ABSTRACT.
A 3.5-year-old male child from Maharashtra, India, presented with features of meningoencephalitis approximately 1 month after sustaining severe bite injuries on the right hand from a stray dog. He had received four doses of post-exposure intradermal rabies vaccination (on days 0, 3, and 7 of the bite and erroneously on day 20, instead of day 28 as recommended in the updated Thai Red Cross regimen) as well as local and systemic injections of equine rabies immune globulin. The child was initially diagnosed with and treated for acute encephalitis syndrome before rabies encephalitis was confirmed by detection of rabies virus neutralizing antibodies in the cerebrospinal fluid. During the emergent period, he also received the antimalarial drug artesunate, recently reported to have antiviral effects against rabies virus. With intensive and supportive care, the child showed substantial clinical improvement over the next few weeks. He has now survived for more than 10 months after disease onset, albeit with severe neurological sequelae including diffuse cerebral and cerebellar atrophy.
CASE REPORT
Rabies is a fatal encephalomyelitis caused by highly neurotropic, bullet-shaped RNA viruses belonging to the genus Lyssavirus within the family Rhabdoviridae. Among the 59,000 human rabies deaths reported worldwide every year, about 40% are in young children.1 Most cases worldwide are caused by Lyssavirus rabies, one of the 17 named species of lyssaviruses. Systematic and timely post-exposure prophylaxis (PEP) is crucial to prevent human rabies deaths, but it faces many challenges in rabies-endemic countries. Rarely, true failure of rabies PEP regimens may occur for various reasons. Despite the generally fatal outcome, there have also been several recent reports of human survival from clinical rabies, especially from India.2–7 Herein, we report a case of recovery from rabies in a young child from western India.
A 3.5-year-old, previously healthy, male child from a rural area in Maharashtra presented with suspected meningoencephalitis on January 23, 2022. Earlier, the child had received treatment in a local hospital for altered sensorium and multiple episodes of vomiting and convulsions of 5 days. Magnetic resonance imaging (MRI) done on January 22, 2022 revealed suspicious T2/fluid-attenuated inversion recovery (FLAIR) hyperintensities in the bilateral basal ganglia; in view of the clinical deterioration, the child was admitted to our hospital.
According to the parents, the child suffered multiple bleeding, deep-bite injuries on the dorsal aspect of the right hand from a stray dog on December 25, 2021. After wound cleansing, the child was taken to a local hospital, where he received treatment for a category III exposure (defined as single/multiple transdermal bites or scratches, contamination of mucous membrane or broken skin with saliva from animal licks, and exposures due to direct contact from bats).8 He received a post-exposure intradermal rabies vaccination (two doses of 0.1 mL of a cell culture rabies vaccine in the deltoid regions on each day) on December 25 (day 0 of bite), 28 (day 3), and 31 (day 7) and on January 14, 2022 (day 20). This involved an erroneous deviation (i.e., administration of the fourth dose on day 20 instead of day 28) from the standard updated Thai Red Cross (TRC) regimen.9 The child also received local infiltration and intramuscular injections of equine rabies immunoglobulin (RIG) within 24 hours. The wound was not sutured.
At the time of admission, the child was afebrile and had a pulse rate of 124/minute, respiratory rate of 24/minute, and blood pressure of 116/79 mm Hg. Clinical examination revealed altered sensorium, deteriorating mental status, signs of meningeal irritation, frothing from the mouth, and hypotonia. No hydrophobia, aerophobia, or photophobia was observed. The Glasgow Coma Scale score was E2V3M4. Superficial and deep tendon reflexes were normal. The pupils were bilaterally equal and reactive to light. A fundus examination and cranial nerve functions were normal.
The child was provisionally diagnosed with acute encephalitis syndrome and treated with intravenous acyclovir (10 mg/kg/dose, every 8 hours), meropenem (20 mg/kg/dose, every 8 hours), artesunate (2.4 mg/kg initially, followed by 1.2 mg/kg after 12 hours and 1.2 mg/kg every day for 3 days), fosphenytoin (7 mg/kg per dose, 12 hourly) and levetiracetam (10 mg/kg/dose, every 12 hours), and 3% saline (anti-edema measure). Owing to clinical deterioration, the child was intubated and started on mechanical ventilation.
The routine clinical and laboratory findings in the patient are summarized in Table 1.
Table 1.
Clinical laboratory findings in the patient
| Test | Findings |
|---|---|
| Hemogram | Within normal limits |
| Renal function tests | Within normal limits |
| Liver function tests | Within normal limits |
| Serum C-reactive protein | 189 mg/dL |
| Serum procalcitonin | 0.21 μg/mL |
| CSF opening pressure | Normal |
| CSF protein | 62.9 mg/dL |
| CSF cell count | 50 cells/mm3 (lymphocytes: 90%) |
| Culture and sensitivity | |
| Blood (January 25 and February 11, 2022) | No growth |
| CSF (January 24, 2022) | No growth |
| Endotracheal aspirate (February 12, 2022) | Significant growth of Staphylococcus aureus |
| Urine (February 12, 2022) | Significant growth of Providencia rettgeri (sensitive only to tigecycline) |
CSF = Cerebrospinal fluid.
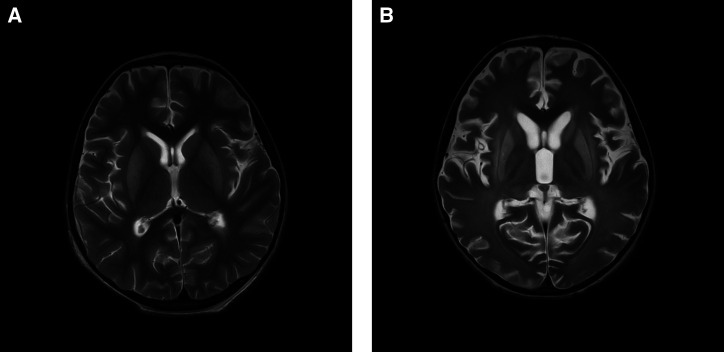
An MRI performed on February 4, 2022 showed symmetrical T2/FLAIR and diffusion-weighted hyperintensities involving the bilateral basal ganglia (mainly caudate nuclei and putamen) and thalami (Figure 1A). Electroencephalography performed on February 5, 2022 showed diffuse theta range intermittent slowing, suggestive of generalized encephalopathy. A repeated MRI was performed on March 14, 2022, which revealed the persistence of T2/FLAIR hyperintensities in the bilateral caudate nuclei, lentiform nuclei, external capsules, and thalami, with reduction in volume of these structures. Mild to moderate diffuse cerebral and cerebellar atrophy was also observed (Figure 1B).
Figure 1.
Serial axial T2 images of the patient taken on (A) February 4, 2022 showing hyperintensities in bilateral basal ganglia and thalami and on (B) March 14, 2022 showing hyperintensities in bilateral caudate nuclei, lentiform nuclei, external capsules, and thalami and moderate diffuse cerebral and cerebellar atrophy.
Immunoglobulin M (IgM) capture ELISA for Japanese encephalitis and reverse transcriptase polymerase chain reaction (RTPCR) assays for Chandipura virus, Japanese encephalitis virus, and enteroviruses performed on the serum and cerebrospinal fluid (CSF) samples yielded negative results. The pooled saliva, CSF, and neck skin biopsy samples tested negative for rabies virus RNA in real-time RTPCR10 and semi-nested RTPCR11 assays. The CSF collected on day 3 of clinical illness tested positive for rabies virus neutralizing antibodies (RVNAs) in a rapid fluorescent focus inhibition test,12 with a 100% neutralization titer of 512, which increased to 4,096 by day 34 (when clinical improvement was noted) and declined to 1,024 by day 86 (at which time there was significant clinical improvement). A high titer (65,536) of RVNA was also detected in a plasma sample collected on day 34.
The child was managed in the pediatric intensive care unit with ventilator support, intravenous levetiracetam (20 mg/kg/day), nasogastric tube feeding, good nursing care, and strict universal precautions. A post-exposure intradermal rabies vaccination (updated TRC regimen) was provided to the parents and caregivers in view of exposure risks. The child developed a catheter-associated urinary tract infection, which was treated with intravenous meropenem (20 mg/kg/dose, every 8 hours) and colistin (2.5 mg/kg/day, every 12 hours). He was weaned off of the ventilator after 4 weeks and was maintained on nasogastric feeding. He subsequently developed dystonia, which was treated with L-dopamine (1 mg/kg/day).
At discharge, the child was on nasogastric feeding, was not obeying verbal commands or making eye contact, and had persistent dystonia and limb contractures. On the latest follow-up at 10 months after disease onset, the child responded to verbal commands and sounds, recognized his mother, and showed less dystonia. He is currently receiving regular physiotherapy for management of limb contractures.
DISCUSSION
At present, around 30 cases of human survival from rabies have been reported worldwide, of which 20 were from India.6,7,13 Maharashtra, a state in western India, reports around 400,000 cases of animal bites and 200 human rabies deaths every year.14 Notably, four cases of human survival (three in children younger than 5 years and one in a 26-year-old male) from clinical rabies were reported from Maharashtra during August to November 2017.4,6
In a case series of true failures of PEP, Wilde15 observed that almost all patients had bite exposures in highly innervated areas, and the present case also matches with this. Post-exposure prophylaxis failures mostly result from inadequate wound care, errors in RIG administration, compromised potency of rabies biologicals, large viral inocula, and infection by neutralization-resistant atypical virus strains.13 In the present case, optimal wound care was provided, though significant deviations from current guidelines occurred in the administration of RIG (local and systemic administration instead of wound infiltration alone) and rabies vaccine (administration of the fourth dose on day 20 instead of day 28 after bite exposure). Because of late presentation, the integrity of the cold chain used for the rabies biological and the accuracy of injection practices could not be evaluated. The infecting rabies virus strain could not be characterized in this case because all clinical samples tested negative for viral RNA. Rabies immunoglobulin may have transient immune suppressive effects on coadministered rabies vaccines,16 and whether its systemic administration contributed to PEP failure in the present case is uncertain. Also, equine RIG prepared by pepsin digestion and/or chromatographic purification may contain split IgG antibody molecules with shorter half-lives and reduced potential for virus neutralization17; however, this could not be investigated in the present case.
Detection of anti-rabies antibodies in the CSF is diagnostic of rabies, regardless of vaccination history,18 and a sequential 4-fold or higher rise in their titer in the CSF and/or serum is confirmative.19,20 As in previous survivors of rabies,21,22 a vigorous early immune response, detection of CSF antibodies, and absence of viral antigen and nucleic acids were noted in the present case as well. Early CSF RVNA levels up to 1 to 10 IU/mL have been reported in rabies survivors23 and were also detected in the present case. Temporal changes in CSF RVNA titers in the present case were suggestive of rabies encephalitis.
Owing to the lack of clinical efficacy24–27 and the absence of national guidelines, experimental treatment regimens for rabies were not used in the present case. However, a factor unique to the present case was the receipt of the anti-malarial drug artesunate intravenously for 3 days during emergent treatment. Artesunate, a semi-synthetic artemisinin derivative, can enhance neutralizing antibody responses to rabies vaccine in mice, inhibit rabies virus replication in vitro, and enhance survival in rabid mice.28,29 No data currently exist on the clinical efficacy of artesunate in the treatment of human rabies; however, the potential effects in this case might have been non-negligible. With intensive and supportive care, the child showed spontaneous clinical improvement and survived, albeit with severe sequelae.
Neurological sequelae of varying severity have been reported in all survivors of human rabies in India.5–7 A follow-up MRI performed in the present case on March 14, 2022 showed moderate, diffuse cerebral and cerebellar atrophy and persistence of abnormal signals, as reported earlier in similar cases.5,30,31
The present case suggests that deviations from standard PEP guidelines may lead to adverse outcomes in young children with a category III exposure to rabies in highly innervated body areas. Anti-rabies clinics should ensure and monitor cold chain conditions for rabies biologicals, strictly follow standard PEP regimens, and administer RIG (or monoclonal globulins) only locally (and not intramuscularly) per latest9 guidelines. Multiple mechanisms could contribute to survival from rabies (including an early intrathecal antibody response and intensive care), which usually occurs with severe sequelae. Future research may address immune profiling in rabies survivors, evaluation of the clinical efficacy of artesunate and other experimental treatment regimens in human rabies, and development of novel antiviral drugs against rabies.
ACKNOWLEDGMENTS
We sincerely acknowledge the father of the child, who provided informed consent to publish the clinical details of the child. We also thank Reeta S. Mani and her team from the Department of Neurovirology (WHO Collaborating Centre for Reference and Research on Rabies), National Institute of Mental Health and Neurosciences, Bengaluru, India, for assistance in validating the results of the rapid fluorescent focus inhibition test on the clinical samples of the patient.
REFERENCES
- 1. Hampson K. et al. , 2015. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis 9: e0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madhusudana SN, Nagaraj D, Uday M, Ratnavalli E, Kumar MV, 2002. Partial recovery from rabies in a six-year-old girl. Int J Infect Dis 6: 85–86. [DOI] [PubMed] [Google Scholar]
- 3. de Souza A, Madhusudana SN, 2014. Survival from rabies encephalitis. J Neurol Sci 339: 8–14. [DOI] [PubMed] [Google Scholar]
- 4. Mani RS. et al. , 2019. Case reports: survival from rabies: case series from India. Am J Trop Med Hyg 100: 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goyal K, Bhagwat C, Suthar R, Saini AG, Ravikumar N, Singh P, Mani RS, Singh M, 2020. Enigma of rabies: prolonged survival in a boy with rabies brachial plexitis and encephalomyelitis. Neurol India 68: 673–676. [DOI] [PubMed] [Google Scholar]
- 6. John B, Kumar S, Kumar S, Dalal SS, Mohimen A, 2020. Child survivor of rabies in India: a case report. Paediatr Int Child Health 40: 255–260. [DOI] [PubMed] [Google Scholar]
- 7. Hamide A, Kaliyappan A, Mani RS, Krishnamurthy A, 2021. Neurological recovery with serological response in a rabies survivor on long-term follow-up. Trop Doct 51: 455–457. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization , 2018. Rabies Vaccines: WHO Position Paper, April 2018. Available at: https://www.who.int/publications/i/item/who-wer9316. Accessed March 7, 2023. [DOI] [PubMed]
- 9. National Centre for Disease Control, Ministry of Health and Family Welfare, Government of India , 2019. National Rabies Control Programme. Available at: www.ncdc.gov.in. Accessed September 11, 2023.
- 10. Nadin-Davis SA, Sheen M, Wandeler AI, 2009. Development of real-time reverse transcriptase polymerase chain reaction methods for human rabies diagnosis. J Med Virol 81: 1484–1497. [DOI] [PubMed] [Google Scholar]
- 11. Tordo N, Sacramento D, Bourhy H, Meslin FX, Kaplan MM, Koprowski H. Laboratory Techniques in Rabies. 4th ed. Geneva, Switzerland: World Health Organization, 157–170. [Google Scholar]
- 12. Smith JS, Yager PA, Baer GM, Meslin FX, Koprowsky H, Kaplan MM. Laboratory Techniques in Rabies. 4th ed. Geneva, Switzerland: World Health Organization, 181–187. [Google Scholar]
- 13.Cases of Human Rabies with Recovery, 2023. UpToDate. Available at: https://www.uptodate.com/contents/image/print?imageKey=ID%2F113372&topicKey=ID%2F16595&source=see_link. Accessed June 13, 2022.
- 14. Isalkar U, 2022, Rabies Cases in Maharashtra Doubled during First Year of Pandemic: RTI Data. Mumbai, India: Times Of India. Available at: https://timesofindia.indiatimes.com/city/pune/rabies-cases-in-maharashtra-doubled-during-first-year-of-pandemic-rti-data/articleshow/90529374.cms. Accessed June 13, 2022.
- 15. Wilde H, 2007. Failures of post exposure rabies prophylaxis. Vaccine 25: 7605–7609. [DOI] [PubMed] [Google Scholar]
- 16. Bernard MC, Boudet F, Pineda-Pena AC, Guinet-Morlot F, 2022. Inhibitory effect of concomitantly administered rabies immunoglobulins on the immunogenicity of commercial and candidate human rabies vaccines in hamsters. Sci Rep 12: 6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanlon CA, Niezgoda M, Morrill PA, Rupprecht CE, 2001. The incurable wound revisited: progress in human rabies prevention? Vaccine 19: 2273–2279. [DOI] [PubMed] [Google Scholar]
- 18. Moore SM, Hanlon CA, 2010. Rabies-specific antibodies: measuring surrogates of protection against a fatal disease. PLoS Negl Trop Dis 4: e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Madhusudana SN, Sukumaran SM, 2008. Ante-mortem diagnosis and prevention of human rabies. Ann Indian Acad Neurol 11: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Damodar T, Mani RS, Prathyusha PV, 2019. Utility of rabies neutralizing antibody detection in cerebrospinal fluid and serum for ante-mortem diagnosis of human rabies. PLoS Negl Trop Dis 13: e0007128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization , 2018. WHO Expert Consultation on Rabies: Third Report. World Health Organization Technical Report Series 1012. Geneva, Switzerland: WHO. [Google Scholar]
- 22. Fooks AR. et al. , 2017. Rabies. Nat Rev Dis Primers 3: 17091. [DOI] [PubMed] [Google Scholar]
- 23. Mani RS, Willoughby RE, Singh SK. Neglected Tropical Diseases—South Asia. New York, NY: Springer Cham, 349–366. [Google Scholar]
- 24. Hemachudha T, Sunsaneewitayakul B, Desudchit T, Suankratay C, Sittipunt C, Wacharapluesadee S, Khawplod P, Wilde H, Jackson AC, 2006. Failure of therapeutic coma and ketamine for therapy of human rabies. J Neurovirol 12: 407–409. [DOI] [PubMed] [Google Scholar]
- 25. Jackson AC, 2011. Rabies in the critical care unit: diagnostic and therapeutic approaches. Can J Neurol Sci 38: 689–695. [DOI] [PubMed] [Google Scholar]
- 26. Aramburo A, Willoughby RE, Bollen AW, Glaser CA, Hsieh CJ, Davis SL, Martin KW, Roy-Burman A, 2011. Failure of the Milwaukee protocol in a child with rabies. Clin Infect Dis 53: 572–574. [DOI] [PubMed] [Google Scholar]
- 27. Manesh A, Mani RS, Pichamuthu K, Jagannati M, Mathew V, Karthik R, Abraham OC, Chacko G, Varghese GM, 2018. Case report: failure of therapeutic coma in rabies encephalitis. Am J Trop Med Hyg 98: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luo J, Zhang Y, He H, Liu Q, Huang S, Guo X, 2019. Artesunate enhances the immune response of rabies vaccine as an adjuvant. Vaccine 37: 7478–7481. [DOI] [PubMed] [Google Scholar]
- 29. Luo J, Zhang Y, Wang Y, Liu Q, Li J, He H, Luo Y, Huang S, Guo X, 2021. Artesunate and dihydroartemisinin inhibit rabies virus replication. Virol Sin 36: 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rao A, Pimpalwar Y, Mukherjee A, Yadu N, 2017. Serial brain MRI findings in a rare survivor of rabies encephalitis. Indian J Radiol Imaging 27: 286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nethravathi M. et al. , 2015. Unique clinical and imaging findings in a first ever documented PCR positive rabies survival patient: a case report. J Clin Virol 70: 83–88. [DOI] [PubMed] [Google Scholar]