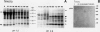Abstract
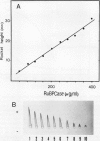
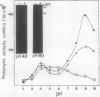
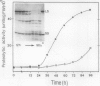
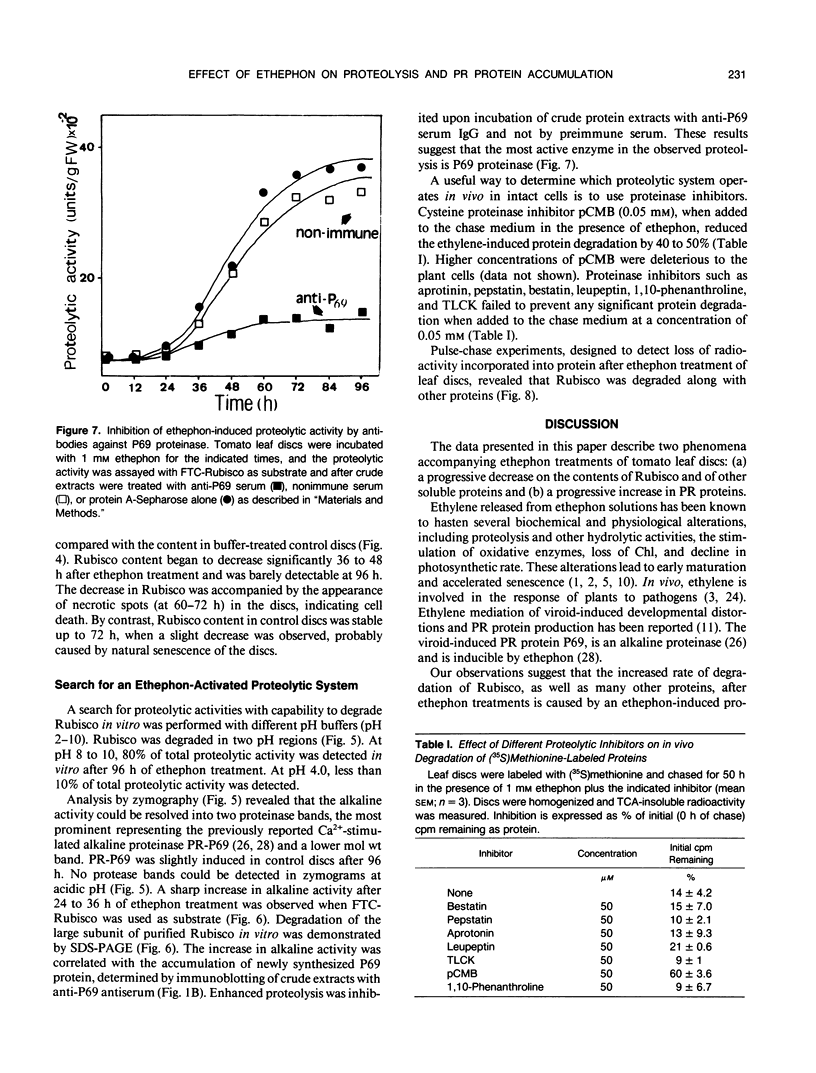
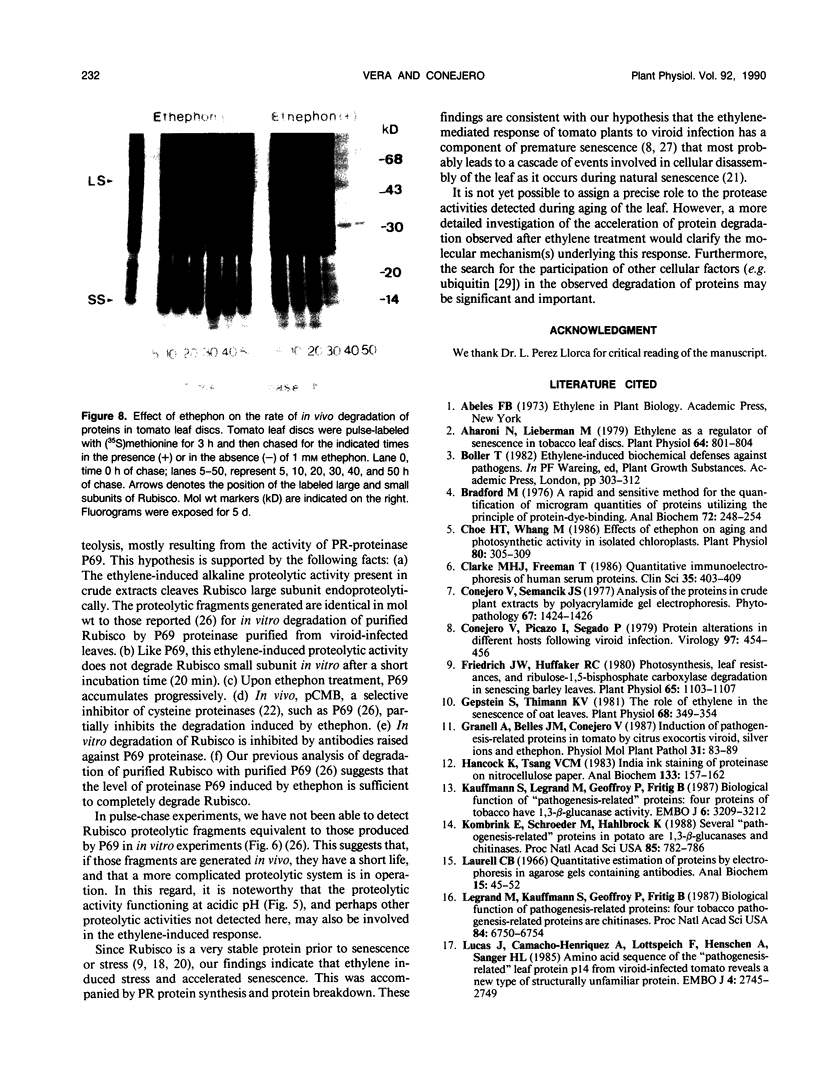
The effect of ethephon (2-chloroetylphosphonic acid) on the degradation of proteins and on the induction of Lycopersicon esculentum pathogenesis-related (PR) proteins was studied in tomato leaf discs. The rate of ribulose, -1,5-bisphosphate carboxylase/oxygenase (Rubisco) degradation was maximal in discs after 48 hours of incubation with 1 millimolar ethephon, leading to complete disappearance of Rubisco after 96 hours. This effect was correlated with an increase in PR protein synthesis and the induction of the previously reported alkaline proteolytic enzyme PR-P69 (P Vera, V Conejero [1988] Plant Physiol 87: 58-63). In vivo pulse-chase experiments demonstrated that ethephon not only affected Rubisco content but that of many other 35S-labeled proteins as well, indicating that ethylene activates a general and nonspecific mechanism of protein degradation. This effect was partially inhibited in vivo by the action of pCMB, a selective inhibitor of cysteine-proteinases such as P69. These data reinforce the hypothesis that P69 and perhaps other PR proteins are involved in the mechanism of accelerated protein degradation activated by ethylene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N., Lieberman M. Ethylene as a regulator of senescence in tobacco leaf discs. Plant Physiol. 1979 Nov;64(5):801–804. doi: 10.1104/pp.64.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Choe H. T., Whang M. Effects of ethephon on aging and photosynthetic activity in isolated chloroplasts. Plant Physiol. 1986 Feb;80(2):305–309. doi: 10.1104/pp.80.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H. G., Freeman T. Quantitative immunoelectrophoresis of human serum proteins. Clin Sci. 1968 Oct;35(2):403–413. [PubMed] [Google Scholar]
- Friedrich J. W., Huffaker R. C. Photosynthesis, leaf resistances, and ribulose-1,5-bisphosphate carboxylase degradation in senescing barley leaves. Plant Physiol. 1980 Jun;65(6):1103–1107. doi: 10.1104/pp.65.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein S., Thimann K. V. The role of ethylene in the senescence of oat leaves. Plant Physiol. 1981 Aug;68(2):349–354. doi: 10.1104/pp.68.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Kauffmann S., Legrand M., Geoffroy P., Fritig B. Biological function of ;pathogenesis-related' proteins: four PR proteins of tobacco have 1,3-beta-glucanase activity. EMBO J. 1987 Nov;6(11):3209–3212. doi: 10.1002/j.1460-2075.1987.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Schröder M., Hahlbrock K. Several "pathogenesis-related" proteins in potato are 1,3-beta-glucanases and chitinases. Proc Natl Acad Sci U S A. 1988 Feb;85(3):782–786. doi: 10.1073/pnas.85.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Legrand M., Kauffmann S., Geoffroy P., Fritig B. Biological function of pathogenesis-related proteins: Four tobacco pathogenesis-related proteins are chitinases. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6750–6754. doi: 10.1073/pnas.84.19.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J., Henriquez A. C., Lottspeich F., Henschen A., Sänger H. L. Amino acid sequence of the ;pathogenesis-related' leaf protein p14 from viroid-infected tomato reveals a new type of structurally unfamiliar proteins. EMBO J. 1985 Nov;4(11):2745–2749. doi: 10.1002/j.1460-2075.1985.tb03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosichan J. L., Huffaker R. C. Source of endoproteolytic activity associated with purified ribulose bisphosphate carboxylase. Plant Physiol. 1984 May;75(1):74–77. doi: 10.1104/pp.75.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera P., Conejero V. Pathogenesis-related proteins of tomato : p-69 as an alkaline endoproteinase. Plant Physiol. 1988 May;87(1):58–63. doi: 10.1104/pp.87.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. L., Leopold A. C. Ethylene evolution from 2-chloroethylphosphonic Acid. Plant Physiol. 1969 Jan;44(1):156–158. doi: 10.1104/pp.44.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. F. Ethylene evolution from 2-chloroethylphosphonic Acid. Plant Physiol. 1969 Aug;44(8):1203–1204. doi: 10.1104/pp.44.8.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]