ABSTRACT.
Chagas disease affects approximately 300,000 patients in the United States. We evaluated a multicenter U.S.-based network to obtain clinical characteristics and outcomes of chronic Chagas disease by disease forms. This was a U.S.-based, multicenter, population-based, retrospective cohort study. We queried TriNetX, a global research network, to identify patients with dual-positive IgG serology for Trypanosoma cruzi. We captured outcomes of interest for up to 5 years. We found 429 patients with evidence of dual-positive T. cruzi IgG out of 19,831 patients with an available test result from 31 U.S. medical centers. The positive proportion for those tested was 2.2%, up to 4.6% among Hispanics. We found a prevalence of a positive Chagas serology of 0.02% among Hispanics. Cardiomyopathy risk reached an annual rate of 1.3% during the initial 5 years of follow-up among patients with the indeterminate form. We found no new events for pulmonary embolism, sudden death, or left ventricular aneurysms at 5 years. Annual risks for arrhythmias and stroke for chronic Chagas cardiomyopathy (CCC) were 1.6% and 0.8%, respectively. The yearly mortality and hospitalization rates for CCC were 2.7% and 17.1%, respectively. Only 13 patients had a documented antitrypanosomal therapy course within 6 months after diagnosis. Of those receiving treatment, 10 patients received benznidazole and three nifurtimox. Chagas disease screening in patients from endemic areas living in the United States remains crucial. Chronic Chagas cardiomyopathy carries a considerable disease burden, translating into increased morbidity and mortality and an enlarging medical health service utilization.
INTRODUCTION
Chagas disease, also known as American trypanosomiasis, is a parasitic infection caused by the protozoan Trypanosoma cruzi. The disease is most commonly transmitted through contact with the feces of an infected triatomine bug, also known as the “kissing bug,” a blood-sucking insect that feeds on humans and animals. Chagas disease can also be transmitted from an infected mother to her unborn baby.1 Chagas disease is endemic in 21 countries of the Western hemisphere. However, owing to migration and travel, cases of Chagas disease have been reported in non-endemic countries, including the United States.2 A recent study that combined American community survey data with age-specific T. cruzi prevalence data estimated that 288,000 people in the United States are infected with Chagas disease, including 57,000 patients with Chagas cardiomyopathy and 43,000 infected women of reproductive age.3 The study also estimated that 10,000 people in the United States have locally acquired T. cruzi infection. Congenital infections occur at a rate of 22 to 108 per year. Several U.S.-based clinical care reports have found a wide range in the estimated prevalence of positive Chagas disease screening. For example, 17% had a positive initial point-of-care test (0% by confirmatory testing) in a women’s health clinic in Atlanta,4 2% at a tertiary center in Colorado,5 and 1.2% among Latin American–born immigrants (5.2% and 7.5% for those with conduction abnormalities or a pacemaker, respectively) in Los Angeles, California.6–8
Chronic Chagas disease is divided into indeterminate (here defined as asymptomatic patients without evidence of clinical, electrocardiographic, and radiological cardiac alterations) and determined (symptomatic cardiac, digestive, or cardiodigestive) forms. The indeterminate form of Chagas disease can lead to the development of Chagas cardiomyopathy at an annual rate of 1.9%.9 Once chronic Chagas cardiomyopathy (CCC) develops, the annual mortality rate is 7.9% in endemic countries.10 Despite the high projected number of patients infected with Chagas disease in the United States, we lack aggregated prevalence data, clinical features, and long-term outcomes on those patients living in the United States. We evaluated a multicenter U.S.-based network to obtain clinical characteristics and outcomes of chronic Chagas disease by disease forms.
MATERIALS AND METHODS
Global federated research network.
The data used in this study were collected on March 2023 from the TriNetX Network, which provided access to electronic medical records (diagnoses, procedures, medications, laboratory values, genomic information) from approximately 100 million patients from 80 healthcare organizations (HCOs). Our group has already published original research using this technology.11–13 TriNetX, LLC complies with the Health Insurance Portability and Accountability Act (HIPAA),14 the U.S. federal law protecting healthcare data privacy and security, and any additional data privacy regulations applicable to the contributing HCOs. Each HCO delivers electronic medical record systems data collected to provide patient care. Received data are either structured or unstructured data processed by Natural Language Processing Technology. Most participating HCOs are large academic medical institutions with inpatient and outpatient facilities. The data they provide represent the entire patient population at the HCO. Most give an average of 7 years of historical data. TriNetX receives data directly from an HCO research repository into the TriNetX environment, or the HCO sends TriNetX data extracts in the form of comma-separated values file (CSV) files coded in the TriNetX Data Dictionary. Healthcare organization and other data providers update their data at various times, with over 80% refreshing in 1-, 2-, or 4-week frequency intervals. The average lag time for an HCO’s source data to refresh is 1 month. TriNetX maps the data to a standard, controlled set of clinical terminologies and transforms it into a proprietary data model. This transformation process includes extensive data quality assessment that includes data cleaning that rejects records that do not meet the TriNetX quality standards.
TriNetX is certified to the ISO 27001:2013 standard and maintains an Information Security Management System to ensure the protection of the healthcare data it has access to and to meet the requirements of the HIPAA Security Rule. Any data displayed on the TriNetX Platform in aggregate form or any patient-level data provided in a dataset generated by the TriNetX Platform contains only de-identified data per the de-identification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. The process of de-identifying data is attested to through a formal determination by a qualified expert as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule.
Study design and population.
We identified adult patients with a double-positive IgG serology for T. cruzi (N = 429) as criteria defined in the WHO guidelines for diagnosing chronic Chagas disease.15 Trypanosoma cruzi IgG antibodies (Ab) (units/volume) was reported in serum by immunoassay (laboratory result: positive or ≥ 1.20 optical density unit). The analysis compared clinical characteristics among patients with the chronic indeterminate form (n = 143) versus those with the determinate cardiac form (CCC) (n = 286). We defined patients with the indeterminate form as those with two positive serologies for T. cruzi IgG without history or evidence of cardiac lesions or cardiomyopathy by International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes only. We defined patients with CCC as those with two positive serologies for T. cruzi IgG with any history or evidence of cardiac lesions or cardiomyopathy by the ICD-10-CM codes only (Supplemental Materials). Definitions are consistent with established expert consensus.1
The query criteria for each cohort were based on ICD-10-CM codes and laboratory results (Supplemental Tables 1–4). The earliest encounter for IgG serology for T. cruzi positivity was identified as the index event. Demographic characteristics, diagnoses, procedures, medications, and measurements (e.g., laboratory test results; see Supplemental Materials) were non-timebound and captured before the index event. The most recent result was chosen when multiple results were available. TriNetX uses the health level 7, version 3, administrative standards codes for ethnicity. The standards define a Hispanic or Latino as a person of “Mexican, Puerto Rican, Cuban, South or Central America, or other Spanish culture or origin, regardless of race.”
Global federated research network outcome measures.
We captured the development of CCC at 1 and 5 years as the primary outcome after a positive Chagas IgG serology. The secondary outcomes included the proportion of patients with the determinate cardiac form of the disease who developed stroke, pulmonary embolism, sudden death, arrhythmias, and left ventricular aneurysm (based on ICD-10 codes; Supplemental Table 4) at 1 and 5 years after the index event. Included arrhythmias or conduction abnormalities were all those available in the ICD-10 code dictionary. To evaluate these outcomes, we excluded their presence individually before and up to 6 months after the diagnosis of CCC. We evaluated the proportion of antitrypanosomal medications, mortality, and hospitalization at 1 and 5 years. We used survival means and 95% CI to construct Kaplan Meier (KM) survival curves at 1 year. We also calculated the annual rates based on the 5-year outcome results. To explore follow-up cardiomyopathy development with the indeterminate form cohort, we defined the cohort as patients with positive serology for T. cruzi IgG who did not have any history or evidence of cardiac lesions or cardiomyopathy before or up to 6 months since the instance of positive serology.
Treatment pathway.
We captured the treatment pathways within the TriNetX platform for all patients diagnosed with a positive IgG serology for Chagas disease. Treatment was recorded within 6 months after the index event. Antitrypanosomal medications were benznidazole and nifurtimox (Supplemental Table 3). A line of treatment was defined as taking the same medication within 6 months from the index event, and it was considered completed once it was absent from the patient’s record for 7 consecutive days.
Prevalence analysis.
The positive test proportion and prevalence analysis were performed in the TriNetX platform. We used all visits for Hispanics within the system. We queried the years 2017 through 2023. Each year was captured from January 1 through December 31.
Statistical analysis.
We completed the statistical analyses of the global federated research network on the TriNetX platform. Descriptive statistics were presented as means and SDs for continuous variables and as frequency and percentages for categorical variables. Additional graphs were designed using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA; www.graphpad.com).
Data access.
The corresponding author had full access to the study’s aggregated data and was responsible for submitting the manuscript for publication. The datasets generated and analyzed in the current study are available from TriNetX for those subscribed to the platform.
RESULTS
Overall annual positive test rate and prevalence of Chagas disease among Hispanics.
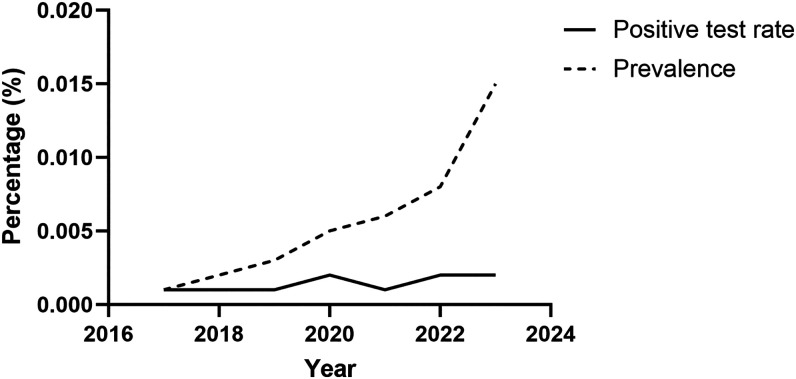
Within the approximately 8 million visits for Hispanics in the TriNetX platform, we found an annual positive proportion of a positive Chagas serology in the range of 0.001–0.002% and an up-trending prevalence to 0.015% in 2023 (Figure 1). The positive test proportion and prevalence increased with age. Prevalence was 0.03% for 75- to 79-year-olds. There were no appreciable gender differences.
Figure 1.
Positive test rate and prevalence of Trypanosoma cruzi IgG among Hispanics.
Clinical characteristics of patients with positive T. cruzi IgG.
We found 429 patients in the system with evidence of a dual-positive T. cruzi IgG out of 19,831 patients with an available test result from 31 U.S. medical centers (59% came from the West and 32% from the Northeast). The positive proportion for those tested was 2.2%. The proportion increased to 4.6% among Hispanics. These patients approached a mean age of 50 years (Table 1), 60% were men, and 58.5% were Hispanic. The most frequent comorbidities were chronic kidney disease, hypertension, type 2 diabetes mellitus, and hyperlipidemia. History of transplantation and HIV infection were relatively infrequent. Common medications recorded in this group included beta-blockers, loop diuretics, and amlodipine. Prior use of specific antitrypanosomal medications was low.
Table 1.
Baseline clinical features of seropositive patients for Chagas disease
| Characteristic name | N | Trypanosoma cruzi IgG+ (n = 429) | Indeterminate (n = 143) | CCC (n = 286) |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 429 | 49.5 ± 16.2 | 44.7 ± 18.8 | 54.4 ± 13.6 |
| Male | 429 | 256 (59.7%) | 74 (51.7%) | 182 (63.6%) |
| Hispanic or Latino | 429 | 251 (58.5%) | 68 (47.6%) | 183 (64%) |
| White | 429 | 165 (38.5%) | 56 (39.2%) | 109 (38.1%) |
| Asian | 429 | 30 (7%) | 10 (7%) | 20 (7%) |
| Native American | 429 | 20 (4.7%) | 10 (7%) | 10 (3.5%) |
| Comorbidities | ||||
| Essential hypertension | 429 | 223 (52%) | 47 (32.9%) | 176 (61.5%) |
| Chronic kidney disease | 429 | 206 (48%) | 62 (43.4%) | 144 (50.3%) |
| Type 2 diabetes mellitus | 429 | 150 (35%) | 29 (20.3%) | 121 (42.3%) |
| Hyperlipidemia | 429 | 136 (31.7%) | 25 (17.5%) | 111 (38.8%) |
| Overweight and obesity | 429 | 114 (26.6%) | 30 (21%) | 84 (29.4%) |
| Neoplasms | 429 | 93 (21.7%) | 19 (13.3%) | 74 (25.9%) |
| Transplant | 429 | 57 (13.3%) | 13 (9.1%) | 44 (15.4%) |
| GERD | 429 | 76 (17.7%) | 10 (7%) | 66 (23.1%) |
| Aplastic anemias | 429 | 24 (5.6%) | 7 (4.9%) | 17 (5.9%) |
| STDs | 429 | 23 (5.4%) | 10 (7%) | 13 (4.5%) |
| HIV | 429 | 20 (4.7%) | 10 (7%) | 10 (3.5%) |
| Strongyloidiasis | 429 | 11 (2.6%) | 0 (0%) | 11 (3.8%) |
| Functional dyspepsia | 429 | 12 (2.8%) | 0 (0%) | 12 (4.2%) |
| Liver fibrosis | 429 | 31 (7.2%) | 10 (7%) | 21 (7.3%) |
| Cerebral infarction | 429 | 21 (4.9%) | 10 (7%) | 11 (3.8%) |
| Pulmonary embolism | 429 | 10 (2.3%) | 0 (0%) | 10 (3.5%) |
| Resuscitated from sudden cardiac arrest | 429 | 10 (2.3%) | 0 (0%) | 10 (3.5%) |
| Toxoplasmosis | 429 | 10 (2.3%) | 0 (0%) | 10 (3.5%) |
| Chronic viral hepatitis | 429 | 20 (4.7%) | 10 (7%) | 10 (3.5%) |
| Pregnancy | 429 | 21 (4.9%) | 11 (7.7%) | 10 (3.5%) |
| LV aneurysm | 429 | 3 (0.7%) | 0 (0%) | 3 (1%) |
| Medications | ||||
| Beta-blockers | 429 | 230 (53.6%) | 40 (28%) | 190 (66.4%) |
| Metoprolol | 429 | 139 (32.4%) | 15 (10.5%) | 124 (43.4%) |
| Carvedilol | 429 | 89 (20.7%) | 13 (9.1%) | 76 (26.6%) |
| Loop diuretics | 429 | 179 (41.7%) | 36 (25.2%) | 143 (50%) |
| Atorvastatin | 429 | 150 (35%) | 23 (16.1%) | 127 (44.4%) |
| ACE inhibitors | 429 | 133 (31%) | 22 (15.4%) | 111 (38.8%) |
| Amlodipine | 429 | 143 (33.3%) | 36 (25.2%) | 107 (37.4%) |
| K+ sparing diuretics | 429 | 65 (15.2%) | 10 (7%) | 55 (19.2%) |
| Amiodarone | 429 | 30 (7%) | 0 (0%) | 30 (10.5%) |
| Adenosine | 429 | 27 (6.3%) | 10 (7%) | 17 (5.9%) |
| Benznidazole | 429 | 20 (4.7%) | 10 (7%) | 10 (3.5%) |
| Nifurtimox | 429 | 10 (2.3%) | 0 (0%) | 10 (3.5%) |
| Posaconazole | 429 | 10 (2.3%) | 10 (7%) | 0 (0%) |
| Laboratory | ||||
| Hematocrit (%) | 398 | 36.9 ± 6.9 | 36.7 ± 7.3 | 37.2 ± 6.6 |
| Creatinine (mg/dL) | 402 | 3.9 ± 3.7 | 4.2 ± 4 | 3.6 ± 3.5 |
| Platelets (103/µL) | 389 | 216.6 ± 86.7 | 225.1 ± 83.9 | 208 ± 89.5 |
| ALT (IU/mL) | 388 | 29.8 ± 54.1 | 21.7 ± 22.9 | 38 ± 85.2 |
| AST (IU/mL) | 291 | 25 ± 21.2 | 23.6 ± 20.7 | 26.3 ± 21.8 |
| Sodium (mmol/L) | 399 | 137 ± 3.6 | 137.1 ± 3.5 | 137 ± 3.7 |
| Leukocytes (103/µL) | 282 | 7.6 ± 5.3 | 7.6 ± 3.6 | 7.7 ± 7.1 |
| Lymphocytes (103/µL) | 360 | 2.2 ± 1.2 | 2.3 ± 1.3 | 2.1 ± 1.2 |
| Hemoglobin (mg/dL) | 272 | 12.6 ± 3 | 12.6 ± 3.5 | 12.5 ± 2.4 |
| Hemoglobin A1c (%) | 277 | 6.4 ± 1.6 | 6.4 ± 1.7 | 6.4 ± 1.5 |
| Cholesterol (mg/dL) | 230 | 155.1 ± 46.9 | 160.6 ± 51.4 | 149.5 ± 42.3 |
| Ferritin (µg/L) | 127 | 428.1 ± 632 | 424.2 ± 630.7 | 431.9 ± 633.3 |
| LDH (IU/mL) | 82 | 252 ± 129.1 | 232.6 ± 126 | 271.4 ± 132.2 |
| BNP (pcg/mL) | 34 | 7,878.3 ± 15,370.2 | – | 7,878.3 ± 15,370.2 |
| Creatine kinase (U/L) | 79 | 207.3 ± 383.2 | 190.7 ± 401.9 | 223.9 ± 364.4 |
| C-reactive protein (mg) | 68 | 34.9 ± 68.6 | 45 ± 88 | 24.8 ± 49.3 |
| Troponin I (ng/mL) | 58 | 0.2 ± 0.5 | 0.1 ± 0 | 0.3 ± 0.9 |
| Others | ||||
| BMI | 335 | 28.4 ± 6 | 28.1 ± 5.9 | 28.7 ± 6 |
| Respiratory rate | 310 | 16.7 ± 2.6 | 16.9 ± 2.3 | 16.6 ± 2.8 |
| Heart rate | 289 | 77 ± 15.9 | 78.9 ± 16 | 75.1 ± 15.9 |
| Corrected QT (mm) | 20 | 426.1 ± 43.7 | 428 ± 38.2 | 424.1 ± 49.2 |
| LVEF (%) | 20 | 57.5 ± 11 | 62 ± 10 | 53 ± 22 |
Data are presented as n (%) or mean ± SD. ALT = alanine transaminase; AST = aspartate transaminase; BMI = body mass index; BNP = brain natriuretic peptide; CCC = chronic Chagas cardiomyopathy; GERD = gastroesophageal reflux disease; K+ = potassium; LDH = lactic dehydrogenase; LV = left ventricle; LVEF = left ventricular ejection fraction; STD = sexually transmitted disease.
The laboratory results were remarkable for an elevated mean creatinine. Hemoglobin was borderline low. The mean Glycosilated hemoglobin (HbA1c) percentage was elevated, with a mean level consistent with prediabetes. Cardiac biomarkers (i.e., mean b-type natriuretic peptide, C-reactive protein, creatine kinase, and troponin I) were available in only ∼10% of the patients and were elevated. The reported mean corrected QT interval of the ECG (QTc) was normal. The mean left ventricular ejection fraction (LVEF) was normal for the overall population.
Among patients with dual-positive serology for Chagas disease, 33.3% had the indeterminate form and 66.6% had a determinate form manifested with cardiomyopathy. Patients with the indeterminate form were younger, with slightly fewer Hispanics and men than patients with CCC (Table 1). Chronic kidney disease was most frequent in the CCC group. Moreover, patients with CCC had higher percentages of heart failure and gastroesophageal reflux. Prior medication use was higher with CCC except for benznidazole. There were no significant laboratory differences except the mean creatinine level being higher in those with the indeterminate form and higher lactate dehydrogenase levels in patients with CCC. The LVEF was mildly reduced, and brain natriuretic peptide was elevated in patients with cardiomyopathy compared with patients with the indeterminate form.
Outcomes for patients by chronic disease forms.
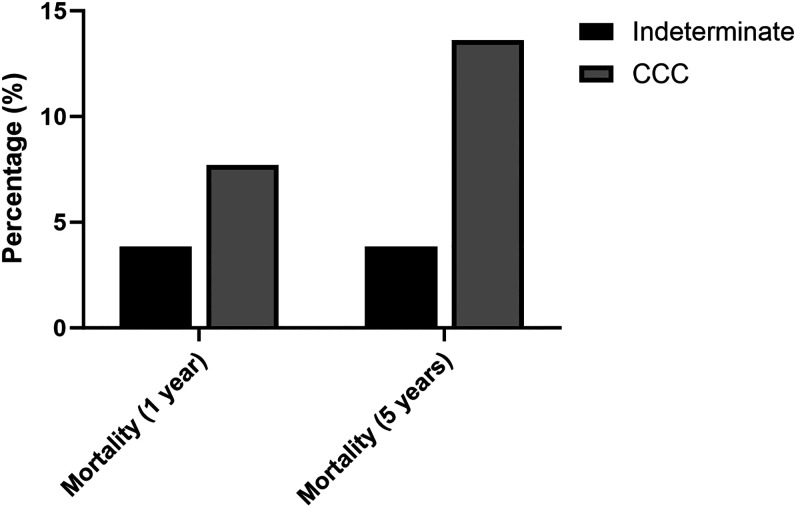
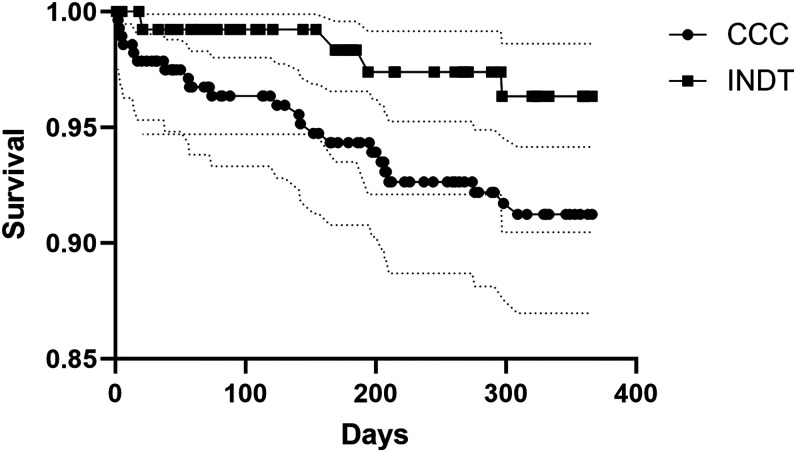
At 5 years, the capture of most cardiovascular complications remained low in patients with CCC. We found no new events for pulmonary embolism, sudden death, or left ventricular aneurysms at 5 years. The rate for new stroke was 3.8% and for new arrhythmias it was 7.8% at 5 years. Type of arrhythmias or conduction abnormalities by frequency were atrial fibrillation and flutter (30%), other cardiac arrhythmias (25%), paroxysmal tachycardia (17%), other conduction abnormalities (12%), atrioventricular block and left bundle-branch block (10%), and cardiac arrest (5%). Annual risk for arrhythmias/conduction abnormalities and stroke for CCC were 1.6% and 0.8%, respectively. Mortality, as expected, was higher in CCC than in patients with the indeterminate form at 1 and 5 years (Figures 2 and 3). The calculated annual mortality rate reached 2.7% among patients with CCC. Up to a fifth (17.1%) of patients with chronic cardiomyopathy required hospitalization due to any cause every year. Among patients with the indeterminate form, the rate of Chagas cardiomyopathy development reached 3.6% at 1 year and 6.6% at 5 years. Cardiomyopathy risk reached an annual rate of 1.3% during the initial 5 years of follow-up among indeterminate form patients. Among transplant patients, the 5-year mortality and hospitalization rates were 17% and 89%, respectively.
Figure 2.
Mortality at 1 and 5 years by chronic disease forms. CCC = chronic Chagas cardiomyopathy.
Figure 3.
Kaplan Meier survival curves at 1 year per disease forms. CCC = chronic Chagas cardiomyopathy; INDT = chronic indeterminate form.
Treatment pathways for patients with Chagas disease.
Within 6 months after the index event of positivity for T. cruzi IgG, only 13 patients (3%) had documented antitrypanosomal therapy. The number decreased to only one among patients with the indeterminate form. Of those receiving therapy, 10 patients received benznidazole and three nifurtimox. The median time from diagnosis until treatment onset was 92 days for benznidazole and 28 days for nifurtimox.
DISCUSSION
Over the last 6 years, we found an estimated prevalence of one to two patients with positive serology for T. cruzi per 10,000 Hispanic patients attending care at a U.S.-based medical center. The overall positive proportion for T. cruzi IgG was 2.2%, and it was 4.6% among Hispanics tested for the disease. The percentage of positive tests ranged between 0% and 7% based on studies done in Colorado, California, Texas, and Georgia.4–6,16 Among blood donors, the positive proportion for T. cruzi ranged from 0.2% in Los Angeles to 0.08% in Miami,17 although blood donor estimates are biased based on who donates blood. Another study found a positive proportion of 0.04% among blood donors in California and Arizona in 2006–2007.18 Significant regional variations of Hispanic populations throughout the continental United States might explain some of the fluctuations of these estimates.
With an estimated Latino population in the United States of 62.5 million, the positive proportion of 0.02% found translates to approximately 12,500 seropositive patients in the United States. However, not all Hispanics are born outside the United States or otherwise at risk. Hispanics attending large U.S. medical centers usually have access to health insurance, and two-thirds are United States born in contrast to Latinos from endemic countries previously living in poverty. Most Hispanics in the United States (U.S.-born, those born in Puerto Rico, the Dominican Republic, or Cuba) do not have an elevated risk compared with non-Hispanics. Most immigrant Hispanics at risk for Chagas disease living in the United States may not be linked to healthcare. U.S. Hispanics may also have low perinatal exposure or come from lower-risk areas in Latin America, and the group may be under-screened because providers have limited awareness of Chagas disease.
Of 429 seropositive patients for Chagas disease, roughly a third had the chronic indeterminate form of infection, and the rest had CCC. Seropositive patients had a high percentage of chronic kidney disease, suggesting that testing was done as part of the evaluation for transplant.
Patients with the indeterminate form of chronic Chagas disease were younger and had a high percentage of chronic kidney disease. These populations may reflect younger Hispanics deemed appropriate for general or kidney/pancreas transplant eligibility screening. Younger women may also be a target for Chagas screening campaigns. The clinical characteristics of those patients may differ from those in endemic countries as patients living in the United States may have a clinical indication for screening instead of asymptomatic screening. By definition, these patients should be asymptomatic and have a normal electrocardiogram (ECG) and normal chest-x-ray. These patients may have other chronic diseases requiring transplant evaluation, which can also be a reason for screening. Notably, patients with the indeterminate form may have subtle or subacute cardiac changes, which can increase the risk of developing Chagas heart disease.19,20
There are scarce longitudinal data and outcomes of patients with Chagas disease living in non-endemic countries. Most observational longitudinal studies have been done in endemic countries. Cardiomyopathy risk reached an annual rate of 1.3% during the initial 5 years of follow-up among indeterminate form patients in our study, slightly lower than the estimated rate in endemic countries (1.9%).9 This rate suggests a possible role for reinfections in cardiomyopathy risk among patients with the indeterminate form in endemic countries.21 The annual mortality rate for CCC was 2.7% during those 5 years, significantly lower than that recently reported by a meta-analysis performed in endemic countries of 7.9%,10 which can partially be explained by the higher mean LVEF encountered in our study (53% versus 38%) and the inclusion of old studies in patients with more severe cardiomyopathy in the meta-analysis above. In addition, advanced cardiac disease requires access to high-technology healthcare, including multidisciplinary assessments, implantable cardiac devices, and heart transplantation. These tools are more commonly present in developed nations. Our study’s annual rate of stroke risk (0.8%) was lower than those in endemic countries (1.4%).22 The rates of arrhythmia development were significant among patients with established Chagas cardiomyopathy. Arrhythmias usually correlate with the degree of heart failure and with motion wall abnormalities.23 The risk of hospitalization was markedly elevated, suggesting a potential role of cardiac-related admissions or pretransplant evaluations among those tested for Chagas disease. Hospital admission rates for Chagas disease due to cardiovascular issues have increased in the United States.24
Despite limited efficacy data or the benefit of antitrypanosomal therapy for Chagas cardiomyopathy or during the indeterminate form of the disease, antiparasitic therapy implementation was very low. This can be due to loss of follow-up, provider unawareness, lack of integrated electronic health records, or confirmed false-positive results. Antiparasitic treatment is usually not recommended for advanced cardiomyopathy. We also could not assess the duration of therapy to ensure completion.
Our study has several limitations. The retrospective nature of the cohort can introduce some selection bias. Diagnosis of the different forms of chronic Chagas disease was performed through ICD code captures, which may be subject to code errors and capture patients not meeting diagnostic criteria. Because we only obtained aggregated data, we could not access specific serology results to confirm the dual-positive confirmatory follow-up testing or the individual threshold used. Missing data could have impaired some of the association’s strengths. Hispanic ethnicity capture could have significant misclassification as we could expect most subjects to fit into this category. Cardiovascular outcomes were obtained 1 and 5 years after diagnosis, but unfortunately, we could not ensure that some were not present before. We may have lost the capture of critical outcomes if they occurred out of network or were not recorded as ICD codes, which may have underestimated the findings. Ascertaining the true incidence of infection would require negative baseline results with confirmed positive results on subsequent follow-up, which were unavailable in our study.
The platform also limited us from running a subgroup analysis by participating centers. However, this is a unique characterization and outcome study of Chagas in the United States.
CONCLUSIONS
The Chagas seropositive prevalence among Hispanics in this multicenter study was 0.02%. Among those tested, the positive proportion of T. cruzi IgG was 4.6%. Cardiomyopathy risk reached an annual rate of 1.3% during the initial 5 years of follow-up among patients with the indeterminate form. The yearly mortality of CCC was 2.7%. The annual rate of new arrhythmias or conduction abnormalities was 1.6% for patients with established Chagas cardiomyopathy. Chagas disease continues to affect thousands of Hispanics in the United States. We should continue advocating for increased recognition and screening efforts for Chagas disease.2 Chronic Chagas cardiomyopathy carries a significant disease burden that translates into increased morbidity and mortality and the use of healthcare resources.
Supplemental Materials
Note: Supplemental material appears at www.ajtmh.org.
REFERENCES
- 1. Rassi A, Jr., Rassi A, Marin-Neto JA, 2010. Chagas disease. Lancet 375: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 2. Marcus R, Henao-Martínez AF, Nolan M, Livingston E, Klotz SA, Gilman RH, Miranda-Schaeubinger M, Meymandi S, 2021. Recognition and screening for chagas disease in the USA. Ther Adv Infect Dis 8: 20499361211046086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Irish A, Whitman JD, Clark EH, Marcus R, Bern C, 2022. Updated estimates and mapping for prevalence of Chagas disease among adults, United States. Emerg Infect Dis 28: 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zamora LE, Palacio F, Kozlowski DS, Doraivelu K, Dude CM, Jamieson DJ, Haddad LB, 2020. Chagas disease screening using point-of-care testing in an at-risk obstetric population. Am J Trop Med Hyg 104: 959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hyson P. et al. , 2021. Experiences with diagnosis and treatment of Chagas disease at a United States teaching hospital–clinical features of patients with positive screening serologic testing. Trop Med Infect Dis 6: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park S, Sanchez DR, Traina MI, Bradfield JS, Hernandez S, Ufion AJA, Dufani J, Bergin P, Wachsner RY, Meymandi SK, 2017. The prevalence of Chagas disease among Latin American immigrants with pacemakers in Los Angeles, California. Am J Trop Med Hyg 96: 1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meymandi SK, Forsyth CJ, Soverow J, Hernandez S, Sanchez D, Montgomery SP, Traina M, 2017. Prevalence of Chagas disease in the Latin American-born population of Los Angeles. Clin Infect Dis 64: 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Traina MI. et al. , 2017. Prevalence of Chagas disease in a U.S. population of Latin American immigrants with conduction abnormalities on electrocardiogram. PLoS Negl Trop Dis 11: e0005244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chadalawada S. et al. , 2020. Risk of chronic cardiomyopathy among patients with the acute phase or indeterminate form of Chagas disease: a systematic review and meta-analysis. JAMA Netw Open 3: e2015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chadalawada S. et al. , 2021. Mortality risk in chronic Chagas cardiomyopathy: a systematic review and meta-analysis. ESC Heart Fail 8: 5466–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vargas Barahona L. et al. , 2023. Previous corticosteroid exposure associates with an increased Pneumocystis jirovecii pneumonia mortality among HIV-negative patients: a global research network with a follow-up multicenter case-control study. Ther Adv Infect Dis 10: 20499361231159481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chastain DB, Motoa G, Ortiz-Martínez Y, Gharamti A, Henao-Martínez AF, 2023. Characteristics and clinical manifestations of monkeypox among people with and without HIV in the United States: a retrospective cohort. AIDS 37: 611–616. [DOI] [PubMed] [Google Scholar]
- 13. Kennis M. et al. , 2022. Seasonal variations and risk factors of Streptococcus pyogenes infection: a multicenter research network study. Ther Adv Infect Dis 9: 20499361221132101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. GovInfo The Health Insurance Portability and Accountability Act of 1996. Public Law 104–191. Available at: https://www.govinfo.gov/app/details/PLAW-104publ191. Accessed August 18, 2023.
- 15. World Health Organization , 2018. Guidelines for the Diagnosis and Treatment of Chagas Disease. Available at: https://www.who.int/publications/i/item/9789275120439. Accessed July 19, 2023.
- 16. Pentima MCD, Hwang L-Y, Skeeter CM, Edwards MS, 1999. Prevalence of antibody to Trypanosoma cruzi in pregnant Hispanic women in Houston. Clin Infect Dis 28: 1281–1285. [DOI] [PubMed] [Google Scholar]
- 17. Leiby DA, Herron RM, Jr., Read EJ, Lenes BA, Stumpf RJ, 2002. Trypanosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion 42: 549–555. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention , 2007. Blood donor screening for Chagas disease – United States, 2006–2007. MMWR Morb Mortal Wkly Rep 56: 141–143. [PubMed] [Google Scholar]
- 19. Hasslocher-Moreno AM, Xavier SS, Saraiva RM, de Sousa AS, 2021. Indeterminate form of Chagas disease: historical, conceptual, clinical, and prognostic aspects. Rev Soc Bras Med Trop 54: e02542021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rabelo DR, da Costa Rocha MO, de Barros MVL, da Silva JLP, Tan TC, Nunes MCP, 2014. Impaired coronary flow reserve in patients with indeterminate form of Chagas’ disease. Echocardiography 31: 67–73. [DOI] [PubMed] [Google Scholar]
- 21. Olivo Freites C, Sy H, Gharamti A, Higuita NIA, Franco-Paredes C, Suárez JA, Henao-Martínez AF, 2022. Chronic Chagas disease – the potential role of reinfections in cardiomyopathy pathogenesis. Curr Heart Fail Rep 19: 279–289. [DOI] [PubMed] [Google Scholar]
- 22. Moreira HT, Volpe GJ, Mesquita GM, Braggion-Santos MF, Pazin-Filho A, Marin-Neto JA, Schmidt A, 2022. Association of left ventricular abnormalities with incident cerebrovascular events and sources of thromboembolism in patients with chronic Chagas cardiomyopathy. J Cardiovasc Magn Reson 24: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Healy C, Viles-Gonzalez JF, Sáenz LC, Soto M, Ramírez JD, d’Avila A, 2015. Arrhythmias in chagasic cardiomyopathy. Card Electrophysiol Clin 7: 251–268. [DOI] [PubMed] [Google Scholar]
- 24. Singh A, Cohen B, Sturzoiu T, Vallabhaneni S, Shirani J, 2020. Recent trends in hospital admissions and outcomes of cardiac Chagas disease in the United States. Int J Crit Illn Inj Sci 10: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.