Abstract
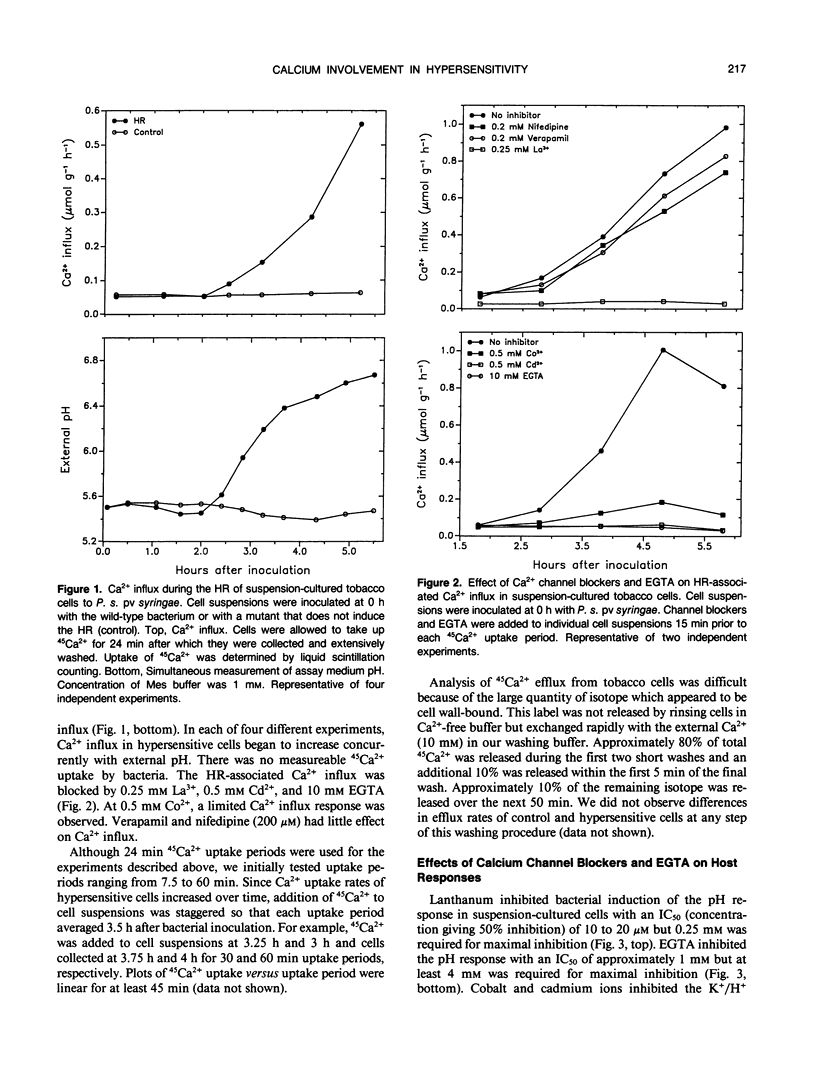
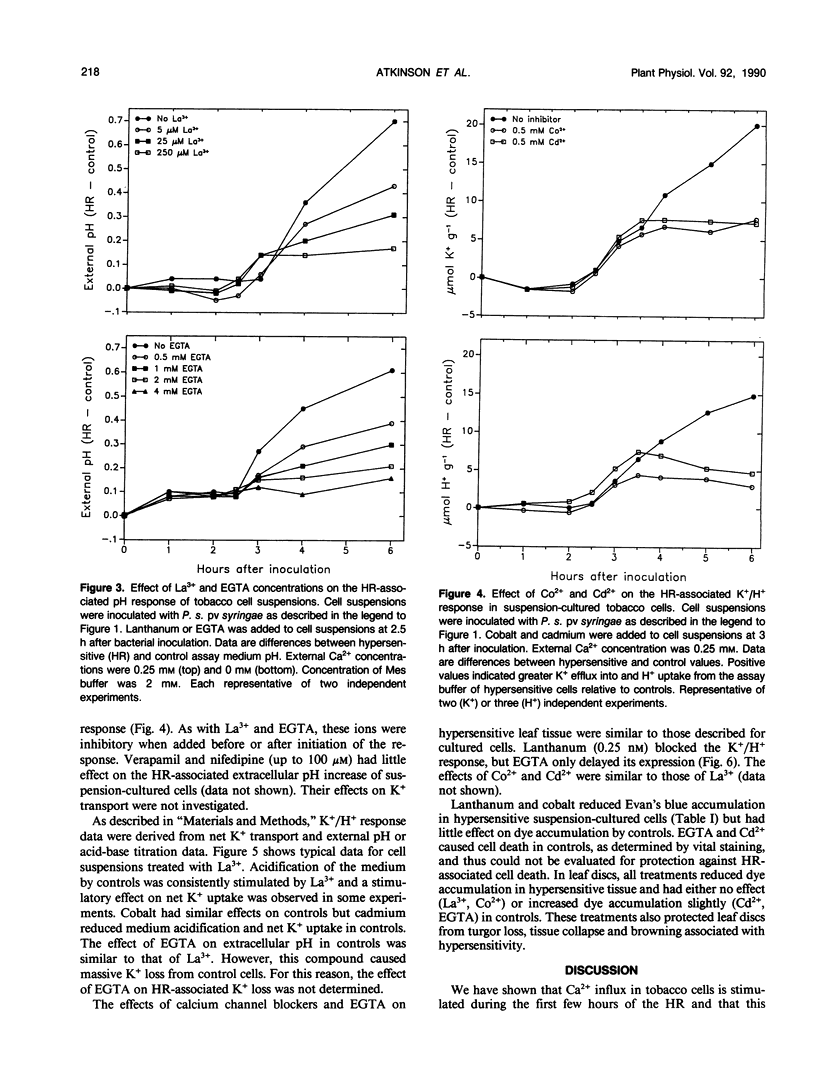
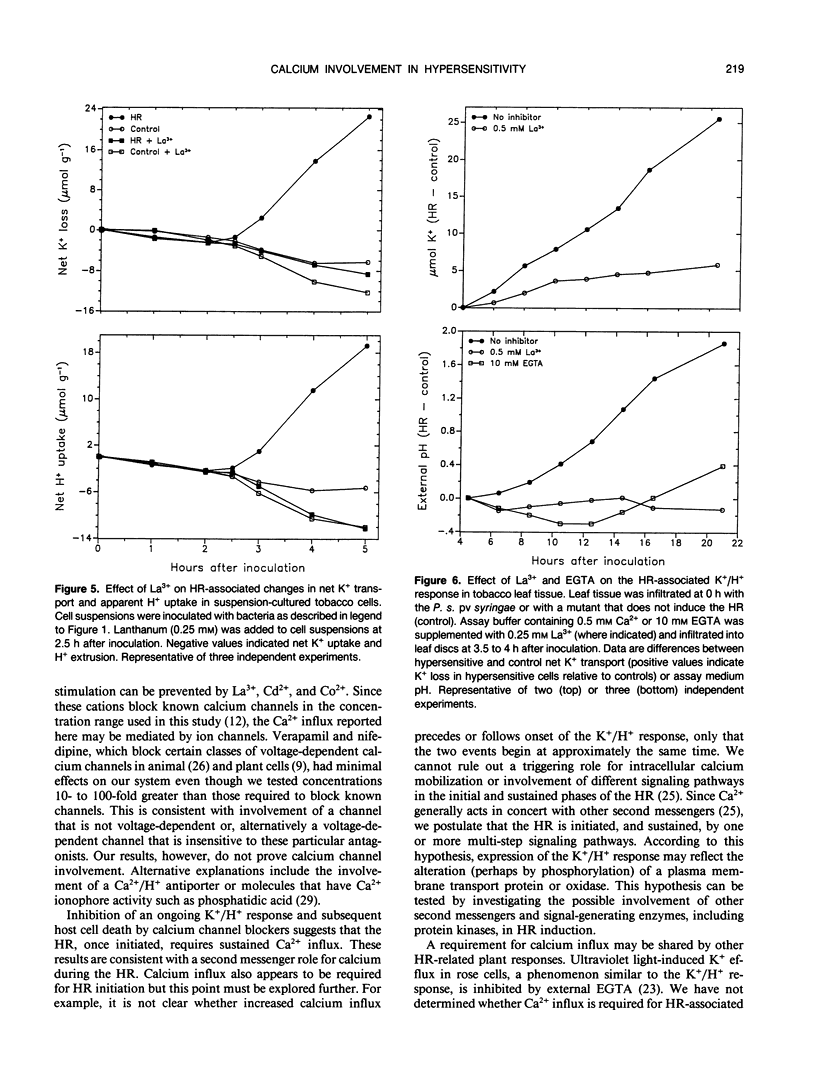
An early event in the hypersensitive response of tobacco to Pseudomonas syringae pv syringae is the initiation of a K+/H+ response characterized by specific plasma membrane K+ efflux, extracellular alkalinization, and intracellular acidification. We investigated the role of calcium in induction of these host responses. Suspension-cultured tobacco cells exhibited a baseline Ca2+ influx of 0.02 to 0.06 micromole per gram per hour as determined from 45Ca2+ uptake. Following bacterial inoculation, uptake rates began to increase coincidently with onset of the K+/H+ response. Rates increased steadily for 2 to 3 hours, reaching 0.5 to 1 micromole per gram per hour. This increased Ca2+ influx was prevented by EGTA and calcium channel blockers such as La3+, Co2+, and Cd2+ but not by verapamil and nifedipine. Lanthanum, cobalt, cadmium, and EGTA inhibited the K+/H+ response in both suspension-cultured cells and leaf discs and prevented hypersensitive cell death in leaf discs. We conclude that increased plasmalemma Ca2+ influx is required for the K+/H+ and hypersensitive responses in tobacco.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. M., Baker C. J. Role of the Plasmalemma H-ATPase in Pseudomonas syringae-Induced K/H Exchange in Suspension-Cultured Tobacco Cells. Plant Physiol. 1989 Sep;91(1):298–303. doi: 10.1104/pp.91.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. M., Huang J. S., Knopp J. A. The Hypersensitive Reaction of Tobacco to Pseudomonas syringae pv. pisi: Activation of a Plasmalemma K/H Exchange Mechanism. Plant Physiol. 1985 Nov;79(3):843–847. doi: 10.1104/pp.79.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blowers D. P., Boss W. F., Trewavas A. J. Rapid Changes in Plasma Membrane Protein Phosphorylation during Initiation of Cell Wall Digestion. Plant Physiol. 1988 Feb;86(2):505–509. doi: 10.1104/pp.86.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Pearce G., Ryan C. A. In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1539–1542. doi: 10.1073/pnas.86.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Huang H. C., Schuurink R., Denny T. P., Atkinson M. M., Baker C. J., Yucel I., Hutcheson S. W., Collmer A. Molecular cloning of a Pseudomonas syringae pv. syringae gene cluster that enables Pseudomonas fluorescens to elicit the hypersensitive response in tobacco plants. J Bacteriol. 1988 Oct;170(10):4748–4756. doi: 10.1128/jb.170.10.4748-4756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta A. J., Murphy T. M. Effects of Extracellular pH on UV-Induced K Efflux from Cultured Rose Cells. Plant Physiol. 1989 Jun;90(2):749–753. doi: 10.1104/pp.90.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki F., Tsurusawa Y., Nishi A. Breakdown of Phosphatidylinositol during the Elicitation of Phytoalexin Production in Cultured Carrot Cells. Plant Physiol. 1987 Nov;85(3):601–604. doi: 10.1104/pp.85.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhle H., Jeblick W., Poten F., Blaschek W., Kauss H. Chitosan-elicited callose synthesis in soybean cells as a ca-dependent process. Plant Physiol. 1985 Mar;77(3):544–551. doi: 10.1104/pp.77.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Murphy T. M., Wilson C. UV-Stimulated K Efflux from Rose Cells: Counterion and Inhibitor Studies. Plant Physiol. 1982 Sep;70(3):709–713. doi: 10.1104/pp.70.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Snyder S. H. Calcium antagonist receptors. Ion Channels. 1988;1:213–249. doi: 10.1007/978-1-4615-7302-9_6. [DOI] [PubMed] [Google Scholar]
- Salzwedel J. L., Daub M. E., Huang J. S. Effects of Singlet Oxygen Quenchers and pH on the Bacterially Induced Hypersensitive Reaction in Tobacco Suspension Cell Cultures. Plant Physiol. 1989 May;90(1):25–28. doi: 10.1104/pp.90.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. E., Ebel J. Specific binding of a fungal glucan phytoalexin elicitor to membrane fractions from soybean Glycine max. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4117–4121. doi: 10.1073/pnas.84.12.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C., Anderson P., Goodman E., Dunham P., Weissmann G. Phosphatidate and oxidized fatty acids are calcium ionophores. Studies employing arsenazo III in liposomes. J Biol Chem. 1981 Mar 25;256(6):2736–2741. [PubMed] [Google Scholar]
- Stäb M. R., Ebel J. Effects of Ca2+ on phytoalexin induction by fungal elicitor in soybean cells. Arch Biochem Biophys. 1987 Sep;257(2):416–423. doi: 10.1016/0003-9861(87)90585-6. [DOI] [PubMed] [Google Scholar]


