ABSTRACT.
Artemisinin-combined treatments are the recommended first-line treatment of Plasmodium falciparum malaria, but they are being threatened by emerging artemisinin resistance. Mutations in pfk13 are the principal molecular marker for artemisinin resistance. This study characterizes the presence of mutations in pfk13 in P. falciparum in Western Equatoria State, South Sudan. We analyzed 468 samples from patients with symptomatic malaria and found 15 mutations (8 nonsynonymous and 7 synonymous). Each mutation appeared only once, and none were validated or candidate markers of artemisinin resistance. However, some mutations were in the same or following position of validated and candidate resistance markers, suggesting instability of the gene that could lead to resistance. The R561L nonsynonymous mutation was found in the same position as the R561H validated mutation. Moreover, the A578S mutation, which is widespread in Africa, was also reported in this study. We found a high diversity of other pfk13 mutations in low frequency. Therefore, routine molecular surveillance of resistance markers is highly recommended to promptly detect the emergence of resistance-related mutations and to limit their spread.
INTRODUCTION
Malaria is a serious disease that continues to pose a global health challenge, particularly in Africa. In 2021, there were an estimated 247 million malaria cases globally, of which 95% were located in African countries.1 One of the key malaria control strategies in endemic areas is artemisinin-based combination therapy (ACT), which includes an artemisinin derivative with another antimalarial drug. Artemisinin-based combination therapy is adopted worldwide as the first-line treatment against uncomplicated falciparum malaria.1 It has demonstrated good efficacy even against parasites resistant to other antimalarials.2
Artemisinin-based combination therapy has successfully contributed to the reduction of the global burden of malaria; however, the emergence and spread of artemisinin resistance threatens its efficacy.1 Artemisinin partial resistance, characterized by delayed parasite clearance after treatment, is widespread in the Greater Mekong subregion in Southeast Asia, and it has been generally absent in Africa.3 However, the WHO recently confirmed the presence of artemisinin partial resistance in Rwanda, Uganda, and Eritrea.4,5
Artemisinin partial resistance has been associated with mutations in the propeller domain of the gene Pfk13, encoding the Kelch 13 (K13) protein, which are associated with resistant phenotypes in vitro and in vivo.6 So far, the WHO has recognized 13 pfk13 nonsynonymous mutations (i.e., resulting in a change of amino acid) as validated molecular markers of resistance and 9 as candidate markers of resistance.3 These mutations are located in the BTB/POZ domain and the C-terminal six-blade propeller regions of the pfK13 protein.6
The majority of the pfK13 mutations that have been identified in Africa are not validated resistance markers.7–9 However, some of the pfK13 validated mutations and their clonal expansion have been reported recently in Plasmodium falciparum parasites in Africa.10 These mutations have been found in different frequencies in Rwanda,11 Tanzania,12 Uganda,13 and Ethiopia14 (the latter two sharing a border with South Sudan). Although Rwanda and Uganda have both confirmed the presence of partial artemisinin resistance, ACTs continue to be effective, and treatment failure rates remain below 10% in these countries.10–15 The WHO African Region has declared surveillance of pfk13 polymorphisms a priority for malaria control.1
Malaria is a major public health problem in South Sudan, where it is the leading cause of illness and death.16 Over 93% of malaria cases in the country are caused by P. falciparum,16 and malaria incidence and mortality rates are on the rise.1 The government health authorities adopted artesunate-amodiaquine as the first-line antimalarial treatment in 2006.17
Given increasing malaria incidence and mortality in South Sudan and the emergence of artemisinin resistance in other countries in east Africa, studies on molecular markers are highly recommended in this country.1
We aimed to investigate the presence of pfk13 gene mutations related to artemisinin resistance in the malaria-endemic region of Yambio County (Western Equatoria State, South Sudan) 14 years after the introduction of ACT in the country.
MATERIALS AND METHODS
Study area and biological samples.
In this retrospective analysis, we assessed samples that had been collected for a study led by Médecins Sans Frontières in collaboration with the South Sudanese Ministry of Health to investigate molecular markers of antimalarial drug resistance after implementation of a seasonal malaria chemoprophylaxis campaign in Western Equatoria State, South Sudan. The study was conducted in a rural, malaria-endemic area in Yambio County at the end of the malaria peak season (from January to February 2019).
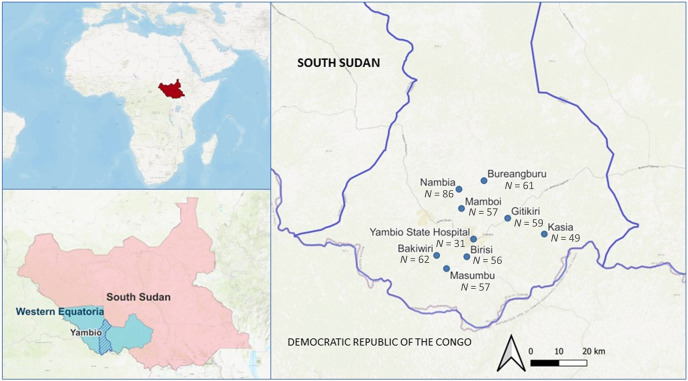
Finger-prick, dried blood spot (DBS) samples were collected using Whatman 903 paper (GE HealthCare Bio-Sciences Corp., Chicago, IL) from the general population aged > 6 months who were presenting symptoms compatible with malaria and for whom malaria infection was confirmed by pan lactate dehydrogenase-based-rapid diagnostic test in eight community-based treatment sites and in the referral hospital (Yambio State Hospital) (Figure 1). Most samples were from patients with uncomplicated malaria, except for the samples from the Yambio State Hospital, which were from patients with severe malaria. The DBS samples were sent for analysis to the National Center of Tropical Medicine (Madrid, Spain) following standard procedures.
Figure 1.
Map of the sample collection sites in Yambio County, Western Equatoria State, South Sudan. N = sample size per collection site.
DNA extraction.
We extracted DNA from the DBS samples using the Saponin/Chelex method with minor modifications from our laboratory.18 We used a punch of 5-mm diameter containing 10 μL of blood. The tube containing the isolated DNA was labeled with the sample number and year and place of collection. These DNA samples were either used immediately for polymerase chain reaction (PCR) or stored at −20°C until further use.
Screening for Pfk13 mutations.
We used nested multiplex PCR to confirm P. falciparum diagnosis for all samples.18,19 Then, we amplified the pfk13 gene using nested PCR, as described by Ariey et al.,6 with minor modifications from our laboratory including the use of another Polymerase HotStart (5 U/mL) (Biotools, Madrid, Spain) and lower annealing temperature (58°C) and MgCl2 concentration (2 mM) for primary PCR. We verified the size of the amplification fragment (±850 bp) by electrophoresis on 2% agarose gel stained with Pronasafe (Pronadisa, Spain).
Next, we purified the PCR products using Illustra ExoProStar 1-step (GE Healthcare Life Sciences, Marlborough, MA) according to the manufacturer’s instructions and sequenced these products from both directions by a standard dye terminator Big Dye Terminator v3.1 Cycle Sequencing kit (Thermo Fisher Scientific, Madrid, Spain) in an ABI PRISM 3730 XL Analyser (Thermo Fisher Scientific). We checked these sequences with BLAST and compared them against a pfk13 3D7 clone using BioEdit 7.2 software to find new and validated mutations.
Ethics.
This study was approved by the internal ethics review board at Médecins Sans Frontières and by the South Sudan Research Ethics Committee. All participants or children’s parents or guardians provided written informed consent.
RESULTS
Of 601 samples analyzed, 518 had a PCR-confirmed P. falciparum mono-infection. Pfk13 was successfully amplified and sequenced in 468 of these samples. Amplification of Pfk13 was unsuccessful for 50 samples.
We detected 15 different mutations in 14 (3%) of the 468 samples (one sample had two mutations). Eight of these 15 mutations were nonsynonymous (53%), and seven were synonymous (47%). None of the eight nonsynonymous mutations were validated or candidate mutations associated with artemisinin resistance listed by the WHO.
The distribution of mutations in the propeller domain of pfK13 revealed that the majority of mutations were in blades 2 (n = 4) and 4 (n = 4). The rest of the blades (blades 3, 5, and 6) had two mutations each, except for blade 1 (n = 1) and BTB/POZ (n = 0).
All geographical locations presented at least one parasite with a mutation in the PfK13 gene (Table 1), with Yambio Hospital being the site with the highest frequency of mutations (8%, 2/26). The R561L nonsynonymous mutation was found in the same position as the R561H validated mutation by the WHO and the S623G nonsynonymous mutation in the following position to the R622I validated mutation. In addition, three synonymous mutations were observed either in the same position or adjacent to a candidate mutation point for resistance (Table 2).
Table 1.
Frequency of pfk13 synonymous and nonsynonymous mutations by location
| Location | N | Codon | Wild allele | AA | Mutant allele | AA | Mutation | Type | Frequency n (%) | Previous identification (WHO region) |
|---|---|---|---|---|---|---|---|---|---|---|
| Sequence (nt) | Sequence (nt) | |||||||||
| Nambia | 86 | 623 | AGT | S | GTT | G | S623G | NS | 4 (4.7%) | Africa* |
| 654 | CCA | Q | CAG | Q | Q654Q | S | – | |||
| 519 | TAT | Y | CAT | H | Y519H | NS | None (novel) | |||
| 561 | CGT | R | CTT | L | R561L | NS | Africa* | |||
| Momboi | 55 | 605 | GAA | E | AAA | K | E605K | NS | 1 (1.8%) | None (novel) |
| Gitikiri | 50 | 486 | GCT | A | TCT | S | A486S | NS | 1 (2.0%) | None (novel) |
| Bakiwiri | 57 | 694 | AAT | N | AAC | N | N694N | S | 2 (3.5%) | – |
| 516 | GAT | D | GAC | D | D516D | S | – | |||
| Yambio State Hospital | 26 | 673 | TTT | F | TTC | F | F673F | S | 2 (7.7%) | Africa† |
| 578 | GCT | A | TCT | S | A578S | NS | Africa | |||
| Masumbu | 50 | 567 | GAG | E | GAA | E | E567E | S | 1 (2.0%) | – |
| Birisi | 50 | 602 | GAA | E | AAA | K | E602K | NS | 1 (2.0%) | None (novel) |
| Bureangburu | 51 | 496 | GGT | G | GGC | G | G496G | S | 3 (5.9%) | Africa† |
| 615 | CCA | P | TCA | S | P615S | NS | Africa* | |||
| 449 | GGT | G | GGC | G | G449G | S | Africa† | |||
| Kasia | 43 | – | – | – | – | – | – | – | 0 | – |
AA = amino acid; NS = not significant; nt = nucleotide; S = significant.
Reported mutation with other change.
Reported NS mutation.
Table 2.
Relatedness between mutations found in South Sudan and mutations associated with resistance to artemisinin
| Validated mutations by the WHO | Candidate mutations by the WHO | Potentially associated mutations* | Mutations South Sudan | Location |
|---|---|---|---|---|
| R561H | – | – | R561L | Nambia |
| R622I | – | – | S623G | Nambia |
| – | G449A | – | G449G | Bureangburu |
| – | V568G | – | E567E | Musumbu |
| – | R515K | – | D516D | Bakiwiri |
| – | – | M579I | A578S | Yambio State Hospital |
| – | – | F673I | F673F | Yambio State Hospital |
These mutations have been associated with partial resistance; however, because of small sample size, the associations were statistically nonsignificant.20
DISCUSSION
This study reveals the presence of 15 mutations, 8 nonsynonymous and 7 synonymous, in pfK13 in South Sudan, some of which are novel. Similar to findings in other sub-Saharan countries,21,22 none of these mutations were in the WHO list of resistance-associated markers. However, any new mutations detected in pfk13 could potentially be a new indicator of drug resistance development and should be reported.23,24 Moreover, the majority of mutations detected were in blades 2 and 4, which are responsible for protein structure.20,25 Although knowledge is still limited, it seems that changes in these blades could alter the integrity of the protein and potentially lead to resistance.6,25,26
Recent reports of emerged mutations in Africa highlight the crucial need for surveillance to limit their spread and try to avoid potential consequences on ACT efficacy.24 Recently, northern Uganda, bordering South Sudan, reported a high presence of molecular markers related to artemisinin partial resistance, including an increasing trend in frequencies for C469Y and A675V, both associated with delayed parasite clearance.13,15 Although these mutations have not been found in South Sudan, there is a risk of introduction and spread to this country.
The A578S mutation, a widespread pfK13 mutation in sub-Saharan Africa including Uganda, Kenya, and Republic of the Congo, was detected in our study.9 This mutation has not been associated with reduced susceptibility to artemisinin,26,27 although one report from Uganda has linked it with possible artemisinin resistance.28 For this reason and because of its extensive distribution and its position near the A580Y validated resistance mutation,29 it would be relevant to continue surveillance to monitor its impact on parasite clearance.
None of the mutations detected in South Sudan correspond to validated or candidate artemisinin-resistance mutations; however, some are either in the same or in an adjacent position as other validated or candidate mutations previously reported elsewhere in Africa. Although there are no data on the biological significance of these mutations, these changes could precede validated mutations or could potentially affect susceptibility to artemisinin.
For example, the nonsynonymous mutation R561L was detected in the same position as the WHO validated R561H resistance marker, which has been found to be present in Rwanda.10 These variants with mutations in the same position as a validated mutation but with a change to a different amino acid need to be carefully monitored and assessed, as they could potentially affect antimalarial activity of artemisinins.26
The synonymous mutation G449G is in the same position as the WHO candidate resistance mutation G449A, and the presence of various mutations (e.g., S623G, A578S) in positions adjacent to those of validated or candidate resistance markers should be highlighted, as they indicate instability in these specific areas of the gene.3,30,31
In addition, we detected the E605K mutation, which was also reported in Ghana.23 This mutation has not been associated with resistance, but it could correspond to an unstable position where mutations occur more easily, albeit without a known biological effect.
Recent reports, including our findings, on the emergence in Africa of spontaneous Pfk13 mutations that can be associated with delayed clearance or even resistance suggest that continued molecular and clinical monitoring is crucial, especially in areas of high transmission such as South Sudan. A high rate of recombination, associated with high transmission, increases the risk of new mutations, although selection may be suppressed by high diversity.23 Some authors suggest that the mutations described in Africa have an independent origin from the ones in Asia, conforming different genetic clusters, so it is expected that more resistance-associated mutations may emerge independently in Africa.8 Moreover, a diversifying selection of parasites with mutations in pfk13, likely due to adaptation of the parasite to ACTs, could possibly endanger the antimalarial activity of these medications, threatening malaria control.32,33 In the case of Yambio County (South Sudan), we found a wide variety of mutations but in low frequency.
This study has some limitations. It is important to highlight that these samples were collected in 2020; hence, the scenario may have changed, especially considering rapid spread in neighboring Uganda. Results cannot be extrapolated to the rest of the country as samples were collected from only one state and areas farther east bordering Uganda could potentially have a higher prevalence of mutations. Moreover, as therapeutic efficacy was not evaluated in this study, it is not possible to determine the impact of the pfk13 mutations reported on parasite clearance half-life.
CONCLUSIONS
We found no validated or candidate markers associated with artemisinin resistance in Yambio County, Western Equatoria State (South Sudan). However, we reported a high diversity of other Pfk13 mutations in low frequency. Some of these mutations were either in the same positions or in adjacent positions as validated or candidate markers of resistance, suggesting instability of the gene that could lead to resistance. Therefore, routine molecular surveillance of resistance markers is highly recommended to promptly detect the emergence of resistance-related mutations and to limit their spread. In addition, routine monitoring of antimalarial drug efficacy is needed.1
ACKNOWLEDGMENTS
We thank the population of Yambio County that participated, the South Sudan Ministry of Health, and MSF Spain teams in Barcelona, Juba, and Yambio for their support in organizing this campaign. Also, we thank Olaya Astudillo for her editing to improve this manuscript.
REFERENCES
- 1. World Health Organization , 2022. World Malaria Report 2022. Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022. Accessed August 21, 2023.
- 2. Nosten F, White NJ, 2007. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg 77: 181–192. [PubMed] [Google Scholar]
- 3. World Health Organization , 2022. Malaria: Artemisinin Partial Resistance. Available at: https://www.who.int/news-room/questions-and-answers/item/artemisinin-resistance. Accessed August 21, 2023.
- 4. Uwimana A. et al. , 2021. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis 21: 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tumwebaze P. et al. , 2017. Changing antimalarial drug resistance patterns identified by surveillance at three sites in Uganda. J Infect Dis 215: 631–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ariey F. et al. , 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stokes BH. et al. , 2021. Plasmodium falciparum K13 mutations in Africa and Asia impact artemisinin resistance and parasite fitness. eLife 10: e66277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pacheco MA, Kadakia ER, Chaudhary Z, Perkins DJ, Kelley J, Ravishankar S, Cranfield M, Talundzic E, Udhayakumar V, Escalante AA, 2019. Evolution and genetic diversity of the k13 gene associated with artemisinin delayed parasite clearance in Plasmodium falciparum. Antimicrob Agents Chemother 63: e02550-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ménard D. et al. , 2016. A worldwide map of Plasmodium falciparum K13-Propeller polymorphisms. N Engl J Med 374: 2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uwimana A. et al. , 2020. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med 26: 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Straimer J, Gandhi P, Renner KC, Schmitt EK, 2022. High prevalence of Plasmodium falciparum K13 mutations in Rwanda is associated with slow parasite clearance after treatment with artemether-lumefantrine. J Infect Dis 225: 1411–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moser KA. et al. , 2021. Describing the current status of Plasmodium falciparum population structure and drug resistance within mainland Tanzania using molecular inversion probes. Mol Ecol 30: 100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asua V, Vinden J, Conrad MD, Legac J, Kigozi SP, Kamya MR, Dorsey G, Nsobya SL, Rosenthal PJ, 2019. Changing molecular markers of antimalarial drug sensitivity across Uganda. Antimicrob Agents Chemother 63: e01818-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bayih AG, Getnet G, Alemu A, Getie S, Mohon AN, Pillai DR, 2016. A unique Plasmodium falciparum K13 gene mutation in Northwest Ethiopia. Am J Trop Med Hyg 94: 132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balikagala B. et al. , 2021. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 385: 1163–1171. [DOI] [PubMed] [Google Scholar]
- 16. Pasquale HA, 2020. South Sudan’s New National Malaria Strategic Plan 2021–2025 is a Game Changer. Available at: http://www.southsudanmedicaljournal.com/archive/december-2020/south-sudans-new-national-malaria-strategic-plan-2021-2025-is-a-game-changer.html. Accessed.
- 17. Pasquale H, Jarvese M, Julla A, Doggale C, Sebit B, Lual MY, Baba SP, Chanda E, 2013. Malaria control in South Sudan, 2006–2013: strategies, progress and challenges. Malar J 12: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berzosa P. et al. , 2021. Temporal evolution of the resistance genotypes of Plasmodium falciparum in isolates from Equatorial Guinea during 20 years (1999 to 2019). Malar J 20: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM, 2014. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J 13: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adams J, Kelso R, Cooley L, 2000. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol 10: 17–24. [DOI] [PubMed] [Google Scholar]
- 21. Mayengue PI. et al. , 2018. No polymorphisms in K13-propeller gene associated with artemisinin resistance in Plasmodium falciparum isolated from Brazzaville, Republic of Congo. BMC Infect Dis 18: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niba PTN. et al. , 2023. Evolution of Plasmodium falciparum antimalarial drug resistance markers post-adoption of artemisinin-based combination therapies in Yaounde, Cameroon. Int J Infect Dis 132: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matrevi SA. et al. , 2022. Novel pfk13 polymorphisms in Plasmodium falciparum population in Ghana. Sci Rep 12: 7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conrad MD, Rosenthal PJ, 2019. Antimalarial drug resistance in Africa: the calm before the storm? Lancet Infect Dis 19: e338–e351. [DOI] [PubMed] [Google Scholar]
- 25. Coppée R, Jeffares DC, Miteva MA, Sabbagh A, Clain J, 2019. Comparative structural and evolutionary analyses predict functional sites in the artemisinin resistance malaria protein K13. Sci Rep 9: 10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chebore W. et al. , 2020. Assessment of molecular markers of anti-malarial drug resistance among children participating in a therapeutic efficacy study in western Kenya. Malar J 19: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mita T. et al. , 2016. Little polymorphism at the K13 propeller locus in worldwide Plasmodium falciparum populations prior to the introduction of artemisinin combination therapies. Antimicrob Agents Chemother 60: 3340–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hawkes M, Conroy AL, Opoka RO, Namasopo S, Zhong K, Liles WC, John CC, Kain KC, 2015. Slow clearance of Plasmodium falciparum in severe pediatric malaria, Uganda, 2011–2013. Emerg Infect Dis 21: 1237–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohon AN, Alam MS, Bayih AG, Folefoc A, Shahinas D, Haque R, Pillai DR, 2014. Mutations in Plasmodium falciparum K13 Propeller gene from Bangladesh (2009–2013). Malar J 13: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Escobar C, Pateira S, Lobo E, Lobo L, Teodosio R, Dias F, Fernandes N, Arez AP, Varandas L, Nogueira F, 2015. Polymorphisms in Plasmodium falciparum K13-Propeller in Angola and Mozambique after the introduction of the ACTs. PLoS One 10: e0119215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He Y. et al. , 2019. Artemisinin resistance-associated markers in Plasmodium falciparum parasites from the China-Myanmar border: predicted structural stability of K13 propeller variants detected in a low-prevalence area. PLoS One 14: e0213686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nyunt MH. et al. , 2017. Clinical and molecular surveillance of artemisinin resistant falciparum malaria in Myanmar (2009–2013). Malar J 16: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dafalla OM. et al. , 2020. Kelch 13-propeller polymorphisms in Plasmodium falciparum from Jazan region, southwest Saudi Arabia. Malar J 19: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]