum inspiratory pressure (normally ranging between 80 and 120 cmH2O depending on body habitus). The plateau pressure achieved by a bucket bong can therefore be estimated at 300 cmH2O, which is indeed several times higher than during conventional mechanical ventilation, which could produce barotrauma at pressures as low as 40 cmH2O.25,26
Nevertheless, the adult population residing in the Top End, NT of Australia are reported to have a much higher burden of chronic respiratory disorders, including complex and advanced respiratory conditions, giving rise to significant morbidity and mortality.27–45 The true prevalence of bucket bong consumption within NT remote communities is not exactly known. However, it is reasonable to speculate that what is represented in this report is only a glimpse of a potentially significant underexplored or underreported problem. In a population with an already higher burden of chronic respiratory disorders,46,47 use of a bucket bong may further add not only to morbidity and mortality but also to healthcare cost and utilization. Hence, the patients presented in this report could be considered a wake-up call to explore avenues to implement patient educational strategies.48,49
CONCLUSION
The cases represented in this report illustrate an acute lung injury that could be caused by utilizing a bucket bong for cannabis consumption and the potential physiological mechanism involved in the development of pneumothorax. We hope that this report prompts future research/reports that can provide further information on the usage patterns and other health-related effects of cannabis use via bucket bong worldwide, with an ultimate goal of facilitating educational interventions to prevent catastrophic consequences.
ACKNOWLEDGMENTS
We thank the remote health practitioners in the Top End NT, emergency department physicians, intensive care medical team, radiology division, and general medical and surgical divisions at the RDH for rather remarkable care provided to the remote community patients described in this report. We also thank Timothy Howarth for his help with this report.
REFERENCES
- 1. United Nations Office on Drugs and Crime , 2022. World Drug. Vienna, Austria: United Nations. Available at: https://www.unodc.org. Accessed August 21, 2023.
- 2. Australian Institute of Health and Welfare , 2022. Alcohol, Tobacco & Other Drugs in Australia. Available at: https://www.aihw.gov.au/reports/alcohol/alcohol-tobacco-other-drugs-australia/contents/drug-types/cannabis. Accessed August 21, 2023.
- 3. Butt J, Wilson M, Jones J, Lenton S, 2022. Review of cannabis use among Aboriginal and Torres Strait Islander people. HealthInfoNet 3, doi: 10.14221/aihjournal.v3n3.1. [DOI] [Google Scholar]
- 4. Yayan J, Rasche K, Pokorski M. Advancements in Clinical Research. Advances in Experimental Medicine and Biology, Vol. 952. Cham: Springer. [Google Scholar]
- 5. Ribeiro L, Ind PW, 2018. Marijuana and the lung: hysteria or cause for concern? Breathe (Sheff) 14: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA, 2016. Toking, vaping, and eating for health or fun: marijuana use patterns in adults, U.S., 2014. Am J Prev Med 50: 1–8. [DOI] [PubMed] [Google Scholar]
- 7. Australian Institute of Health and Welfare , 2020. National Drug Strategy Household Survey 2019. Cat. No.: PHE 270. Available at: https://www.aihw.gov.au/reports/illicit-use-of-drugs/national-drug-strategy-household-survey-2019/data. Accessed August 21, 2023.
- 8. Wikipedia , 2007. Gravity Bong. Available at: https://en.wikipedia.org/wiki/Gravity_bong. Accessed August 21, 2023.
- 9. Moore D, 1995. Drug Use and Western Selfhood: An Ethnography of Urban Paths in Perth. Dissertation, University of Western Australia, Perth Australia. [Google Scholar]
- 10. Tashkin DP, Roth MD, 2019. Pulmonary effects of inhaled cannabis smoke. Am J Drug Alcohol Abuse 45: 596–609. [DOI] [PubMed] [Google Scholar]
- 11. Tashkin DP, 2013. Effects of marijuana smoking on the lung. Ann Am Thorac Soc 10: 239–247. [DOI] [PubMed] [Google Scholar]
- 12. Aldington S, Williams M, Nowitz M, Weatherall M, Pritchard A, McNaughton A, Robinson G, Beasley R, 2007. Effects of cannabis on pulmonary structure, function and symptoms. Thorax 62: 1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haddad I, Al-Ghzawi F, Karakattu SM, Musa R, Hoskere G, 2021. Dabbing-induced hypersensitivity pneumonitis. Cureus 13: e16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McMahon MJ, Bhatt NA, Stahlmann CG, Philip AI, 2016. Severe pneumonitis after inhalation of butane hash oil. Ann Am Thorac Soc 13: 991–992. [DOI] [PubMed] [Google Scholar]
- 15. Gill A, 2005. Bong lung: regular smokers of cannabis show relatively distinctive histologic changes that predispose to pneumothorax. Am J Surg Pathol 29: 980–982. [DOI] [PubMed] [Google Scholar]
- 16. Weiss ZF, Gore S, Foderaro A, 2019. Pneumomediastinum in marijuana users: a retrospective review of 14 cases. BMJ Open Respir Res 6: e000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hancox RJ, Gray AR, Zhang X, Poulton R, Moffitt TE, Caspi A, Sears MR, 2022. Differential effects of cannabis and tobacco on lung function in mid-adult life. Am J Respir Crit Care Med 205: 1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghasemiesfe M, Ravi D, Vali M, Korenstein D, Arjomandi M, Frank J, Austin PC, Keyhani S, 2018. Marijuana use, respiratory symptoms, and pulmonary function: a systematic review and meta-analysis. Ann Intern Med 169: 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gracie K, Hancox RJ, 2021. Cannabis use disorder and the lungs. Addiction 116: 182–190. [DOI] [PubMed] [Google Scholar]
- 20. Marcus HSL, Hancox RJ, 2011. Effects of smoking cannabis on lung function. Expert Rev Respir Med 5: 537–547. [DOI] [PubMed] [Google Scholar]
- 21. Ribeiro LIG, Ind PW, 2016. Effect of cannabis smoking on lung function and respiratory symptoms: a structured literature review. NPJ Prim Care Respir Med 26: 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murtha L, Sathiadoss P, Salameh JP, Mcinnes MDF, Revah G, Chest CT, 2023. Findings in marijuana smokers. Radiology 307: e212611. [DOI] [PubMed] [Google Scholar]
- 23. Leb JS, D’Souza B, Steiner RM, 2018. Images in COPD: marijuana lung. Chronic Obstr Pulm Dis (Miami) 5: 81–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilgus M-L, 2022. Bong Lung: A Case of Massive Lower Lobe Lung Bullae Associated with Cannabis Smoking. 26. Available at: https://proceedings.med.ucla.edu/index.php/2022/09/29/bong-lung-a-case-of-massive-lower-lobe-lung-bullae-associated-with-cannabis-smoking/. Accessed August 21, 2023. [Google Scholar]
- 25. Warner MA, Patel B, 2013. Mechanical ventilation. Hagberg CA, Benumof J, ed. Benumof and Hagberg’s Airway Management. Philadelphia, PA: Elsevier/Saunders, 981–997. [Google Scholar]
- 26. Ioannidis G. et al. , 2015. Barotrauma and pneumothorax. J Thorac Dis 7 (Suppl 1): S38–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kruavit A, Fox M, Pearson R, Heraganahally S, 2017. Chronic respiratory disease in the regional and remote population of the Northern Territory Top End: a perspective from the specialist respiratory outreach service. Aust J Rural Health 25: 275–284. [DOI] [PubMed] [Google Scholar]
- 28. Heraganahally SS, Wasgewatta SL, McNamara K, Eisemberg CC, Budd RC, Mehra S, Sajkov D, 2019. Chronic obstructive pulmonary disease in Aboriginal patients of the Northern Territory of Australia: a landscape perspective. Int J Chron Obstruct Pulmon Dis 14: 2205–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehra S, Chang AB, Lam CK, Campbell S, Mingi JJ, Thomas I, Harwood S, Maguire G, Heraganahally S, 2021. Bronchiectasis among Australian Aboriginal and non-Aboriginal patients in the regional and remote population of the Northern Territory of Australia. Rural Remote Health 21: 6390. [DOI] [PubMed] [Google Scholar]
- 30. Heraganahally SS, Wasgewatta SL, McNamara K, Mingi JJ, Mehra S, Eisemberg CC, Maguire G, 2020. Chronic obstructive pulmonary disease with and without bronchiectasis in Aboriginal Australians – a comparative study. Int Med J 50: 1505–1513. [DOI] [PubMed] [Google Scholar]
- 31. Heraganahally S, Digges M, Haygarth M, Liyanaarachchi K, Kalro A, Mehra S, 2019. Pulmonary AL – amyloidosis masquerading as lung malignancy in an Australian Indigenous patient with Sjogren’s syndrome. Respir Med Case Rep 26: 94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heraganahally SS, Howarth TP, Lloyd A, White E, Veale A, Ben Saad H, 2022. The prevalence of bronchodilator responsiveness “asthma” among adult Indigenous Australians referred for lung function testing in the Top End Northern Territory of Australia. J Asthma Allergy 15: 1305–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heraganahally SS, Timothy TP, Sorger L, 2022. Chest computed tomography findings among adult Indigenous Australians in the Northern Territory of Australia. J Med Imaging Radiat Oncol 66: 337–344. [DOI] [PubMed] [Google Scholar]
- 34. Schubert J, Kruavit A, Mehra S, Wasgewatta S, Chang AB, Heraganahally S, 2019. Prevalence and nature of lung function abnormalities among Indigenous Australians referred to specialist respiratory outreach clinics in the Northern Territory. Int Med J 49: 217–224. [DOI] [PubMed] [Google Scholar]
- 35. Heraganahally SS, Howarth T, White E, Sorger L, Biancardi E, Ben Saad H, 2020. Lung function parameters among Australian Aboriginal “apparently healthy” adults: an Australian Caucasian and Global Lung Function Initiative (GLI-2012) various ethnic norms comparative study. Expert Rev Respir Med 23: 1–11. [DOI] [PubMed] [Google Scholar]
- 36. Sze DFL, Howarth TP, Lake CD, Ben Saad H, Heraganahally SS, 2022. Differences in the spirometry parameters between Indigenous and non-Indigenous patients with COPD: a matched control study. Int J Chron Obstruct Pulmon Dis 17: 869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heraganahally SS, Ponneri TR, Howarth TP, Saad HB, 2021. The effects of inhaled airway directed pharmacotherapy on decline in lung function parameters among Indigenous Australian adults with and without underlying airway disease. Int J Chron Obstruct Pulmon Dis 16: 2707–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Howarth T, Gahreman D, Ben Saad H, Ng L, Heraganahally SS, 2023. Correlation of spirometry indices to chest radiology in the diagnosis of chronic airway disease among regional and rural Indigenous Australians. Int Med J. doi: 10.1111/imj.16023. [DOI] [PubMed] [Google Scholar]
- 39. Heraganahally SS, Howarth T, Mo L, Sorger L, Ben Saad H, 2021. Critical analysis of spirometric patterns in correlation to chest computed tomography among adult Indigenous Australians with chronic airway diseases. Expert Rev Respir Med 15: 1229–1238. [DOI] [PubMed] [Google Scholar]
- 40. Heraganahally SS, Rajaratnam B, Silva SAAS, Robinson N, Oguoma VM, Naing P, Kangaharan N, Ilton M, 2021. Obstructive sleep apnoea and cardiac disease among Aboriginal patients in the Northern Territory of Australia. Heart Lung Circ 30: 1184–1192. [DOI] [PubMed] [Google Scholar]
- 41. Heraganahally SS, Kruavit A, Oguoma VM, Gokula C, Mehra S, Judge D, Sajkov D, 2020. Sleep apnoea among Australian Aboriginal and non-Aboriginal patients in the Northern Territory of Australia – a comparative study. Sleep (Basel) 43: zsz248. [DOI] [PubMed] [Google Scholar]
- 42. Howarth T, Ben Saad H, Heraganahally SS, 2023. The impact of lung function parameters on sleep among Aboriginal Australians – a polysomnography and spirometry relationship study. Nat Sci Sleep 15: 449–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heraganahally SS, Mortimer N, Howarth T, Messenger R, Issac S, Thomas I, Brannelly C, 2021. Utility and outcomes among Indigenous and non-Indigenous patients requiring domiciliary oxygen therapy in the regional and rural Australian population. Aust J Rural Health 29: 918–926. [DOI] [PubMed] [Google Scholar]
- 44. Heraganahally SS, Ghimire RM, Howarth T, Kankanamalage OM, Palmer D, Falhammar H, 2022. Comparison and outcomes of emergency department presentations with respiratory disorders among Australian Indigenous and non-Indigenous patients. BMC Emerg Med 22: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heraganahally S, Howarth TP, Issac S, Lloyd A, Ravichandran SJ, Abeyaratne A, Patel B, 2023. Exploring the appropriateness of prescribing practice of inhaled pharmacotherapy among Aboriginal Australians in the Top End Northern Territory of Australia: a retrospective cohort study. BMJ Open Respir Res 10: e001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Howarth TP, Jersmann HPA, Majoni SW, Mo L, Ben Saad H, Ford LP, Heraganahally SS, 2023. The “ABC” of respiratory disorders among adult Indigenous people – asthma, bronchiectasis and COPD among Aboriginal Australians – a systematic review. BMJ Open Respir Res 10: e001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Howarth T, Heraganahally S, Heraganahally S, 2023. Bronchiectasis among adult First Nations Indigenous people – a scoping review. Curr Respir Med Rev 19: 36–51. [Google Scholar]
- 48. Kaplan AG, 2021. Cannabis and lung health: does the bad outweigh the good? Pulm Ther 7: 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pal A, Howarth TP, Rissel C, Messenger RL, Issac S, Ford L, Connors C, Heraganahally SS, 2022. COPD disease knowledge, self-awareness and reasons for hospital presentations among a predominately Indigenous Australian cohort – a study to explore preventable hospitalization. BMJ Open Respir Res 9: e001295. [DOI] [PMC free article] [PubMed] [Google Scholar]