ABSTRACT.
Poor sanitation and hygiene practices and inadequate diets can contribute to environmental enteric dysfunction (EED). We evaluated the impact of a combined homestead food production and food hygiene intervention on EED biomarkers in young children in rural Bangladesh. The analysis was conducted within the Food and Agricultural Approaches to Reducing Malnutrition (FAARM) cluster-randomized trial in Sylhet, Bangladesh. The FAARM trial enrolled 2,705 married women and their children younger than 3 years of age in 96 settlements (geographic clusters): 48 intervention and 48 control. The 3-year intervention (2015–2018) included training on gardening, poultry rearing, and improved nutrition practices and was supplemented by an 8-month food hygiene behavior change component, implemented from mid-2017. We analyzed data on 574 children age 0 to 24 months with multilevel linear regression. We assessed fecal myeloperoxidase (MPO), neopterin (NEO), and alpha-1-antitrypsin (AAT) as biomarkers of EED, and serum C-reactive protein (CRP) and alpha-1-acid glycoprotein (AGP) as biomarkers of systemic inflammation, using ELISA. There was no intervention effect on NEO, AAT, CRP, and AGP concentrations, but, surprisingly, MPO levels were increased in children of the intervention group (0.11 log ng/mL; 95% CI, 0.001–0.22). This increase was greater with increasing child age and among intervention households with poultry that were not kept in a shed. A combined homestead food production and food hygiene intervention did not decrease EED in children in our study setting. Small-scale poultry rearing promoted by the intervention might be a risk factor for EED.
INTRODUCTION
Worldwide, nearly one quarter of children younger than 5 years are too short for their age, which indicates widespread undernutrition. The developmental consequences of undernutrition include compromised immune function and impaired cognitive development.1,2 Alongside insufficient intake of nutritious food and recurring diarrheal diseases, environmental enteric dysfunction (EED) is hypothesized to be a major cause of undernutrition.3–5
EED is a subclinical disorder of the small intestine characterized by villous atrophy, crypt hyperplasia, and lymphocyte infiltration, which result in intestinal permeability, nutrient malabsorption, and intestinal and systemic inflammation.3,6–9 EED is thought to contribute to undernutrition through decreased nutrient absorption, as well as increased energy requirements resulting from ongoing intestinal and systemic inflammation and immune activation.4,6,10–14 There is no agreed-upon case definition for EED. As the manifestation of EED is highly variable among individuals, studies usually measure a combination of biomarkers that reflect the different domains of EED: intestinal damage and repair (e.g., measured by Regenerating [REG] family proteins), permeability and absorption (e.g., fecal alpha-1-antitrypsin [AAT] or zonulin), microbial translocation (e.g., serum lipopolysaccharides [LPS] or anti-LPS IgG), intestinal inflammation (e.g., fecal myeloperoxidase [MPO] and neopterin (NEO]), and systemic inflammation (e.g., C-reactive protein [CRP] or alpha-1-acid glycoprotein [AGP]).15 Several of these biomarkers have been associated with poorer child growth outcomes.16–25
Environmental enteric dysfunction often begins in early infancy. Repeated exposure to enteric pathogens from contaminated food, water, or the environment resulting, for example, from poor water, sanitation, and hygiene (WASH) conditions or exposure to livestock in the household setting, is thought to contribute to EED.26–30 In addition, poor infant feeding practices and intake of food with low nutrient densities have been associated with an increased risk of developing EED14,31—for example, by weakening intestinal barrier function and mucosal repair.32
Therefore, a combined nutrition and hygiene intervention may have the potential to reduce or prevent EED in children. However, recent trials investigating combined WASH and nutrition interventions could not report a clear and consistent effect on EED biomarkers.33,34 Although the WASH Benefits trial33 in Bangladesh observed lower concentrations for some biomarkers of intestinal permeability and inflammation among the intervention arms during the course of the study (3- and 14-month assessments), by the age of 28 months, children in the intervention arms showed higher biomarkers of EED compared to control subjects. Also, the SHINE trial34 in Zimbabwe observed only minor, inconsistent effects of its nutrition and WASH interventions on EED biomarkers. A study in India25 assessing the effect of a household-level water and sanitation infrastructure intervention observed a decrease in intestinal permeability but not intestinal inflammation in children from intervention households compared with control subjects. Findings from these studies suggest that the interventions did not interrupt contamination pathways sufficiently, or that other unaddressed pathways, such as livestock exposure and food contamination, exposed children to pathogens.
Microbial contamination of food is highly prevalent in resource-poor settings and is a known contributor to intestinal infection and diarrheal disease.35–38 There is evidence that suboptimal household hygiene around food preparation and feeding leads to contamination of complementary foods.39–44 Therefore, good caregiver food hygiene practices could contribute to a reduction in complementary food contamination and intestinal disease. Recent studies45–47 showed that interventions using social and behavior change techniques were successful in improving caregiver food hygiene practices in low-income settings in sub-Saharan Africa and South Asia. However, there is currently no evidence on whether food hygiene behavior change interventions can improve child health outcomes, or if a combined approach to improve food hygiene behavior and dietary intake through nutrition diversification can affect EED in children.
The Food Hygiene to reduce Environmental Enteric Dysfunction (FHEED) study was designed to evaluate a behavior change intervention within the Food and Agricultural Approaches to Reducing Malnutrition (FAARM) cluster-randomized controlled trial in Sylhet, Bangladesh. In this study, we assess the effect of an intervention that trained women’s groups in gardening, poultry rearing, and food hygiene on EED in children younger than 24 months.
MATERIALS AND METHODS
Study design and participants.
Data analyzed for this study are from the FAARM cluster-randomized controlled trial (ClinicalTrials.gov, ID: NCT02505711) conducted from 2015 to 2020, and the FHEED substudy. Detailed information about the FAARM trial design, study setting, and study population can be found in the study protocol.48 In brief, the FAARM trial evaluated the impact of a homestead food production (HFP) program, implemented through Helen Keller International from mid 2015 to late 2018, on child undernutrition in the rural Habiganj District, Sylhet Division, northeastern Bangladesh. The trial enrolled 2,705 married women, with a self-reported age of 30 years or younger at enrollment, in 96 settlements (geographic clusters). Covariate-constrained randomization was used to assign 48 settlements to the intervention and 48 to the control group.
The HFP program included training on year-round home gardening, small-scale poultry rearing, and improved nutrition and hygiene practices. The project staff conducted training sessions with woman farmer groups at least every 2 months, supplemented by individual household visits every 2 months.48 The HFP program had a strong focus on diversified food production and improved nutrition practices; improving hygiene was limited to messages around handwashing and instructions to build handwashing stations. Therefore, an additional food hygiene module was added (implemented from June 2017 to February 2018) to strengthen household food hygiene practices, especially around food preparation and child feeding.
The food hygiene module was based on a Nepali intervention,45 adapted to the FAARM population, and integrated to fit the ongoing intervention activities and study setting. Using a behavior-centered design and emotional drivers, the module promoted four specific food hygiene behaviors: washing hands with soap and water, washing feeding utensils with soap and water, cooking fresh food or reheating leftover food before feeding, and storing food and water safely. This was done under the hypothesis that improved food hygiene practices would prevent or decrease complementary food contamination and thereby reduce the risk of undernutrition from intestinal infections and disease. A detailed description of the design, implementation, and uptake of the food hygiene module has been published.49
The FHEED substudy was designed to analyze the impact of the combined HFP and food hygiene intervention on household food hygiene practices, contamination of complementary foods, and intestinal infection and inflammation among children 0 to 18 months old at the trial’s endline assessment in 2019. All children enrolled in the FHEED substudy were thus born during or after delivery of the food hygiene module, ensuring they could benefit from that intervention throughout infancy. Supplemental Figure 1 depicts the timeline of the FAARM trial and FHEED substudy.
Sample size.
The main outcome variables were fecal biomarker concentrations of MPO, AAT, and NEO at endline. To detect intervention-related differences with a minimal standardized effect size of 0.3 for these biomarkers, a sample size of ∼300 children per study group (on average, six children per cluster) was deemed necessary (α = 0.05, β = 0.20). To achieve the required sample size, we included all children born between November 2017 and October 2019 in the endline assessment, which increased the age range of the final sample from 0 to 18 to ∼0 to 24 months.
Randomization and masking.
The randomization procedure is explained in detail in the FAARM study protocol.48 The nature of the intervention did not allow masking of the implementation team and participants in intervention settlements. A distance of at least 400 m between settlements was chosen to avoid spillover of intervention activities to control settlements. Data collectors and laboratory staff of the FHEED substudy were not engaged in any intervention activities and were not informed about settlement allocation. However, data collectors could have observed intervention materials during data and sample collection in the intervention settlements.
Data collection.
For this analysis, we used data from the following FAARM surveys: 1) demographic characteristics at baseline (March to May 2015), 2) data on poultry ownership from the routine assessment conducted as part of the FAARM surveillance system (May to September 2019), 3) characteristics and child health status from the FAARM endline survey (September 2019 to April 2020), 4) child health status during collection of stool samples at the FAARM endline survey (September to December 2019).
The FAARM baseline and endline surveys collected data on women and household characteristics such as education, age, religion, and household wealth, as well as on sanitation facilities of all households. Education was measured as the number of school years completed and was grouped into none, some or completed primary education, and some or completed secondary education. At baseline and endline, we calculated wealth quintiles using principal component analysis of household assets, in line with the Bangladesh Demographic and Health Survey.50 A handwashing station was considered functional when it was equipped with water, a cleaning agent, and a pouring device. Access to basic sanitation facilities was defined as the presence of a flush/pour flush toilet connected to a piped sewer system, septic tank, or pit latrine; a pit latrine with a slab (including a ventilated pit latrine); and a composting toilet, not shared with other households.51
During the surveillance system’s routine assessment in May to September 2019, data on household poultry ownership, including the reported number of poultry in a household, reported ownership of a poultry shed (traditional or improved), and observed use of the poultry shed (for keeping poultry or other) were collected as prespecified pathway indicators of the FAARM trial.
At the time of serum and fecal sample collection during the FAARM endline survey, data on child health were collected. Caregivers were asked whether their child was sick with loose or mushy stools within the past 7 days prior to the survey. If caregivers reported loose or mushy stools, they were asked for the number of stools passed on the worst day. A child was considered sick with diarrhea when a caregiver reported three or more loose, mushy stools on at least 1 day within the 7 days prior to the survey. We also collected information on disease severity, including caregivers’ report of blood in the stool or a fever. Questionnaires were administered by trained data collection officers (holding at least a bachelor’s degree) who conducted face-to-face interviews with the respondents. All survey data were collected using the tablet-based application Open Data Kit.52
Biological sample collection.
Fecal samples of participating children were collected by their caregivers at endline. Caregivers were provided with a sterile stool collection tube, gloves, and instructions on how to collect the stool sample from their children (infographic as well as verbal explanation and practical demonstration by the sample collector). If possible, mothers collected the first stool sample in the morning. The field team collected the stool samples from the households and stored them in a cool box with icepacks, monitoring the temperature in the cool box (8–10°C) at all times. If a child did not pass stool in the morning, the sample collector returned to the household later that day to collect the sample. Samples were brought to the field laboratory by noon, when they were aliquoted and stored without additives at −20°C until shipment to Germany, where they were stored at −80°C until further analysis.
Serum samples of participating children older than 6 months were collected by trained and experienced phlebotomists at endline. Approximately 3 mL of venous blood was drawn from the arm vein and collected into a serum tube (BD Vacutainer trace element serum tubes, BD, Franklin Lakes, NJ). Samples were stored in a cool box with icepacks and brought to the field laboratory within 5 hours after sample collection. For serum separation, samples were centrifuged for 15 minutes at 1,500 × g. Serum was aliquoted and stored at −20°C until further analysis.
Biomarker analyses.
Biomarkers of EED (MPO and NEO measuring intestinal inflammation, AAT measuring intestinal permeability) were measured in stool samples by ELISA. Commercially available ELISA kits for MPO (R&D Systems, Minneapolis, MN), AAT (R&D Systems, Minneapolis, MN), and NEO (IBL International, Hamburg, Germany) were used according to the manufacturer’s instructions. A detailed description of the assay procedures can be found in Supplemental Appendix 1. In brief, samples were homogenized in assay buffer (Ref. KENO751, IBL International) for 2 × 1 minute using a bead beater. Samples were centrifuged at 2,500 × g at 4°C to clear the samples. Supernatants were diluted 1:5,000 (MPO), 1:20,000 (AAT), and 1:100 (NEO) with the respective assay buffer. ELISAs were run in technical duplicates. Samples with out-of-range values were retested at an appropriate dilution.
Biomarkers of systemic inflammation (CRP as a marker for acute inflammation and AGP as a marker for chronic inflammation) were measured in serum samples using a combined sandwich ELISA technique by the VitMin Laboratory (Willstaett, Germany).53
Outcomes.
Prespecified outcomes were fecal concentrations of MPO, AAT, and NEO and serum concentrations of CRP and AGP in children younger than 2 years of age. For their use in regression analyses, biomarker concentrations were log-transformed to achieve a normal distribution. Additional outcomes of interest in this sample were 7-day period prevalence of caregiver-reported diarrhea in children and diarrhea severity.
Statistical analysis.
All data analyses were performed in Stata SE version 14.2 (StataCorp, College Station, TX). Biomarker concentrations were summarized as median and interquartile range or were log- transformed and summarized as mean and SE. In addition, we calculated Pearson correlations between concentrations of each biomarker. Other continuous variables were summarized using mean and SD, and categorical variables using proportions.
We used mixed-effects linear regression models to estimate the intervention effect on each log-transformed biomarker, with settlement-level random effects and adjustments for age and sex of the child to increase precision. Intervention effects by child age were calculated using an interaction term between intervention allocation and grouped child age (0–5 months, 6–11 months, 12–17 months, and 18–24 months ). Predicted biomarker concentrations were calculated using the margins command, and the lincom command was used to calculate point estimates and 95% CIs. Mixed-effects linear regression models also adjusting for baseline household wealth were run as sensitivity analyses, as household wealth was slightly imbalanced between the intervention and control groups. All analyses estimating the intervention effect on EED biomarkers were intention-to-treat.
We also conducted post hoc analyses to explore whether the intervention’s promotion of poultry may have increased EED biomarkers. To assess associations between poultry ownership and log-transformed biomarkers, we used mixed-effects linear regression models, with settlement-level random effects and adjusting for age and sex of the child 1) including only poultry variables as the exposure, 2) including intervention allocation as a covariate, and 3) including an interaction term between intervention allocation and poultry variables to calculate effects stratified by poultry ownership and poultry location, or including the settlement-level average number of poultry. The Stata command margins dydx was used to calculate marginal effects. A likelihood ratio test was used to compare models with and without the interaction term.
RESULTS
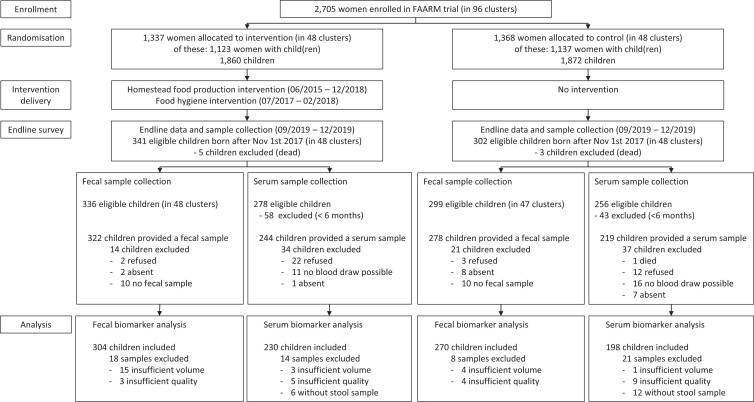
Our analysis includes data from 574 children 0 to 24 months old and their households, collected during the FAARM endline survey in 2019 (90% of the 635 children born between November 1, 2017 and October 31, 2019). Analyses of the serum biomarkers CRP and AGP were performed on a smaller subset of 428 children, as blood was collected only from children 6 months or older. Figure 1 provides details on how many children were included in the analysis of different biomarkers, as well as reasons for exclusion.
Figure 1.
Study flow diagram. FAARM = Food and Agricultural Approaches to Reducing Malnutrition.
Background characteristics of the intervention and control households were largely similar regarding household size, religion, and wealth, with a slight imbalance in that there were slightly fewer intervention households in the least-wealthy quintiles (Table 1). At endline in 2019, ∼60% of households had access to a functional handwashing facility, equipped with soap and water, and more than 50% had access to a basic sanitation facility that was not shared with another household. Approximately 85% of women had at least some primary education, and the index child was 13 months old on average on the day of stool collection (Table 1).
Table 1.
Characteristics of households, women, and children of the study population in rural Sylhet, Bangladesh
| Characteristic* | Overall, % or mean (SD) | Control, % or mean (SD) | Intervention, % or mean (SD) |
|---|---|---|---|
| Household characteristics | |||
| Intervention allocation | |||
| Control | 47 | – | – |
| Intervention | 53 | – | – |
| Religion | |||
| Muslim | 75 | 73 | 77 |
| Hindu | 25 | 27 | 23 |
| No. of household members† | 7.2 (3.4) | 7.3 (3.3) | 7.0 (3.4) |
| Wealth at baseline‡ | |||
| Poorest | 25 | 26 | 23 |
| Lower | 23 | 26 | 21 |
| Middle | 19 | 18 | 20 |
| Upper | 19 | 17 | 22 |
| Wealthiest | 14 | 13 | 14 |
| Wealth at endline§ | |||
| Poorest | 29 | 32 | 26 |
| Lower | 19 | 21 | 18 |
| Middle | 19 | 16 | 22 |
| Upper | 19 | 17 | 20 |
| Wealthiest | 14 | 14 | 14 |
| Access to a functional handwashing station at endlineǁ | 58 | 56 | 60 |
| Access to basic sanitation facilities at endline¶ | 57 | 56 | 57 |
| Women characteristics | |||
| Age at baseline, years | 22.8 (3.9) | 22.7 (3.8) | 23.0 (3.9) |
| Education# | |||
| None | 14 | 14 | 15 |
| Partial/compete primary | 47 | 48 | 46 |
| Partial secondary or more | 39 | 38 | 39 |
| Children characteristics | |||
| Age at endline stool collection survey, months | 12.9 (6.5) | 12.5 (6.3) | 13.2 (6.5) |
| Sex | |||
| Male | 54 | 55 | 54 |
| Female | 46 | 45 | 46 |
N = 574; n = 270 (control), n = 304 (intervention).
If not indicated otherwise, characteristics were collected during the baseline survey in 2015.
For number of household members, n = 546; missing data, n = 28.
For wealth at baseline, n = 562; missing data, n = 12.
For wealth at endline, functional handwashing station, and access to latrines, n = 570; missing data, n = 4.
A functional handwashing station is equipped with water, detergent, and a pouring device.
Access to basic sanitation facilities includes flush/pour flush toilets connected to a piped sewer system, septic tanks or pit latrines, pit latrines with slabs (including ventilated pit latrines), and composting toilets not shared with other households.
Partial/complete primary education consists of up to 5 years of schooling. Partial secondary or more education consists of up to 10 years of schooling or higher education.
The median values for biomarkers of enteric dysfunction were 23,233 ng/mL MPO, 422 μg/mL AAT, and 1,534 nmol/L NEO. Median values for biomarkers of systemic inflammation were 0.62 mg/L CRP and 0.77 g/L AGP (Table 2). MPO, NEO, and CRP showed a decreasing trend in concentration with increasing child age, whereas median AAT concentrations were slightly higher in girls (Table 2). Pairwise correlations between log MPO, log AAT, and log NEO were rather weak, with the greatest correlation between MPO and AAT (correlation coefficient r = 0.4). C-reactive protein and AGP showed a high correlation (r = 0.66). No correlation was observed between biomarkers of enteric dysfunction and systemic inflammation (Supplemental Table 1).
Table 2.
Summary measures of EED and systemic inflammation biomarker concentrations overall by child age and sex
| Variable | Biomarker in stool | Biomarker in serum | |||||
|---|---|---|---|---|---|---|---|
| n | MPO, ng/mL; median (IQR) | AAT, μg/mL; median (IQR) | NEO, nmol/L; median (IQR) | n | CRP, mg/L; median (IQR) | AGP, g/L; median (IQR) | |
| Overall | 574 | 23,233 (9,481–55,227) | 422 (233–720) | 1,534 (753–3,089) | 428 | 0.62 (0.14–2.42) | 0.77 (0.58–1.11) |
| Child age, months | |||||||
| 0–5 | 79 | 39,686 (21,105–92,967) | 455 (228–731) | 1,920 (1,265–3,412) | * | * | * |
| 6–11 | 179 | 28,691 (12,746–56,629) | 425 (232–707) | 2,064 (1,175–3,441) | 148 | 0.84 (0.17–2.86) | 0.84 (0.59–1.18) |
| 12–17 | 134 | 21,740 (8,194–44,635) | 398 (225–654) | 1,367 (701–2,718) | 119 | 0.61 (0.10–2.0) | 0.73 (0.58–1.09) |
| 18–24 | 182 | 17,170 (6,701–39,899) | 412 (246–747) | 922 (395–1,872) | 161 | 0.51 (0.09–2.02) | 0.75 (0.57–1.01) |
| Child sex | |||||||
| Male | 312 | 22,935 (8,841–53,546) | 399 (216–669) | 1,557 (795–3,126) | 230 | 0.69 (0.17–2.25) | 0.78 (0.59–1.08) |
| Female | 262 | 23,323 (10,472–57,474) | 454 (262–788) | 1,455 (717–2,956) | 198 | 0.55 (0.12–2.56) | 0.76 (0.57–1.14) |
AAT = alpha-1-antitrypsin; AGP = alpha-1-acid glycoprotein; CRP = C-reactive protein; EED = environmental enteric dysfunction; IQR = interquartile range; MPO = myeloperoxidase; NEO = neopterin.
Serum was not collected from children younger than 6 months of age.
Approximately 6% of mothers reported that their children had diarrhea within the past 7 days, and the prevalence did not differ between children in the intervention and control groups (Supplemental Table 2). Biomarker levels of EED were not elevated in children with reported diarrhea in the past 7 days; children with diarrhea even showed lower levels of MPO and AAT compared to children without diarrhea (Supplemental Table 3).
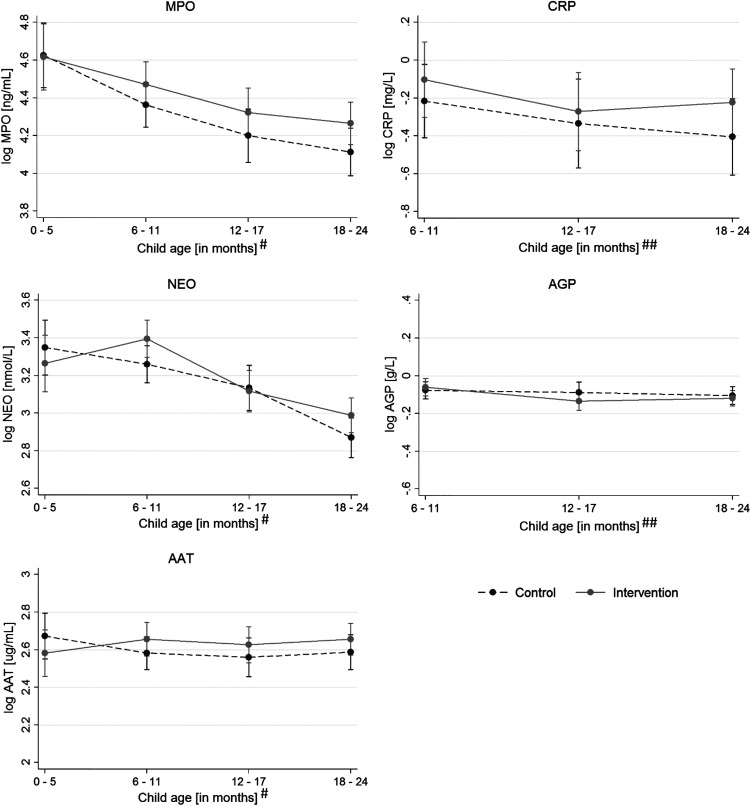
There was no effect of the intervention on AAT and NEO concentrations nor on CRP and AGP concentrations among children younger than 24 months of age using linear mixed-effect models adjusting for clustering, child age, and sex (Table 3). There was some evidence that the intervention led to an increase in MPO levels (0.11 log ng/mL; 95% CI, 0.001–0.22; P = 0.047; Table 3), with MPO concentrations in intervention children ∼11% greater than in control children. The increase in MPO concentration was dependent on child age. Although MPO concentrations were similar in children 0 to 5 months old, they diverged with increasing child age and showed the greatest difference in children 18 to 24 months of age (Figure 2 and Supplemental Table 4).
Table 3.
Effect of the intervention on of EED and systemic inflammation biomarker concentrations
| Inflammatory marker | n | Mean | Robust SE | Coefficient (95% CI)* | P value* |
|---|---|---|---|---|---|
| log MPO ng/mL | |||||
| Control | 270 | 4.28 | 0.044 | 0.12 (0.001 to 0.22) | 0.047 |
| Intervention | 304 | 4.38 | 0.038 | ||
| log AAT μg/mL | |||||
| Control | 270 | 2.58 | 0.033 | 0.05 (−0.04 to 0.14) | 0.29 |
| Intervention | 304 | 2.63 | 0.033 | ||
| log NEO nmol/L | |||||
| Control | 270 | 3.13 | 0.030 | 0.06 (−0.02 to 0.15) | 0.13 |
| Intervention | 304 | 3.18 | 0.032 | ||
| log CRP mg/L# | |||||
| Control | 198 | −0.31 | 0.069 | 0.13 (−0.04 to 0.29) | 0.13 |
| Intervention | 230 | −0.20 | 0.052 | ||
| log AGP g/L# | |||||
| Control | 198 | −0.09 | 0.017 | −0.01 (−0.05 to 0.03) | 0.55 |
| Intervention | 230 | −0.11 | 0.013 | ||
AAT = alpha-1-antitrypsin; AGP = alpha-1-acid glycoprotein; CRP = C-reactive protein; EED = environmental enteric dysfunction; MPO = myeloperoxidase; NEO = neopterin.
All children younger than 6 months on the day of blood collection were excluded from sampling because they did not provide a serum sample.
From multilevel regression models, adjusted for child age and sex, with settlement random effects.
Figure 2.
Concentration of biomarkers for environmental enteric dysfunction (EED) and systemic inflammation by intervention group and child age. The graphs show predicted concentrations and 95% CIs using marginal standardization from multilevel regression models, adjusted for child sex, with settlement random effects (n = 574 for log myeloperoxidase [MPO], log neopterin [NEO], log alpha-1-antitrypsin [AAT]; n = 428 for log C-reactive protein [CRP] and log alpha-1-acid glycoprotein [AGP]). #Child age at the time of stool sample collection. ##Child age at the time of blood sample collection. Light-gray solid lines indicate intervention; dark-gray dashed lines indicate control.
As the FHEED study was originally designed to investigate the effect of the food hygiene and HFP intervention on children 0 to 18 months old, additional regression models were run, including only children younger than 18 months. For this subgroup, the intervention had no effect on any of the inflammatory biomarkers, including MPO (Supplemental Table 5).
Given the slight imbalance in household wealth at baseline between study groups, we also assessed the intervention effects on each EED biomarker while adjusting not only for age and sex of the child, but also for baseline wealth as a sensitivity analysis. We found no major differences in the effect estimates compared with the models not adjusting for baseline wealth (Supplemental Table 5).
Among other activities, the HFP intervention promoted small-scale poultry rearing in the intervention households. Accordingly, in 2019, in the intervention arm, more children were in households that owned poultry (62% versus 56%, intervention versus control), and among poultry owners, intervention households had a slightly greater number of poultry (3.9 versus 3.3). The average number of poultry per settlement was also slightly greater in the intervention settlements compared with control settlements (2.9 versus 2.0). More intervention households owned a modern poultry shed (56% versus 0.4%) and kept poultry in a shed for at least some time during the day or night (34% versus 4%, Supplemental Table 6).
As previous studies showed an association between poultry ownership and increased EED biomarkers, enteric infections, and diarrheal disease,28,54,55 we investigated the association of MPO and poultry ownership in a post hoc analysis as a potential explanation for the increase in MPO levels in children’s stool samples. Overall, there was rather weak evidence that MPO levels were increased by poultry ownership (0.07 log ng/mL; 95% CI, −0.02 to 0.16; P = 0.11) or not keeping poultry in a shed (versus no poultry: 0.09 log ng/mL; 95% CI, −0.009 to 0.19; P = 0.07). Poultry tend to roam; therefore, we examined not only household poultry ownership, but also the average number of poultry at the settlement level.56 There was also weak evidence for increased MPO concentrations with a greater average number of poultry at the settlement level (0.04 log ng/mL; 95% CI, −0.003 to 0.08; P = 0.07; Supplemental Table 7).
Evidence for an association between poultry location and MPO levels was slightly stronger when accounting for intervention allocation as a covariate (poultry not in shed versus no poultry: 0.1 log ng/mL MPO; 95% CI, 0.002–0.20, P = 0.05). When stratifying the intervention effect on MPO by poultry ownership or poultry location, using an interaction term, we found no effect on MPO concentrations among intervention households without poultry, weak evidence for an increase in MPO among intervention households owning poultry (0.13 log ng/mL; 95% CI, −0.006 to 0.26; P = 0.06), and some evidence for an increase among intervention households not keeping poultry in a shed (versus no poultry: 0.2 log ng/mL; 95% CI, 0.05–0.36; P = 0.01); however, there was no evidence of an interaction of these subgroups with the intervention (Supplemental Table 8).
DISCUSSION
Our results suggest that a combined nutrition and food hygiene intervention integrated in an HFP program in rural Bangladesh had no beneficial impact on biomarkers of EED and systemic inflammation in children younger than 24 months of age. There was no evidence of an intervention effect on concentrations of AAT, NEO, CRP, and AGP. For MPO, we found weak evidence for an adverse effect of the intervention, possibly on older children of the study population, and in households that did not keep poultry in a shed.
Approximately 15% of study children had increased levels of CRP and 30% increased levels of AGP (> 5 mg /L and > 1 g/L, respectively; the defined cutoff values indicative of inflammation57), indicating ongoing systemic immune activation in a substantial proportion of children in our study population. There are no agreed-upon reference values for MPO, AAT, and NEO concentrations in children of this age group. The median concentrations for AAT and NEO measured in our study were comparable to concentrations reported in the MAL-ED study17 and WASH Benefits trial33 in Bangladesh. However, our MPO concentrations were greater and more comparable to concentrations reported from MAL-ED sites in South Africa or Tanzania.17 As described in previous studies,33,34,58 we also observed a decrease in NEO and MPO concentrations with increasing child age.
Similar to our study, two previous large-scale cluster-randomized trials that evaluated combined nutrition and WASH interventions on EED biomarkers in children failed to show clear beneficial effects.33,34 The SHINE trial34 in Zimbabwe, assessing the effect of a combined infant and young child feeding (IYCF) and WASH intervention on biomarkers of EED in children 1, 3, 6, 12, and 18 months old observed a slight decrease in NEO concentrations in children of 18 months in the IYCF group compared with the non-IYCF group. There was also a small decrease in kynurenine in children 12 months old and an increase in insulin-like growth factor 1 in children 18 months of age in the WASH group compared with the non-WASH group, but no effects on other EED biomarkers.34 The WASH Benefits trial in Bangladesh,33 which evaluated a WASH intervention with or without nutrition supplementation, observed lower concentrations for some biomarkers of intestinal permeability (lactulose and mannitol) and inflammation (NEO) in children 3 and 14 months in the intervention arms; however, by the age of 28 months, they showed higher biomarker levels of intestinal inflammation (MPO) than controls.33 A matched cohort study25 in India that assessed the effect of a household-level water and sanitation infrastructure intervention observed a decrease in intestinal permeability (AAT) but not intestinal inflammation in children from intervention households compared with control households. Similarly, a substudy59 of the WASH for WORMS cluster-randomized trial, evaluating a community-wide WASH intervention in Timor-Leste, reported slightly lower MPO concentrations in children 1 to 5 years of age from intervention households, but no effect on other biomarkers that were measured.
In contrast to the previously mentioned studies, the FAARM trial focused on skill building and only supplied assets for HFP. The additional food hygiene intervention did not provide any infrastructure, but rather concentrated on changing caregivers’ household food hygiene practices through social and behavior change techniques. Therefore, the success of the intervention relied mainly on faithful implementation of the intervention curriculum, high uptake of intervention messages, and consistent practice of the promoted behaviors by the women and households. According to our theory of change, the two possible pathways through which the intervention could have affected EED positively are through its food hygiene component and through improved consumption of nutrient-rich foods.
An effect through nutrient-rich food consumption could be expected, given that the HFP intervention had a rich training curriculum on diversified food production and improved nutrition practices. Attendance in the HFP training sessions was 80% on average, and pathway indicators collected through the program monitoring system show improvement for intervention households. Women from intervention households breastfed their children exclusively for approximately half a month longer, and children’s dietary diversity increased by 0.3 food groups (Waid et al., under review). Despite the improvement in diets, we did not see a decrease in EED biomarkers, which is in contrast to two previous studies14,31 that found some evidence for a decrease in EED biomarkers with increased nutrient intake from complementary foods.
Regarding the impact pathway through food hygiene, the rather light hygiene component from the HFP program had been enhanced by an additional dedicated food hygiene module. Attendance and participation in the food hygiene training sessions was high (87% on average),49 and knowledge of promoted food hygiene behaviors was widespread (Huda et al., manuscript in preparation). We also saw improvements of several food hygiene behaviors, including using clean feeding utensils, preparing food fresh or reheating stored food before feeding, and washing hands before food preparation and child feeding. However, handwashing was rare overall, and most behaviors were practiced inconsistently (Sobhan et al., under review). This indicates an attenuation of the intervention effect along the causal pathway from participation via knowledge to practice in a “funnel of attrition.”60 The observed improvements in food hygiene practice were not sufficient to reduce complementary food contamination (Huda et al., manuscript in preparation), which makes an impact on EED through the food hygiene pathway rather unlikely. This is in line with the lack of impact we found in our study, and with the observed null effect of the intervention on diarrhea prevalence.61
Young children in resource-poor settings are likely to come into contact with fecal pathogens in their home environment from a range of sources. Besides pathogen exposure through poor sanitation infrastructure or contaminated foods and water, frequent contact with animals, or unknowingly touching and ingesting feces while crawling or playing might present an equally important source of pathogen contact.27,28,30,62 Interventions such as WASH (or other hygiene interventions), which target only one or a few pathways of exposure to fecal contamination, might thus not be able to reduce the level of exposure sufficiently to translate into a beneficial health outcome (such as reduction of EED or diarrhea). Therefore, comprehensive WASH interventions, including profound, long-lasting changes in the physical household environment that allow a drastic reduction of fecal contamination, might be needed to achieve health impacts.63–65
Although the intervention did not affect most EED biomarkers, we found increased levels of MPO. The difference in MPO increased with child age and was most pronounced in children 18 to 24 months of age. Interestingly, the WASH Benefits trial33 in Bangladesh observed a similar effect in 28-month-old children. When thinking about possible reasons for this potentially harmful effect, greater mobility of children with increasing age could play a role in that it leads to greater pathogen exposure from the environment—if the intervention increases pathogen loads. As our intervention increased poultry numbers somewhat, both at the household and settlement levels (household: 3.9 birds versus 3.3 in controls (among poultry-owning households); settlement-level average: 2.9 birds versus 2.0 in controls), and handwashing practices are very poor in this population, this may be a possible mechanism for the increase in MPO levels. Although our exploratory analysis found only weak evidence linking higher average poultry numbers at the settlement level to higher MPO levels, we found that exposure to poultry in the household, if not kept in a shed, was associated with higher MPO levels, and that any harmful intervention effect on MPO was in this subgroup, but without any evidence for a statistical interaction.
The HFP intervention promoted small-scale poultry rearing in combination with good poultry management techniques, including improved poultry sheds. In 2019, approximately two thirds of intervention households that kept poultry owned an improved poultry shed, and more than half of the intervention households with poultry used a shed for keeping them (compared with less than 10% in controls). A study in Peru66 found that an intervention to promote poultry corralling, surprisingly, increased the risk of Campylobacter infection—a pathogen that has been associated with EED.67 However, in our study households, greater shed use in the intervention group seemed to have had a protective effect, possibly counteracting any negative effects from the moderate increase in poultry numbers. Poultry in our study were kept in the shed primarily overnight and roamed freely during the day in almost all households. In households without a shed, poultry were mostly kept inside the house overnight, in the kitchen or sleeping area. Intervention households that did not opt to build a shed, or did not keep their poultry in the shed, may thus have had increased exposure to poultry inside the house overnight; however, this exposure was also true for controls and to an even greater extent. We are thus not sure which feature of our poultry intervention, if any, may have contributed to potentially increased intestinal inflammation in the intervention children. Although the evidence is not very strong, we feel it is important to highlight this potential harm that might originate from poultry. Previous research in Ethiopia68 found that keeping poultry inside the house overnight decreased children’s height-for-age z-score, likely because of an increased risk of infection. Also, other studies28,55,69,70 provide evidence that exposure to poultry or other livestock in the household setting is associated with enteric infections, diarrheal disease, and EED in children. Unfortunately, the FHEED study was not designed to look at these associations in more detail. Future studies are needed to understand more fully the risk of poultry interventions for intestinal health as well as safety measures to reduce potential risk factors.
Our study has several strengths and limitations. Its cluster-randomized design, the use of covariate-constrained randomization, and its relatively large number of clusters helped to balance observed and unobserved characteristics that could otherwise confound the results. Moreover, the high completion of ∼90% of the attempted sample minimizes the risk of a selection bias. In addition, the rich data collected during the course of the FAARM trial as part of the surveillance system enabled us to explore poultry as a potential cause of increased MPO levels, thereby aiding the interpretation of study findings, despite not being the focus of this study. However, the study also has limitations. Because of financial constraints, we did not analyze EED biomarkers over time for all children included in the FHEED study, but only once at endline, which did not allow us to investigate changes in EED over time. In addition, we did not analyze enteropathogens for all FHEED children, which could have potentially provided an explanation for the increased MPO levels found. However, longitudinal data on EED biomarkers and enteropathogens has been analyzed in a smaller subset of children, and will be reported in a forthcoming article. The chosen age cutoff of 24 months for the FHEED study excludes the older FAARM children, who may have also shown an increase in MPO levels. This limited our sample size for examining the association between poultry and intestinal inflammation, or other potential contributors to increased MPO levels, in more detail. Finally, our analyses did not address intervention fidelity and adoption of intervention messages, and how these influenced the overall results, leaving these aspects to be addressed quantitatively in future work.
In conclusion, our results suggest that a food hygiene intervention integrated in an HFP program did not succeed in reducing biomarkers of EED and systemic inflammation among young children in rural Bangladesh. Surprisingly, children of intervention households showed increased levels of MPO compared with control households, which may be because of greater poultry ownership in the intervention group, particularly when poultry were not kept in a shed. Although data were collected only from two rural subdistricts in the Sylhet Division, the household environment resembles typical rural areas in Bangladesh. It is thus likely that our results are relevant for regions with similar demographic characteristics across the country, and other countries in South Asia. Future nutrition and hygiene interventions might need to incorporate more comprehensive WASH components, with profound, long-lasting changes in the household and community environment to achieve an impact on health outcomes. A better understanding of the potential benefits and risks of complex interventions is also needed to increase knowledge of potential risk factors associated with adopted intervention practices (such as increased poultry production), to identify potential mitigation strategies, and to tailor intervention components to individual contexts.
Supplemental Materials
ACKNOWLEDGMENTS
We thank the FAARM households for their valuable time and participation. We also thank Dr. Jesmin Sultana and Dr. Wolf-Peter Schmidt for their valuable feedback and support in the design of the FHEED study, and Sushobhan Sarker for his support in the preparation of data collection, biosampling, training, and procurement. We thank our collaboration partners: Helen Keller International and icddr,b in Bangladesh. We are grateful to the survey staff and supervisors, especially to Saheen Hossein, for their time and effort in sample and data collection; and to Abdul Kader for his support in questionnaire adaptation and training. Furthermore, we thank the Medical Microbiology Laboratory staff, Heidelberg University Hospital, especially Dr. Dennis Nurjadi, Aline Sähr, and Sandra Förmer for their kind support. The authors confirm that all ongoing and related trials for this intervention are registered (#NCT02505711). This trial is registered at ClinicalTrials.gov (#NCT02505711, https://clinicaltrials.gov/ct2/show/NCT02505711).
Note: Supplemental material appears at www.ajtmh.org.
REFERENCES
- 1. UNICEF, World Health Organization, International Bank for Reconstruction and Development/The World Bank , 2021. Levels and Trends in Child Malnutrition: Key Findings of the 2021 Edition of the Joint Child Malnutrition Estimates. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2. Dewey KG, Begum K, 2011. Long-term consequences of stunting in early life. Matern Child Nutr 7 ( Suppl 3 ): 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lunn PG, Northrop-Clewes CA, Downes RM, 1991. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 338: 907–910. [DOI] [PubMed] [Google Scholar]
- 4. Campbell DI, Elia M, Lunn PG, 2003. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr 133: 1332–1338. [DOI] [PubMed] [Google Scholar]
- 5. Humphrey JH, 2009. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 374: 1032–1035. [DOI] [PubMed] [Google Scholar]
- 6. Cook GC, Kajubi SK, Lee FD, 1969. Jejunal morphology of the African in Uganda. J Pathol 98: 157–169. [DOI] [PubMed] [Google Scholar]
- 7. Kelly P. et al. , 2004. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am J Trop Med Hyg 70: 412–419. [PubMed] [Google Scholar]
- 8. Syed S, Ali A, Duggan C, 2016. Environmental enteric dysfunction in children. J Pediatr Gastroenterol Nutr 63: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu TC. et al. , 2020. A novel histological index for evaluation of environmental enteric dysfunction identifies geographic-specific features of enteropathy among children with suboptimal growth. PLoS Negl Trop Dis 14: e0007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell DI, Murch SH, Elia M, Sullivan PB, Sanyang MS, Jobarteh B, Lunn PG, 2003. Chronic T cell-mediated enteropathy in rural West African children: relationship with nutritional status and small bowel function. Pediatr Res 54: 306–311. [DOI] [PubMed] [Google Scholar]
- 11. Prendergast A, Kelly P, 2012. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg 86: 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu J, Ordiz MI, Stauber J, Shaikh N, Trehan I, Barnell E, Head RD, Maleta K, Tarr PI, Manary MJ, 2016. Environmental enteric dysfunction includes a broad spectrum of inflammatory responses and epithelial repair processes. Cell Mol Gastroenterol Hepatol 2: 158–174.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhoades NS, Hendrickson SM, Prongay K, Haertel A, Gill L, Edwards RA, Garzel L, Slifka MK, Messaoudi I, 2021. Growth faltering regardless of chronic diarrhea is associated with mucosal immune dysfunction and microbial dysbiosis in the gut lumen. Mucosal Immunol 14: 1113–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCormick BJJ. et al. , 2019. Intestinal permeability and inflammation mediate the association between nutrient density of complementary foods and biochemical measures of micronutrient status in young children: results from the MAL-ED study. Am J Clin Nutr 110: 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harper KM, Mutasa M, Prendergast AJ, Humphrey J, Manges AR, 2018. Environmental enteric dysfunction pathways and child stunting: a systematic review. PLoS Negl Trop Dis 12: e0006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peterson KM. et al. , 2013. REG1B as a predictor of childhood stunting in Bangladesh and Peru. Am J Clin Nutr 97: 1129–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kosek M. et al. , 2013. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg 88: 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arndt MB, Richardson BA, Ahmed T, Mahfuz M, Haque R, John-Stewart GC, Denno DM, Petri WA, Jr., Kosek M, Walson JL; in coordination with the MAL-ED Network Project , 2016. Fecal markers of environmental enteropathy and subsequent growth in Bangladeshi children. Am J Trop Med Hyg 95: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guerrant RL. et al. , 2016. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in Northeast Brazil. PLoS One 11: e0158772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kosek MN. et al. , 2016. Plasma tryptophan and the kynurenine-tryptophan ratio are associated with the acquisition of statural growth deficits and oral vaccine underperformance in populations with environmental enteropathy. Am J Trop Med Hyg 95: 928–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prata MM, Havt A, Bolick DT, Pinkerton R, Lima A, Guerrant RL, 2016. Comparisons between myeloperoxidase, lactoferrin, calprotectin and lipocalin-2, as fecal biomarkers of intestinal inflammation in malnourished children. J Transl Sci 2: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kosek MN, MAL-ED Network Investigators , 2017. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. EBioMedicine 18: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iqbal NT. et al. , 2018. Promising biomarkers of environmental enteric dysfunction: a prospective cohort study in Pakistani children. Sci Rep 8: 2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richard SA. et al. , 2019. Enteric dysfunction and other factors associated with attained size at 5 years: MAL-ED birth cohort study findings. Am J Clin Nutr 110: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sinharoy SS, Reese HE, Praharaj I, Chang HH, Clasen T, 2021. Effects of a combined water and sanitation intervention on biomarkers of child environmental enteric dysfunction and associations with height-for-age z-score: a matched cohort study in rural Odisha, India. PLoS Negl Trop Dis 15: e0009198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin A. et al. , 2013. Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. Am J Trop Med Hyg 89: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. George CM. et al. , 2015. Geophagy is associated with environmental enteropathy and stunting in children in rural Bangladesh. Am J Trop Med Hyg 92: 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. George CM. et al. , 2015. Fecal markers of environmental enteropathy are associated with animal exposure and caregiver hygiene in Bangladesh. Am J Trop Med Hyg 93: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. George CM. et al. , 2016. Unsafe child feces disposal is associated with environmental enteropathy and impaired growth. J Pediatr 176: 43–49. [DOI] [PubMed] [Google Scholar]
- 30. Lauer JM, Duggan CP, Ausman LM, Griffiths JK, Webb P, Bashaasha B, Agaba E, Turyashemererwa FM, Ghosh S, 2018. Unsafe drinking water is associated with environmental enteric dysfunction and poor growth outcomes in young children in rural southwestern Uganda. Am J Trop Med Hyg 99: 1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morseth MS, Strand TA, Torheim LE, Chandyo RK, Ulak M, Shrestha SK, Shrestha B, Henjum S, 2018. Nutrient intake and environmental enteric dysfunction among Nepalese children 9–24 months old: the MAL-ED birth cohort study. Pediatr Res 84: 509–515. [DOI] [PubMed] [Google Scholar]
- 32. Ziegler TR, Evans ME, Fernández-Estívariz C, Jones DP, 2003. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr 23: 229–261. [DOI] [PubMed] [Google Scholar]
- 33. Lin A. et al. , 2020. Effects of water, sanitation, handwashing, and nutritional interventions on environmental enteric dysfunction in young children: a cluster-randomized, controlled trial in rural Bangladesh. Clin Infect Dis 70: 738–747. [DOI] [PubMed] [Google Scholar]
- 34. Gough EK. et al. , 2020. Effects of improved water, sanitation, and hygiene and improved complementary feeding on environmental enteric dysfunction in children in rural Zimbabwe: a cluster-randomized controlled trial. PLoS Negl Trop Dis 14: e0007963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Islam MA, Ahmed T, Faruque AS, Rahman S, Das SK, Ahmed D, Fattori V, Clarke R, Endtz HP, Cravioto A, 2012. Microbiological quality of complementary foods and its association with diarrhoeal morbidity and nutritional status of Bangladeshi children. Eur J Clin Nutr 66: 1242–1246. [DOI] [PubMed] [Google Scholar]
- 36. Kirk MD, Angulo FJ, Havelaar AH, Black RE, 2017. Diarrhoeal disease in children due to contaminated food. Bull World Health Organ 95: 233–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kung’u JK, Boor KJ, Ame SM, Ali NS, Jackson AE, Stoltzfus RJ, 2009. Bacterial populations in complementary foods and drinking-water in households with children aged 10–15 months in Zanzibar, Tanzania. J Health Popul Nutr 27: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Motarjemi Y, Käferstein F, Moy G, Quevedo F, 1993. Contaminated weaning food: a major risk factor for diarrhoea and associated malnutrition. Bull World Health Organ 71: 79–92. [PMC free article] [PubMed] [Google Scholar]
- 39. Parvez SM, Kwong L, Rahman MJ, Ercumen A, Pickering AJ, Ghosh PK, Rahman MZ, Das KK, Luby SP, Unicomb L, 2017. Escherichia coli contamination of child complementary foods and association with domestic hygiene in rural Bangladesh. Trop Med Int Health 22: 547–557. [DOI] [PubMed] [Google Scholar]
- 40. Ehiri JE, Azubuike MC, Ubbaonu CN, Anyanwu EC, Ibe KM, Ogbonna MO, 2001. Critical control points of complementary food preparation and handling in eastern Nigeria. Bull World Health Organ 79: 423–433. [PMC free article] [PubMed] [Google Scholar]
- 41. Islam MS. et al. , 2013. Hygiene intervention reduces contamination of weaning food in Bangladesh. Trop Med Int Health 18: 250–258. [DOI] [PubMed] [Google Scholar]
- 42. Chidziwisano K, Tilley E, Malolo R, Kumwenda S, Musaya J, Morse T, 2019. Risk factors associated with feeding children under 2 years in rural Malawi: a formative study. Int J Environ Res Public Health 16: 2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Touré O, Coulibaly S, Arby A, Maiga F, Cairncross S, 2011. Improving microbiological food safety in peri-urban Mali: an experimental study. Food Control 22: 1565–1572. [Google Scholar]
- 44. Muller-Hauser AA, Sobhan S, Huda TMN, Waid JL, Wendt AS, Islam MA, Rahman M, Gabrysch S, 2022. Key food hygiene behaviors to reduce microbial contamination of complementary foods in rural Bangladesh. Am J Trop Med Hyg 107: 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gautam OP, Schmidt WP, Cairncross S, Cavill S, Curtis V, 2017. Trial of a novel intervention to improve multiple food hygiene behaviors in Nepal. Am J Trop Med Hyg 96: 1415–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Manaseki-Holland S. et al. , 2021. Effects on childhood infections of promoting safe and hygienic complementary-food handling practices through a community-based programme: a cluster randomised controlled trial in a rural area of The Gambia. PLoS Med 18: e1003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chidziwisano K, Slekiene J, Mosler HJ, Morse T, 2020. Improving complementary food hygiene behaviors using the risk, attitude, norms, ability, and self-regulation approach in rural Malawi. Am J Trop Med Hyg 102: 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wendt AS, Sparling TM, Waid JL, Mueller AA, Gabrysch S, 2019. Food and Agricultural Approaches to Reducing Malnutrition (FAARM): protocol for a cluster-randomised controlled trial to evaluate the impact of a homestead food production programme on undernutrition in rural Bangladesh. BMJ Open 9: e031037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sobhan S, Muller-Hauser AA, Huda TMN, Waid JL, Gautam OP, Gon G, Wendt AS, Gabrysch S, 2022. Design, delivery, and determinants of uptake: findings from a food hygiene behavior change intervention in rural Bangladesh. BMC Public Health 22: 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. National Institute of Population Research and Training (NIPORT), Mitra and Associates, and ICF International 2016. Bangladesh Demographic and Health Survey 2014. Dhaka, Bangladesh, and Rockville, Maryland, USA: NIPORT, Mitra and Associates, and ICF International. [Google Scholar]
- 51. World Health Organization, UNICEF , 2017. JMP Methodology: 2017 Update & SDG Baselines. Geneva, Switzerland: World Health Organization.
- 52. Hartung C, Lerer A, Anokwa Y, Tseng C, Brunette W, Borriello G, 2010. Open data kit: tools to build information services for developing regions. Proceedings of the 4th ACM/IEEE International Conference on Information and Communication Technologies and Development, December, 2010, London, UK.
- 53. Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE, 2004. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr 134: 3127–3132. [DOI] [PubMed] [Google Scholar]
- 54. Zambrano LD, Levy K, Menezes NP, Freeman MC, 2014. Human diarrhea infections associated with domestic animal husbandry: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg 108: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vasco K, Graham JP, Trueba G, 2016. Detection of zoonotic enteropathogens in children and domestic animals in a semirural community in Ecuador. Appl Environ Microbiol 82: 4218–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hedman HD. et al. , 2020. Spatial exposure of agricultural antimicrobial resistance in relation to free-ranging domestic chicken movement patterns among agricultural communities in Ecuador. Am J Trop Med Hyg 103: 1803–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Raiten DJ, Ashour FAS, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev PS, van Ommen B, INSPIRE Consultative Group , 2015. Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J Nutr 145: 1039S–1108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McCormick BJJ. et al. , 2017. Dynamics and trends in fecal biomarkers of gut function in children from 1–24 months in the MAL-ED study. Am J Trop Med Hyg 96: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vaz Nery S, Bennett I, Clarke NE, Lin A, Rahman Z, Rahman M, Clements ACA, 2018. Characterisation of environmental enteropathy biomarkers and associated risk factors in children in the context of a WASH trial in Timor-Leste. Int J Hyg Environ Health 221: 901–906. [DOI] [PubMed] [Google Scholar]
- 60. White H, Raitzer DA, 2017. Impact Evaluation of Development Interventions: A Practical Guide. Mandaluyong City, Philippines: Asian Development Bank. [Google Scholar]
- 61. Lambrecht NJ, Müller-Hauser AA, Sobhan S, Schmidt W-P, Huda TMN, Waid JL, Wendt AS, Kader A, Gabrysch S, 2023. Effect of a Homestead Food Production Program on the Prevalence of Diarrhea and Acute Respiratory Infection in Children in Sylhet, Bangladesh: A Cluster-Randomized Controlled Trial. Am J Trop Med Hyg 109: 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ercumen A. et al. , 2017. Animal feces contribute to domestic fecal contamination: evidence from E. coli measured in water, hands, food, flies, and soil in Bangladesh. Environ Sci Technol 51: 8725–8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vila-Guilera J, Parikh P, Chaturvedi H, Ciric L, Lakhanpaul M, 2021. Towards transformative WASH: an integrated case study exploring environmental, sociocultural, economic and institutional risk factors contributing to infant enteric infections in rural tribal India. BMC Public Health 21: 1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cumming O. et al. , 2019. The implications of three major new trials for the effect of water, sanitation and hygiene on childhood diarrhea and stunting: a consensus statement. BMC Med 17: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pickering AJ. et al. , 2019. The WASH Benefits and SHINE trials: interpretation of WASH intervention effects on linear growth and diarrhoea. Lancet Glob Health 7: e1139–e1146. [DOI] [PubMed] [Google Scholar]
- 66. Harvey SA, Winch PJ, Leontsini E, Torres Gayoso C, López Romero S, Gilman RH, Oberhelman RA, 2003. Domestic poultry-raising practices in a Peruvian shantytown: implications for control of Campylobacter jejuni–associated diarrhea. Acta Trop 86: 41–54. [DOI] [PubMed] [Google Scholar]
- 67. Amour C. et al. , 2016. Epidemiology and impact of Campylobacter infection in children in 8 low-resource settings: results from the MAL-ED study. Clin Infect Dis 63: 1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Headey D, Hirvonen K, 2016. Is exposure to poultry harmful to child nutrition? An observational analysis for rural Ethiopia. PLoS One 11: e0160590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ercumen A, Prottas C, Harris A, Dioguardi A, Dowd G, Guiteras R, 2020. Poultry ownership associated with increased risk of child diarrhea: cross-sectional evidence from Uganda. Am J Trop Med Hyg 102: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lambrecht NJ, Wilson ML, Bridges D, Eisenberg JNS, Adu B, Baylin A, Folson G, Jones AD, 2021. Ruminant-related risk factors are associated with Shiga toxin–producing Escherichia coli infection in children in southern Ghana. Am J Trop Med Hyg 106: 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.