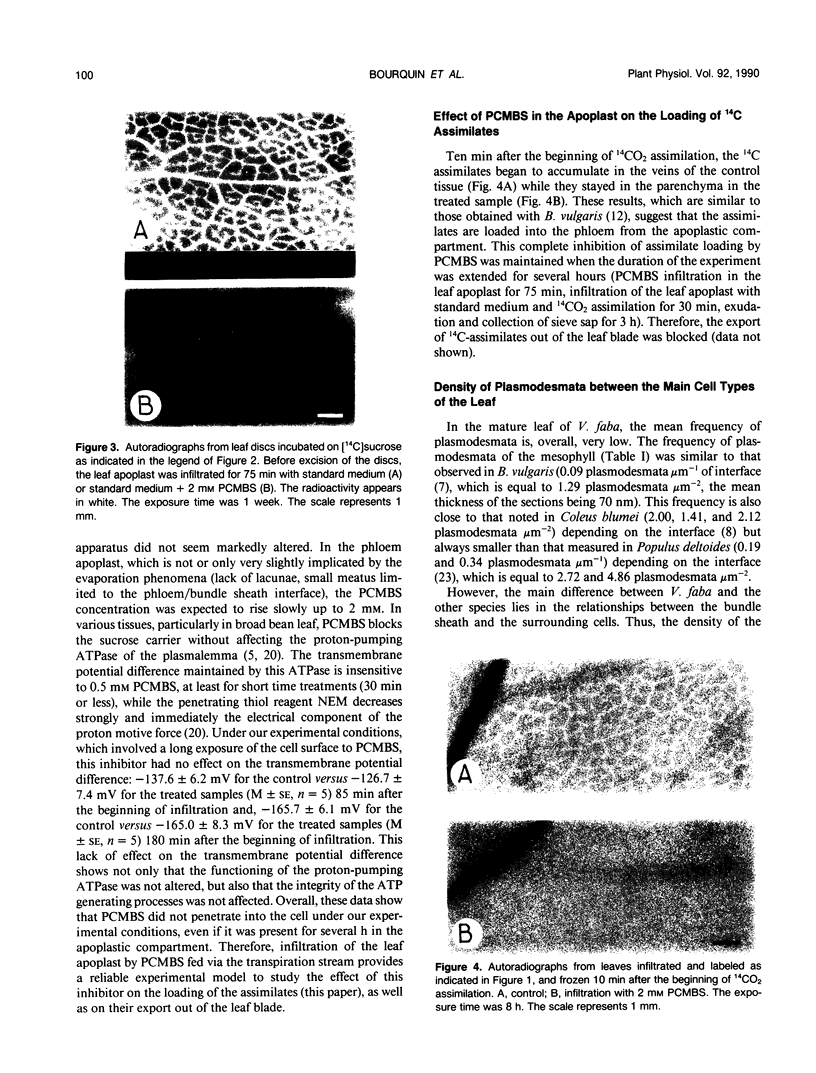
Abstract
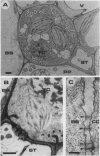
The apoplast of mature leaves excised from broadbean (Vicia faba L.) plants was infiltrated with 2 millimolar p-chloromercuribenzenesulfonic acid (PCMBS) via the transpiration stream, and the ability of the tissues to take up sugars was tested. An infiltration time of 75 minutes was sufficient to obtain a maximal (75%) inhibition of exogenous [14C]sucrose (1 millimolar) uptake. This infiltration affected neither CO2 assimilation nor the transmembrane potential difference of leaf cells but strongly inhibited phloem loading of endogenous [14C] assimilates. The study of the symplastic relations between the different cell types of the mature leaf showed that the density of the plasmodesmata is generally very low in comparison with other species investigated so far, particularly when considering the mesophyll/bundle sheath and the bundle sheath/phloem cells connections, as well as the connections of the transfer cell-sieve tube complex with the surrounding cells. These three successive barriers therefore strongly limit the possibilities of symplastic transit of the assimilates to the conducting cells. The comparison of the densities of plasmodesmata in an importing and an exporting leaf suggests that the maturation of the leaf is characterized by a marked symplastic isolation of the phloem, and, within the phloem itself, by the isolation of the conducting complex. As a consequence, these physiological and cytological data demonstrate the apoplastic nature of loading in the mature leaf of Vicia faba, this species undoubtedly presenting a typical model for apoplastic loading.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Geiger D. R., Giaquinta R. T., Sovonick S. A., Fellows R. J. Solute distribution in sugar beet leaves in relation to Phloem loading and translocation. Plant Physiol. 1973 Dec;52(6):585–589. doi: 10.1104/pp.52.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning B. E., Pate J. S., Minchin F. R., Marks I. Quantitative aspects of transfer cell structure in relation to vein loading in leaves and solute transport in legume nodules. Symp Soc Exp Biol. 1974;(28):87–126. [PubMed] [Google Scholar]
- M'batchi B., Delrot S. Parachloromercuribenzenesulfonic Acid : a potential tool for differential labeling of the sucrose transporter. Plant Physiol. 1984 May;75(1):154–160. doi: 10.1104/pp.75.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore M. A., Oross J. W., Lucas W. J. Symplastic Transport in Ipomea tricolor Source Leaves : Demonstration of Functional Symplastic Connections from Mesophyll to Minor Veins by a Novel Dye-Tracer Method. Plant Physiol. 1986 Oct;82(2):432–442. doi: 10.1104/pp.82.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalstig J. G., Geiger D. R. Phloem Unloading in Developing Leaves of Sugar Beet : I. Evidence for Pathway through the Symplast. Plant Physiol. 1985 Sep;79(1):237–241. doi: 10.1104/pp.79.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalstig J. G., Geiger D. R. Phloem Unloading in Developing Leaves of Sugar Beet : II. Termination of Phloem Unloading. Plant Physiol. 1987 Jan;83(1):49–52. doi: 10.1104/pp.83.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R., Wimmers L. E. Different Patterns of Vein Loading of Exogenous [C]Sucrose in Leaves of Pisum sativum and Coleus blumei. Plant Physiol. 1988 May;87(1):179–182. doi: 10.1104/pp.87.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]