Abstract
Introduction
We report a case of an adult hematopoietic stem cell donor who developed active severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during the donation of stem cells, the final transplantation was successfully completed without SARS-CoV-2 transmission.
Case report
We report on a 34-year-old female diagnosed with acute lymphoblastic leukemia who underwent hemiploid hematopoietic stem cell transplantation (HSCT). Both patient and donor received three doses of inactivated SARS-CoV-2 vaccine before transplantation. PB-HSC was collected by the donor during the process of infection with SARS-CoV-2 (mild), and the patient did not show symptoms related to SARS-CoV-2 after transplantation. Nucleic acid and antigen were negative in regular tests.
Conclusion
In the context of the current Omicron epidemic and high vaccination rate in the population, it is feasible to receive PB-HSC from infected donors even for immunocompromised patients. This also provides some references for our later donor selection.
Keywords: SARS-CoV-2, HSCT, Donor
Introduction
How to ensure the safety of hematopoietic stem cell transplantation (HSCT) patients without delaying the treatment of patients in the era of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak is the topic we are discussing. When a patient cannot delay hematopoietic stem cell transplantation due to medical needs and there is no other suitable donor, will stem cell donation from a SARS-CoV-2 infected donor be fatal to the patient?
Case report
Here we report on a 34-year-old female diagnosed with acute lymphoblastic leukemia (Common B) in 2022.3. The patient reached partial response (PR) after two courses of standard chemotherapy, complete response (CR) after CD19 CAR-T cell treatment on 2022.8.18, and Minimal residual disease (MRD) turned negative. The patient started conditioning regimen to benefit from hematopoietic stem cell transplantation (HSCT) with her HLA-haploidentical younger brother in 2022.12.
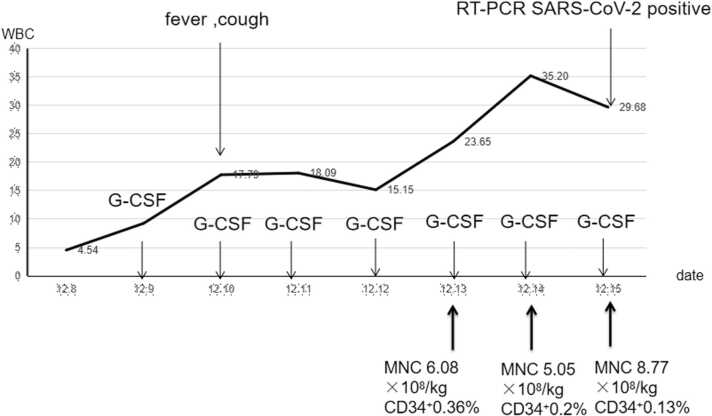
Both patient and donor received three doses of inactivated SARS-CoV-2 vaccine (Vero cells for virus culture amplification and inactivation) in 2021. In the process of mobilizing stem cells, the donor presented dry throat, fever, cough on 2022.12.12, and had positive ( cycle threshold is N 29.69, ORF 32.2) nasopharyngeal RT-PCR severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on 2022.12.15. The patient has completed the total body irradiation total dose (FTBI) of 9.99GY at this time. There was no suitable alternative donor and after evaluating the risk-benefit ratio, we proceeded with the transplantation as planned. Donor stem cells were mobilized well and collected in sufficient quantities (Fig. 1). The donor's cough and fever improved and the RT-PCR SARS-CoV-2 turned negative on 2022.12.22.
Fig. 1.
Donor stem cell collection.
The conditioning regimen was myeloablative (consisting in FTBI 9.99GY, melphalan 100 mg/m2 cyclophosphamide 80 mg/kg and anti-thymocyte globulins 10 mg/kg). From 2022.12.13 to 2022.12.15 (the donor collected fresh stem cells every day and transfused them to the patient on the same day, the donor collected for a total of 3 days, and the patient also received infusion for 3 days), a total of stem cell MNC 14.13 × 108/kg, CD34+ 3.59 × 106/kg was infused in 3 days. The patient did not develop any respiratory symptom and fever following the transplant. Nasopharyngeal SARS-CoV-2 antigen was negative on day + 7 and + 14, nasopharyngeal RT-PCR SARS-CoV-2 was also negative on day + 20. Neutrophil and platelet engraftment occurred on day + 14 and + 13, respectively. Chest CT showed no abnormalities after engraftment.
Discussion
Respiratory route was demonstrated as the most common way of transmission of SARS-CoV-2[1]. However, whether SARS-CoV-2 is transmitted through peripheral blood hematopoietic stem cells (PB-HSC) remains inconclusive. Early researchers, based on patient safety concerns, it advocates to exclude donors if SARS-CoV-2 issuspected, to test the donor prior mobilization and to cryopreserve the product at least 14 days [2], [3], [4], [5]. However, the donation delay and/or the use of an alternative donor can represent a real loss of chance for the recipient. The use of frozen stem cells will also reduce the number of stem cells and increase the risk of patients infusion of frozen cells. In recent years, some case reports show that hematopoietic stem cells (HSC) are safe in the context of SARS-CoV-2 pandemic when the donor is asymptomatic [1], [2], [6], [7], [8]. Our donor suffered from SARS-CoV-2 infection (mild) when the patient had undergone myeloablative conditioning regimen. At this time, the patient's transplantation process could not be stopped, and there were no other suitable donors. Both patient and donor received three doses of inactivated SARS-CoV-2 vaccine before transplantation. PB-HSC was collected by the donor during the process of infection with SARS-CoV-2 (mild), and the patient did not show symptoms related to SARS-CoV-2 after transplantation. Nucleic acid and antigen were negative in regular tests. Our case showed that in the context of the current Omicron epidemic and high vaccination in the population, Even immunocompromised patients did not develop symptoms after receiving PB-HSC from infected donors. Therefore, when the patient cannot delay the transplantation, it may not increase the risk of patients suffering from SARS-CoV-2 to infuse infected donor PB-HSC.
In addition, in our case, the donor can collect enough stem cells when suffering from SARS-CoV-2(mild). Patients' neutrophil and platelet can also be implanted smoothly at normal time. (The patient's neutrophil and platelet engraftment occurred on day +14 and +13, respectively). Therefore, SARS-CoV-2 may have no significant impact on the quality and quantity of PB-HSC, nor dose it increase the risk of poor implantation of patients after infusion.
This is the case report of an adult hematopoietic cell donor with SARS-CoV-2 in the active infection period where the transplant is successfully completed with no transmission of SARS-CoV-2. In the context of the current Omicron epidemic and high vaccination rate in the population, it is feasible to receive PB-HSC from infected donors even for immunocompromised patients. This also provides some references for our later donor selection.
Ethics approval and consent to participate
Ethics Committee of Hematology Hospital, Chinese Academy of Medical Sciences IIT2021011-EC-1.
Role of the funding source
The funding source had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Author Statement
Xin Chen collected cases and write articles. Sizhou Feng instructed patient in diagnosis and treatment, guided article ideas. Qiaoling Ma, Aiming Pang, Donglin Yang, Chen Liang, Qingzhen Liu, Xin Liu and Xiaohui Zheng assisted in managing patients. Erlie Jiang and Mingzhe Han instructed patient in diagnosis and treatment.
Declaration of Competing Interest
The authors declared that they have no commercial, proprietary, or financial interest in the producers or companies described in this manuscript.
Acknowledgements
The authors would like to thank all the doctors and nurses in Centre of Hematopoietic Stem Cell Transplantation for their professional assistance. This research received grant from funding of Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (grant numbers 2021-I2M-1–017, 2021-I2M-C&T-B-080), Tianjin Municipal Science and Technology Commission Grant (grant numbers 21JCZDJC01170), Haihe Laboratory of Cell Ecosystem Innovation Fund (grant numbers HH22KYZX0036) and CAMS Innovation Fund for Medical Sciences (CIFMS) (2022–12 M-C&T-B-092).
Patient consent for publication
The patient knows and agrees to publish.
Author Agreement
All authors have signed a statement that they have reviewed the manuscript, agree with its contents, and consent to its submission to IDCases, and I will email a pdf of the signed form to you soon latter.
References
- 1.Blandin Lucie, Tolmer Elise, Hermet Eric, et al. COVID-19 systematic screening of asymptomatic haematopoietic stem cell donors: Less if often more. EJHaem. 2022;3(4):1381–1384. doi: 10.1002/jha2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lázaro Del Campo P., de Paz Arias R., Ramírez López A., et al. No transmission of SARS-CoV-2 in a patient undergoing allogeneic hematopoietic cell transplantation from a matched-related donor with unknown COVID-19. Transfus Apher Sci. 2020;59(6) doi: 10.1016/j.transci.2020.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaussen Amaury, Hornby Laura, Rockl Gary, et al. Evidence of SARS-CoV-2 infection in cells, tissues, and organs and the risk of transmission through transplantation. Transplantation. 2021;105(7):1405–1422. doi: 10.1097/TP.0000000000003744. [DOI] [PubMed] [Google Scholar]
- 4.Ljungman Per, Mikulska Malgorzata, de la Camara Rafael, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transpl. 2020;55(11):2071–2076. doi: 10.1038/s41409-020-0919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 rapid guideline: haematopoietic stem cell transplantation. London: National Institute for Health and Care Excellence (NICE); 2021 Feb 10. [PubMed]
- 6.Mawalla William Frank, Njiro Belinda J., Bwire George M., et al. No evidence of SARS-CoV-2 transmission through transfusion of human blood products: a systematic review. EJHaem. 2021;2(3):601–606. doi: 10.1002/jha2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anurathapan Usanarat, Apiwattanakul Nopporn, Pakakasama Samart, et al. Hematopoietic stem cell transplantation from an infected SARS-CoV2 donor sibling. Bone Marrow Transpl. 2020;55(12):2359–2360. doi: 10.1038/s41409-020-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coll Elisabeth, Fernández-Ruiz Mario, Sánchez-Álvarez J.Emilio, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transpl. 2021;21(5):1825–1837. doi: 10.1111/ajt.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]