Abstract
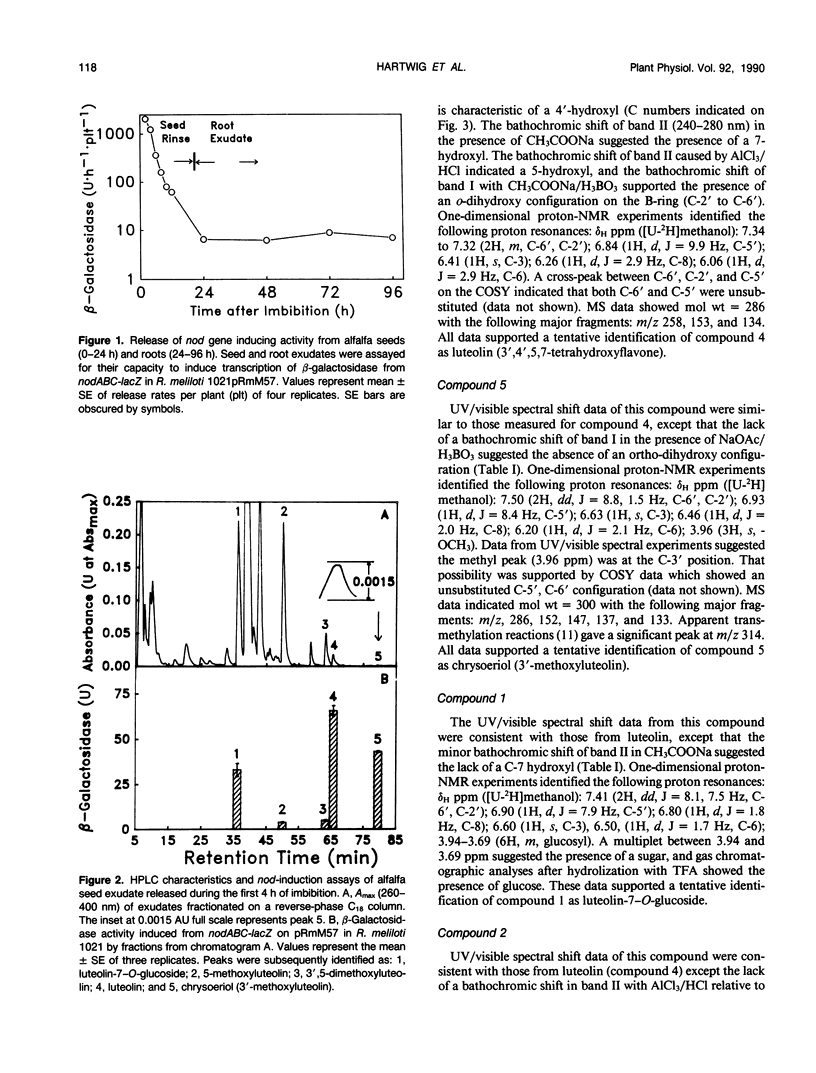
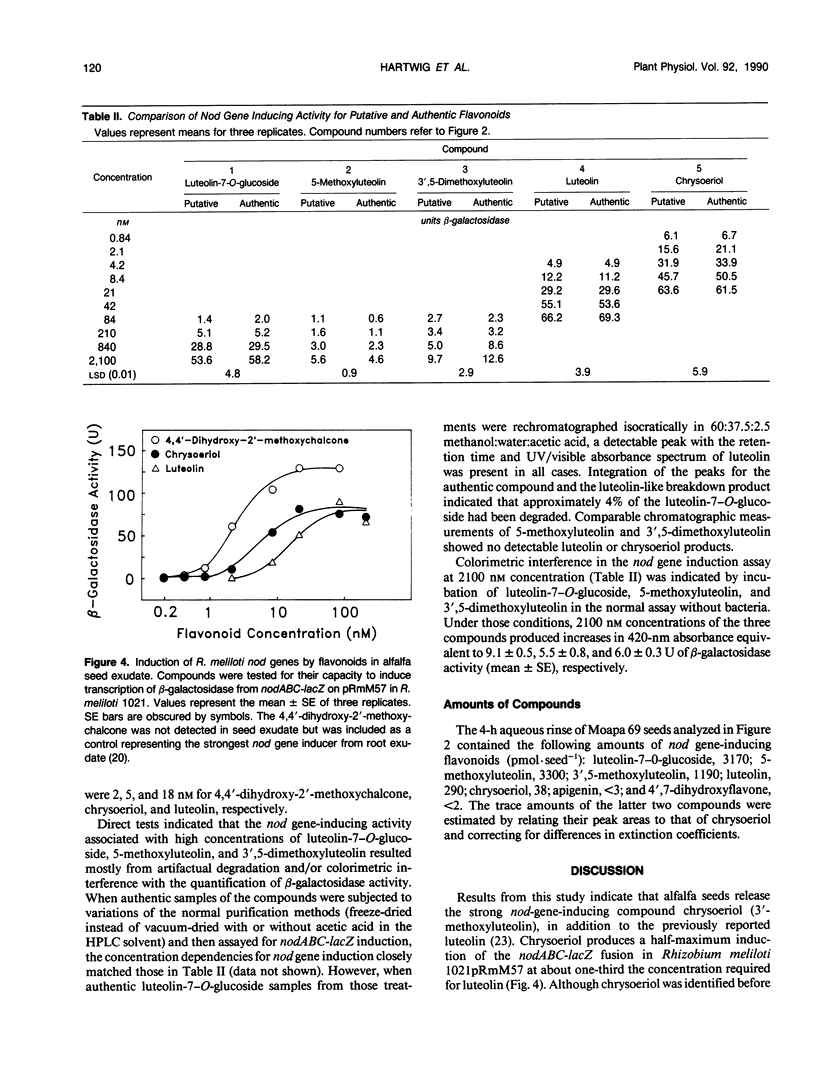
Flavonoid signals from alfalfa (Medicago sativa L.) seed and root exudates induce transcription of nodulation (nod) genes in Rhizobium meliloti. The flavone luteolin previously was isolated from alfalfa seeds by other workers and identified as the first nod gene inducer for R. meliloti. Our recent study of `Moapa 69' alfalfa root exudates found no luteolin but did identify three other nod gene inducers: 4,4′-dihydroxy-2′-methoxychalcone, 4′,7-dihydroxyflavone, and 4′,7-dihydroxyflavanone. The goal of the current study was to identify and quantify nod gene-inducing flavonoids that may influence Rhizobium populations around a germinating alfalfa seed. Aqueous rinses of Moapa 69 alfalfa seeds were collected and assayed for induction of a nodABC-lacZ fusion in R. meliloti. During the first 4 hours of imbibition, total nod gene-inducing activity was released from seeds at 100-fold higher rates than from roots of 72-hour-old seedlings. Five flavonoids were purified and identified by spectroscopic analyses (ultraviolet/visible absorbance, proton nuclear magnetic resonance, and mass spectroscopy) and comparison with authentic standards. Two very active nod gene-inducing flavonoids, chrysoeriol (3′-methoxyluteolin) and luteolin, were identified in seed rinses. Luteolin required a higher concentration (18 nanomolar) than chrysoeriol (5 nanomolar) for half-maximum induction of nodABC-lacZ in R. meliloti, and both were less active than 4,4′-dihydroxy-2′-methoxychalcone (2 nanomolar) from root exudates. Seeds exuded three other luteolin derivatives: luteolin-7-O-glucoside, 5-methoxyluteolin, and 3′,5-dimethoxyluteolin. Their combined quantities were 24-fold greater than that of luteolin plus chrysoeriol. Most nod gene-inducing activity of these luteolin derivatives apparently is associated with degradation to luteolin and chrysoeriol. However, their presence in large quantities suggests that they may contribute significantly to nod gene-inducing activity in the soil. These results indicate the importance of germinating seeds as a source of nod gene-inducing flavonoids and emphasize the quantitative and qualitative differences in those compounds around the seed and root.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassam B. J., Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Identification of a nodD-dependent locus in the Rhizobium strain NGR234 activated by phenolic factors secreted by soybeans and other legumes. Mol Plant Microbe Interact. 1988 Apr;1(4):161–168. doi: 10.1094/mpmi-1-161. [DOI] [PubMed] [Google Scholar]
- Caetano-Anollés G., Crist-Estes D. K., Bauer W. D. Chemotaxis of Rhizobium meliloti to the plant flavone luteolin requires functional nodulation genes. J Bacteriol. 1988 Jul;170(7):3164–3169. doi: 10.1128/jb.170.7.3164-3169.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright N. J., Smith A. R. Bacterial attack on phenolic ethers: An enzyme system demethylating vanillic acid. Biochem J. 1967 Mar;102(3):826–841. doi: 10.1042/bj1020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig U. A., Maxwell C. A., Joseph C. M., Phillips D. A. Interactions among Flavonoid nod Gene Inducers Released from Alfalfa Seeds and Roots. Plant Physiol. 1989 Nov;91(3):1138–1142. doi: 10.1104/pp.91.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M. A., Ausubel F. M. Rhizobium meliloti has three functional copies of the nodD symbiotic regulatory gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8558–8562. doi: 10.1073/pnas.84.23.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartnig T., Böhm J., Hiermann A. Flavonoide in den Blättern von Digitalis purpurea. Planta Med. 1977 Dec;32(20-21):347–349. doi: 10.1055/s-0028-1097610. [DOI] [PubMed] [Google Scholar]
- Kosslak R. M., Bookland R., Barkei J., Paaren H. E., Appelbaum E. R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell C. A., Hartwig U. A., Joseph C. M., Phillips D. A. A Chalcone and Two Related Flavonoids Released from Alfalfa Roots Induce nod Genes of Rhizobium meliloti. Plant Physiol. 1989 Nov;91(3):842–847. doi: 10.1104/pp.91.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Sadowsky M. J., Olson E. R., Foster V. E., Kosslak R. M., Verma D. P. Two host-inducible genes of Rhizobium fredii and characterization of the inducing compound. J Bacteriol. 1988 Jan;170(1):171–178. doi: 10.1128/jb.170.1.171-178.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Mulligan J. T., Long S. R. Expression of Rhizobium meliloti nod genes in Rhizobium and Agrobacterium backgrounds. J Bacteriol. 1987 Jul;169(7):3094–3098. doi: 10.1128/jb.169.7.3094-3098.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaat S. A., Schripsema J., Wijffelman C. A., van Brussel A. A., Lugtenberg B. J. Analysis of the major inducers of the Rhizobium nodA promoter from Vicia sativa root exudate and their activity with different nodD genes. Plant Mol Biol. 1989 Aug;13(2):175–188. doi: 10.1007/BF00016136. [DOI] [PubMed] [Google Scholar]


