Abstract
Tissue engineering and regenerative medicine have shown potential in the repair and regeneration of tissues and organs via the use of engineered biomaterials and scaffolds. However, current constructs face limitations in replicating the intricate native microenvironment and achieving optimal regenerative capacity and functional recovery. To address these challenges, the utilization of decellularized tissues and cell-derived extracellular matrix (ECM) has emerged as a promising approach. These biocompatible and bioactive biomaterials can be engineered into porous scaffolds and grafts that mimic the structural and compositional aspects of the native tissue or organ microenvironment, both in vitro and in vivo. Bioactive dECM materials provide a unique tissue-specific microenvironment that can regulate and guide cellular processes, thereby enhancing regenerative therapies. In this review, we explore the emerging frontiers of decellularized tissue-derived and cell-derived biomaterials and bio-inks in the field of tissue engineering and regenerative medicine. We discuss the need for further improvements in decellularization methods and techniques to retain structural, biological, and physicochemical characteristics of the dECM products in a way to mimic native tissues and organs. This article underscores the potential of dECM biomaterials to stimulate in situ tissue repair through chemotactic effects for the development of growth factor and cell-free tissue engineering strategies. The article also identifies the challenges and opportunities in developing sterilization and preservation methods applicable for decellularized biomaterials and grafts and their translation into clinical products.
Keywords: Non-immune biomaterials and grafts, Native micro-environment, Bio-chemical cues, Chemotactic ability, Tissue-inks, Clinical therapies
Graphical abstract
Highlights
-
•
Decellularized tissue-/cell-derived non-immune biomaterials with tissue-specific microenvironment, structure and mechanics
-
•
Decellularized grafts potential to stimulate in situ tissue regeneration through chemotactic effects and presence of native GFs.
-
•
The use of dECM biomaterials as “Tissue-Inks” for the bio-fabrication of engineered grafts.
-
•
Sterilization/preservation importance and need to establish Good Manufacturing Practice standards for clinical translation
-
•
Highlights recent developments, contemporary challenges, and decellularized-based grafts for regenerative therapies.
1. Introduction
Repair and regeneration of damaged, diseased or loss of tissues and organs remains a significant clinical challenge and costs more than $400 billion in US on a yearly basis [1]. Tissue engineering (TE) has emerged as an alternative to current treatment options for the development of engineered structures to improve and restore affected tissues and organs [2,3]. TE strategies involve the use of biomaterials along with relevant tissue-specific cells and/or growth factors (GFs). This triad work alone or in combination to provide the structural and biochemical cues to guide/regulate cell behavior and tissue development. Various natural and synthetic biomaterials have been utilized for the development of biodegradable three-dimensional (3D) and porous scaffolds that are capable of supporting cell in-growth and de novo tissue formation [[4], [5], [6]].
Tissue engineering efforts are focused on the development of biomimetic scaffold systems that can more closely replicate the complex microenvironment of native tissues and organs. Conventional TE scaffold fabrication methods including porogen-leaching, freeze-drying, phase-separation and electrospinning can generate 3D-porous structures with tissue-like microenvironment [7,8]. Advanced scaffold fabrication methods such as additive manufacturing helped to form structures with tunable porosity and gradient along the scaffold length to support tissue-tissue interfacial engineering [7,[9], [10], [11], [12]]. Significant progress has been made in the development of scaffolds that incorporate ECM-like structures with desired nano/micro topography that mimic the target tissue in terms of bio-chemical composition and mechanical performance [13,14]. Also, recent efforts resulted in more sophisticated scaffolds that can be built using synthetic ECM formed with bioactive domains [[15], [16], [17]]. However, despite this progress the engineered structures still lack the complex microenvironment and composition of the native ECM that is required to achieve optimal regenerative capacity [18].
The complex microenvironment of native tissues presents many challenges with recapitulating in vivo cell interactions and conditions to restore normal function, often varying with type of tissue, and state of health condition. The extracellular matrix (ECM) contains functional characteristics and structures including the complex establishment of proteins and matrix components of the target tissue/organ microenvironment such as collagen, proteoglycans, fibronectin, laminin, elastin, and other glycoproteins [19,20]. Moreover, the ECM also serves as a reservoir of essential GFs and signaling molecules and plays a vital role in regulating and maintaining tissue homeostasis, growth, differentiation, vascularization and maturation [21]. These properties of native ECM present challenges in reproducing the complex 3D ultrastructure with appropriate compositional arrangement and are often difficult to achieve with conventional fabrication methods and biomaterials [22].
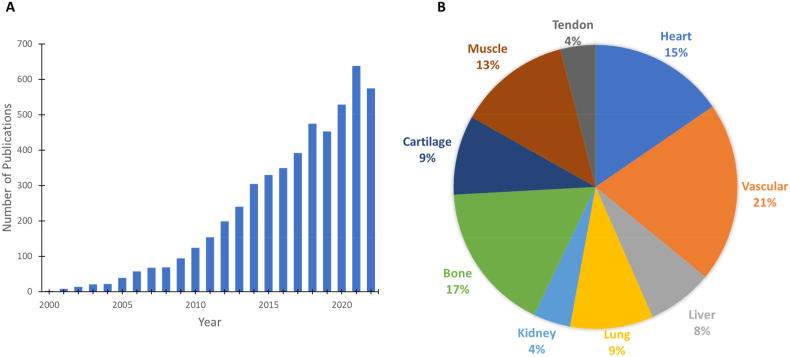
Decellularized tissue has recently gained considerable notice as a potential biological scaffold. The use of decellularized tissue involves processing for the removal of cellular components whilst preserving/minimizing loss of tissue- and/or organ-specific ECM properties and in some cases, the preservation of vascular and neural networks and architecture. The maintenance of the intrinsic biochemical and biophysical cues of the native ECM provides structural and chemical signaling cues for regulating cellular behavior in terms of supporting cellular adhesion, migration, proliferation and differentiation [23]. These include biochemical cues such as structural proteins, peptides and cytokines/GFs as well as biophysical and material properties such as 3D structure, porosity and mechanical properties similar to native tissue. Additionally, decellularized ECM (dECM) also contains an abundant supply of tissue-specific GFs and other signaling molecules [24,25]. Together these play an important role in the development of cellular microenvironment niche and holds great promise for decellularized tissue biomaterials as natural bio-instructive materials/grafts to regulate and facilitate tissue-specific cellular behavior and stimulate regeneration. As such, there has been an exponential growth in research interest and progression with the use of decellularized biomaterials/grafts since 2000s (Fig. 1A).
Fig. 1.
Decellularized tissue biomaterials/grafts. (A) SciFinder published article results for “Decellularized Tissue” by year since 2000, showing exponential increase in research interest and progression over the years. (B) Break-down of the articles published according to tissue/whole organ application. Heart, vascular and bone tissue engineering are the top three areas where dECM is used as a biomaterial or graft. We used a total of 5386 published articles to develop the presented pie chart.
The development of dECM for use as natural instructive scaffolds and their functional outcome involves efficient decellularization of the donor tissue which can later be re-seeded with various types of cells. Simple tissues, such as bone, cartilage, muscle, tendon, vascular, and complex organs, such as heart, liver, lung, kidney are decellularized for tissue engineering and regenerative medicine applications (Fig. 1B) [[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]]. A number of reviews on this topic covered decellularized biomaterials in various forms (hydrogels, bio-inks, and organized porous structures) and their biological, and medical engineering applications including disease modeling and drug screening platforms [31,[45], [46], [47], [48], [49], [50], [51], [52], [53], [54]].
This comprehensive review focuses on decellularized tissue-/organ-derived biomaterials and cell-derived biomaterials as an emerging frontier in tissue engineering and regenerative medicine. We identify the decellularization effects on mechanical as well as biological properties and discuss mitigation techniques with the goal of mimicking native tissue and organ structure as well as biological function. We discuss the need for sterilization and preservation methods of dECM products that are often ignored but have clinical prominence to meet GMP standards. A special attention is given to the emerging area of research that explores the chemotactic ability of the decellularized tissue/organ and their use in developing in situ tissue regeneration strategies. The review also highlights recent trends and developments, contemporary challenges, and clinical prospects of the dECM biomaterials and grafts.
2. Decellularization methods
Allogenic/xenogeneic sourced tissue-/organ-derived materials have been widely studied for use in tissue repair and organ transplantation strategies [23]. However, the presence of foreign cellular material and antigens such as alpha-gal epitope can lead to adverse cell/host responses leading to rejection and thus requires the minimization/elimination of immunogenic risk before use as a biomaterial or graft for TE [55]. For that reason, numerous methods have been developed for tissue/organ decellularization for the removal of cellular and genetic material. The goal of the decellularization process is to completely remove cellular material while preserving ECM structure and bio-chemical composition. The three classifications of common methods of decellularization include: physical, chemical and enzymatic methods. Tissue or organ decellularization methods rely on immersion or perfusion of solutions containing chemical or biological agents, or physical stresses for cellular membrane disruption, to induce cell death and removal. The choice of decellularization agents, duration of treatment and protocol involvement/complexity is dependent on the target tissue/organ properties such as the tissue thickness, density, structure, origin and lipid content along with its intended use [19]. Commonly used tissue/organ decellularization, sterilization and preservation methods, along with their effects on ECM are shown in Table 1 and Table 2.
Table 1.
Physical, Enzymatic, and Chemical Methods commonly applied to decellularize tissues and organs.
| Category |
Treatment/Technique |
Effect on Tissue |
Ref. |
|---|---|---|---|
| Decellularization Method | |||
| Chemical | |||
| Acids & Bases |
|
[252] | |
| Hypotonic/Hypertonic Solutions |
|
[253,254] | |
| Detergents |
|
||
| Non-ionic Detergents (e.g. Triton X-100) |
|
[254,255] | |
| Ionic Detergents (e.g. SDS) |
|
[256,257] | |
| Zwitterionic Detergents (e.g. CHAPS) |
|
[118] | |
| Enzymatic | |||
| Nucleases (DNase, RNase, etc.) |
|
[258] | |
| Trypsin |
|
[259,260] | |
| Lipase |
|
[261,262] | |
| Dispase |
|
[263,264] | |
| Collagenase |
|
[265,266] | |
| Physical | |||
| Freeze-Thaw Cycling |
|
[[267], [268], [269], [270], [271]] | |
| High Hydrostatic Pressure |
|
[[272], [273], [274], [275], [276]] | |
Table 2.
Decellularization Tissue Sterilization and Preservation methods for developing dECM biomaterials/grafts for clinical use.
| Category | Treatment/Technique | Effect on Tissue | Ref. |
|---|---|---|---|
| Sterilization Methods | |||
| Gamma and Electron irradiation |
|
[277,278] | |
| Ethylene oxide (EtO) |
|
[58,60] | |
| Supercritical carbon dioxide (scCO2) |
|
[279,280] | |
| Preservation Methods | |||
| Lyophilization |
|
[65,262] | |
| Cryopreservation |
|
[281] | |
| Antibiotics and Antimycotics in −20/-80 °C |
|
[282] | |
Chemical reagents are commonly employed for the solubilization of cellular membranes, dissociation of DNA and disruption of lipid-protein interactions. Chemical reagents for decellularization include acids and bases, hypo/hypertonic solutions and detergents (ionic/non-ionic). Unlike chemical reagents, the implementation of enzymatic methods to decellularize tissue allows for the removal of undesirable components and cellular residue with high specificity for biological molecules. Enzymes are commonly implemented alongside other decellularization methods to accentuate cellular and genetic material removal by cleavage of cell-cell/ECM interactions and nucleic acids. Deleterious effects towards critical ECM components and substantial toxicity of commonly used chemical and enzymatic decellularization methods have incentivized the potential efficacy of other methods utilizing physical modalities to be investigated. Physical methods can prevent disruption of ECM structure, however, are typically ineffective for efficient decellularization on their own. It is commonly seen for physical methods to be combined with chemical and/or enzymatic modalities to accentuate the removal of cellular and genetic material by improving the penetration of other decellularization agents [56]. The improvement of decellularization agent infiltration is paramount for avascular tissues, such as hyaline cartilage and fibrocartilage. Additionally, all physical methods require the rinsing of the tissue to flush the structure of cellular and genetic material present after the physical method is implemented. Physical methods for decellularization include the use of freeze-thaw cycling, and high hydrostatic pressure.
3. Decellularized tissue preparation for medical use
3.1. Sterilization
Preceding implantation, it is paramount that biological constructs are sterilized and rid of existing genetic material and residual bacterial and viral content to minimize immunogenicity risk [57]. Commonly implemented terminal sterilization techniques include utilization of gamma or electron beam radiation, ethylene oxide and supercritical CO2. In addition, antibiotics/antimycotics are routinely used sterilization techniques during decellularization and handling in aseptic conditions. Moreover, the method of choice is dependent on size and complexity of the decellularized tissue graft and must prevent structural damage and ECM changes. Gamma irradiation (GI) is a cold process where the temperature of the sterilized product does not increase substantially, thus making it a suitable option for sterilizing biologically relevant materials. The source used for the sterilization process is the radioactive isotope, cobalt-60 [58]. Due to the insufficient energy, products treated with gamma irradiation via the cobalt-60 isotope do not become radioactive, resulting only in the destruction of existing microbes [59]. Electron irradiation (EI) is another cold sterilization process that utilizes radiation. Conversely, EI uses an electron accelerator as a source for its radiation. Both processes damage DNA and thus prevent replication of microorganisms that exist in the graft, however, can cause damage to the ECM collagen network. Processing materials with ethylene oxide (EtO) involves the exposure of the material to ethylene oxide gas. EtO acts as an alkylating agent that prevents the replication of microorganisms by damaging DNA and prevents cellular metabolism and division [60]. EtO is limited in terms of how much it penetrates the material, thus it only affects the surface. As well as being used as a means to decellularize tissue, supercritical CO2 (scCO2) has also been observed to have a sterilizing effect and has been used to sterilize natural [61] and synthetic [62] biomaterials. Due to the low viscosity and high diffusion coefficients, scCO2 liquid is able to penetrate biological grafts and extract undesirable material without causing substantial disruption of structural integrity and mechanical performance of the tissue [61]. This benefit is accentuated in thick tissues that require adequate penetration to decellularize and sterilize effectively. Moreover, the implementation of scCO2 is relatively non-toxic, making it an attractive decellularization/sterilization method for producing a construct with no immunogenicity [63].
3.2. Preservation
In addition to sterilization of final decellularized tissue constructs, preservation is also an important step as freshly decellularized constructs are often not feasible due to shortage of supply, requiring long-term preservation techniques. The goal of preservation is to have on-shelf products for clinical use. Considerations must be taken for developing appropriate preservation techniques for quality assurance and clinical translation of these tissue-derived products. Commonly implemented preservation techniques include lyophilization, cryopreservation and utilization of anitbioitics and antimycotics stored at −20 °C/-80 °C. For long-term storage of decellularized tissue grafts, they may be lyophilized to better preserve the material without causing substantial damage to the construct during processing. In order to prevent the formation of large ice-crystals, which may cause physical damage to tissues, low pressure is used to hasten the freezing process. By being performed at low pressures, the majority of the water present in the material is sublimated. Proceeding from the sublimation phase, the temperature is raised to break bonds between water ionically bounded to the construct, further drying the construct. This method is performed with non-toxic protective agents and eliminates need of low temperature storage [64]. Moreover, cryopreservation which involves the use of 10% DMSO and slow-rate of freezing or snap freezing in liquid nitrogen has also led to the preservation of tissue grafts with histological resemblance to non-preserved grafts [65]. Other methods of long-term preservation include the maintenance of decellularized tissue constructs in PBS containing antibiotics and antimycotics and stored at 4 °C, however only suitable for short-term preservation as well as stored at − 20 °C and −80 °C for longer term preservation [66,67]. Nevertheless, current methods lead to a progressive degradation of tissue architecture with compromising biomechanical properties limits clinical applicability and warrants further investigations [68,69].
4. Decellularized ECM-based grafts: various forms
Similar to the plurality of possible decellularization methods, there are also many possible graft forms that can be prepared from decellularized tissue. Common varieties include so-called “2D” scaffolds, ECM powders, hydrogels, composite grafts, and whole organs. Physical and chemical properties vary between graft types - the desired application dictates which type of graft form is used.
Clinical success has been shown for more “simple” graft architectures such as skin grafts and vessels, while complex grafts such as whole organs remain a challenge [70]. In clinical settings, intact tissue remains the most commonly used variety of decellularized graft tissue. Intact tissue refers to a graft that maintains its geometry once decellularized (it is not turned into a powder or hydrogel). The most popular clinically available intact grafts are decellularized dermis products such as GraftJacket®, Integra®, Dermagraft®, Apligraft® and Allopatch®. These decellularized dermis grafts are largely composed of collagen, making them versatile skin grafts. Intact decellularized dermis grafts are also used in tendon repairs, bone regeneration, hernia repair, wound healing etc.
4.1. Intact/powder ECM
Decellularization often compromises mechanical properties of tissues depending on the decellularization protocol employed; consequently, it is critical that most of the native ECM is not disrupted or removed while processing. Post-decellularization, tissues can be used for tissue remodeling and regeneration as grafts provided the ECM retains its functionality and provides necessary cues to support cellular proliferation and differentiation for guiding de novo tissue formation.
In a study using a decellularized vein as a graft for vascular tissue engineering, strategies were used to decellularize human greater saphenous veins, and its structure and composition were evaluated. Ultimately, the scaffold should retain enough of its native ECM for strength and stiff environment for cell attachment. Vascular application of this graft requires maintenance of structural and mechanical integrity to endure arterial implantation for its initial strength and pressure that occurs post-implantation [71]. The vein was prepared using a chemical detergent SDS to remove cells and washed with PBS. In vitro mechanical integrity assessment showed insignificant alterations to burst and suture strength given the decellularization processing; as a result, strengths were similar to that of the fresh vein. In applications demanding structure and mechanical integrity, intact decellularized tissue ECM is preferred over further processed tissue/organ ECM.
While intact tissues provide original tissue vasculature, the shape and form of the original tissue, and retained mechanical strength, decellularized tissue processed as a powder offers benefits that intact tissue cannot. Fabrication of tissue ECM powder consists of freezing and lyophilization of the decellularized tissue followed by pulverization and milling [72]. Additionally, powder can be formed by snap freezing the lyophilized tissue and grinding the product in a mill. The sample endures temperatures below −70 °C by using liquid nitrogen for the purpose of preserving the tissue. Prior to snap freezing, samples can also be saturated in NaCl to promote salt crystal precipitation; as a result, the powder shows a more uniform distribution once ground. Powdered tissue can fill areas and mold with the shape of the tissue defect; its ability to conform allows for minimally invasive procedures for implantation. ECM powders offer versatility in that they are often converted into gels and inks to be used as injectables. When constructing these powders and considering their applications, special attention is given to particle size, powder solubilization, and ECM crosslinking. For example, a range of particle sizes in a powder will still allow for cell growth in applications, but providing uniformly sized and distributed particles promotes homogeneous tissue formation. Attention to powder solubilization is also critical if the application requires processing these powders into injectable hydrogels. The derived powder is generally solubilized using hydrochloric acid to ensure proper enzymatic digestion. For in vivo use, the acidic pH from solubilization must be neutralized to a pH naturally found in the area of implantation. Because processing decellularized tissues into powders compromises its mechanical integrity, ECM protein crosslinking is usually performed to account for the lost strength.
Decellularized tissue powders were applied in an approach to create “tissue papers” using dECM powders obtained from porcine and bovine tissues and organs [73]. The powder was prepared through a variety of processes including dicing, decellularizing, washing, lyophilizing, and mechanically milling tissue. The use of this dECM powder allowed for control of the desired “tissue papers” as the powdered suspension was converted into an ink to be poured and dried for maximum control of size and shape. The conversion of powder to ink consisted of introducing the powder to a solvent mixture including evaporants, surfactants, plasticizers, and solubilized PLGA. Because mechanical integrity can be lost as a disadvantage in creating dECM powders, the ECM was mechanically milled into approximately 200 μm particles which showed to be large enough to retain mechanical properties in its native structure. In vitro results showed the adhesion, viability, and proliferation of human MSCs on “tissue papers” derived from decellularized porcine heart, kidney, liver, muscle, bovine ovary, and uterus.
In another study, Mazzitelli et al. utilized a powdered form of a decellularized urinary bladder to co-exist with Sertoli cells, or epithelial cells of testes, encapsulated within alginate based microparticles. This study was designed to investigate the effect of urinary bladder matrix (UBM) decellularized powder on the morphology of the alginate based microparticles, cell viability and behavior of Sertoli cells [74]. The incorporation of UBM in powder form was used to increase cell survival and function. Preparing UBM powder consisted of decellularizing porcine urinary bladder, which was prepared in sheets and then lyophilized, and mechanically milled into particles. Results showed increased levels of laminin, and integrin expressions for Sertoli cells encapsulated in UBM powder.
4.2. Decellularized tissue-derived hydrogels
Decellularized ECM tissue powder can be further solubilized and manipulated to form hydrogels. Decellularized tissue-derived hydrogels have expanded the potential use of dECM in vitro and in vivo as culture substrates that are both injectable and 3D printable [75,76]. This allows for their use to fill irregularly-shaped defects in a minimally invasive manner and to create precisely controlled decellularized tissue grafts [77].
The formation of hydrogels derived from decellularized tissue/organ typically involves the pepsin-solubilization of the dECM followed by physical crosslinking or the self-assembly of the collagen fibers to form a 3D network [78]. Smooth muscle ECM from caprine esophageal tissue was decellularized using hypo and hyper-molar sodium chloride solutions alternatingly, solubilized and then constructed into a hydrogel after adjusted to physiological pH and temperature [76]. The detergent-free method of decellularization improved the retention of sGAG and collagen content and ECM constituents. Moreover, the study demonstrated that the hydrogel induced differentiation of encapsulated adipose-derived mesenchymal stem cells towards smooth muscle cells (SMCs) without externally added factors, through expression of alpha-smooth muscle actin and myosin heavy chain, the hallmarks of SMCs. ECM-derived hydrogels are also utilized to deliver soluble factors, such as growth factors/biologics, and improve retention of transplanted cells [79]. Wu et al. utilized decellularized cardiac muscle tissue which self-assembled into a nanofibrous hydrogel at physiological temperature and was used to encapsulate cardiomyocytes (CMs) and loaded with SDF-1α [75]. The hydrogel improved retention of transplanted CMs and the GF promoted recruitment of endogenous cells. Intramyocardial injection of the hydrogel solution to the infarcted area led to the promotion of angiogenesis, inhibition of fibrosis, reduced infraction size and improved cardiac function. ECM-based hydrogels have the ability to mimic the physiological matrix environment, promote cellular adhesion, infiltration and proliferation. However, they often exhibit poor mechanical strength, rapid degradation and poor stability [80]. In efforts to mitigate these, researchers developed graded-concentration hydrogels composed of porcine urinary bladder matrix (UBM) as a dermal scaffold for chronic wound treatment [81]. Hydrogels were designed as a three-tiered gradient hydrogel of different concentration (low and high). The gradient dUBM hydrogel showed stability of cross-sectional area during collagenase degradation despite considerable loss of mass, as well as resisted fibroblast mediated contraction while supporting high surface cell viability through mechanical support provided by the denser layers of the dUBM.
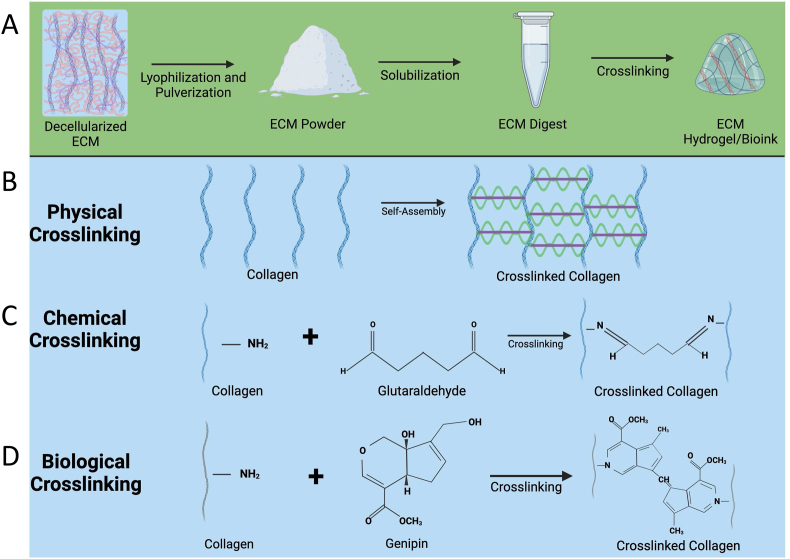
The self-assembly of hydrogels allow for milder crosslinking with improved cytocompatibility, however, these hydrogels are often mechanically weaker, have decreased stability and undergo rapid degradation, hindering their applications for tissue engineering. To improve these properties, hydrogels are often modified and subjected to chemical or biological crosslinking methods, as depicted in Fig. 2. In one study, human bone fragments were demineralized, decellularized and further processed by functionalizing with methacrylate groups to form a photocrosslinkable methacrylate bone ECM hydrogel [82,83]. The mechanical properties of the hydrogel demonstrated tunable mechanical strength with elastic modulus increasing as a function of photocrosslinking time while still retaining the nanoscale feature of the polymer networks. The resulting hydrogel demonstrated high cytocompatibility that supports vascularization of endothelial cells and led to the formation of an interconnected vascular network likely due to the presence of pro-angiogenic biomolecules in the bone ECM. Polyethylene glycol diacrylate (PEGDA) and decellularized annulus fibrosus matrix (DAFM) was combined to develop an injectable photocurable hydrogel for annulus fibrous repair [84]. The addition of PEGDA improved the mechanical strength of the DAFM hydrogels whilst maintaining the porous structure. The hydrogels were loaded with TGF-β1 and in vivo repair performance was assessed using a rat annulus fibrosus (AF) defect model. The implantation of the hydrogel sealed the AF defect, prevented nucleus pulposus atrophy, retained disc height and partially restored the disc biomechanical properties. Other chemical/covalent crosslinking agents, such as glutaraldehyde (GA), and carbodiimides or the use of secondary components that are capable of chemical crosslinking can be used to develop dECM biomaterials and grafts with the desired physio-chemical characteristics [84,85]. Compared to physical crosslinkers, chemical crosslinking creates more stable hydrogels, however there are concerns of possible cytotoxicity. The large volume of hydrogel biomaterial data that is available may help us to determine the safe chemical-crosslinkers to develop relatively stable and functional dECM biomaterials and grafts [86,87].
Fig. 2.
Development of dECM hydrogels/bio-inks. In the schematic representation (A), decellularized tissue undergoes a series of processing steps to form hydrogels/bio-inks. Initially, the tissue is decellularized, followed by conversion into a powder form. Subsequently, the powder is solubilized and digested to obtain dECM hydrogel. Cross-linking of dECM is essential to maintain mechanical strength and the stability of the structure in a biological environment. Different crosslinking methods can be employed for dECM cross-linking, as illustrated in panels (B), (C), and (D). Physical crosslinking (B) involves self-assembly under physiological conditions. Chemical crosslinking (C) can be achieved using agents like Glutaraldehyde. Biological/enzymatic crosslinking (D) can be facilitated using biological agents or naturally available materials such as genipin.
Since dECM is a tissue-derived material, researchers have also looked at using biological crosslinking methods. Common biological crosslinking agents include genipin and transglutaminase (TG) [86,88]. Genipin is derived from gardenia fruit and can bind with free amine groups of lysine or hydroxylysine to crosslink collagen hydrogels. Biological crosslinkers are naturally available chemical agents that can create cross-links and form covalent bonds with dECM. Due to their biological origin, they are proven to show improved biocompatibility. Genipin crosslinked decellularized nucleus pulposus hydrogels were developed and evaluated in a rat degenerated coccygeal intervertebral disc model [89]. The hydrogel developed with optimal genipin concentration (0.02%) demonstrated similar elastic modulus to human nucleus pulposus (NP), good biocompatibility and inducibility of expressing NP-related genes. In vivo studies showed that the hydrogel supported the survival of adipose-derived mesenchymal stem cells, improved the intervertebral height, and histological grading score. In another study, genipin-terminated 4 arm-poly(ethylene glycol) (GeniPEG) was synthesized. dECM-based hydrogels were formed by mixing GeniPEG and dECM at an optimum pH through crosslinking of dECM and self-crosslinking between the GeniPEG molecules [90]. The hydrogels crosslinked with GeniPEG exhibited greater tissue adhesive strength to porcine-derived aorta tissue compared to genipin crosslinking. In vivo studies demonstrated biocompatibility and biodegradability of the hydrogel which later expanded to be used for dECMs-GeniPEG hydrogels for sealing wounds and preventing post-operative complications.
In a systematic study, physical, chemical and enzymatic crosslinking methods were compared in developing dECM hydrogels [87]. Hydrogel derived from human umbilical cord were subjected to genipin or EDC crosslinking and the mechanical, degradation stability and biocompatibility were evaluated. Genipin and EDC crosslinking slowed the gelation time and increased the resistance against in vitro enzymatic degradation compared to physically crosslinked hydrogels, with genipin being more effective. Genipin crosslinking also revealed improved rheological properties compared to physical crosslinking. Both genipin and EDC crosslinking also enhanced the bio-stability without affecting mesenchymal stem cell proliferation, and neural stem cell growth and differentiation. In vivo studies demonstrated that genipin crosslinking allowed for in situ gelation and improved ECM retention for up to 2 weeks without any adverse tissue response or enhanced inflammatory reaction. These studies suggest that decellularized tissue can be modified via physical, chemical, or enzymatic cross-linking based on the need. For instance, temperature induced cross-linking (hydrogen bonding/collagen self-assembly) is used to form decellularized tissue gels for short-term or in vitro evaluation studies. On the other hand, dECM can be modified with chemical/biological cross-linking agent (covalent bonding) to develop decellularized tissue biomaterials/grafts for tissue repair and regeneration studies.
4.3. Composite grafts
Tissue-derived grafts are often used in the composite form to address loss of mechanical strength or inadequate mechanical behavior of the produced grafts. Additionally, tissue engineered grafts are designed to provide an environment in which cell-cell interactions as well as the surrounding matrix in terms of the bio-chemical composition can be controlled for a desired cell behavior and performance.
Of materials that are incorporated to assist the decellularized tissue, synthetic and natural polymers show promising features allowing for enhanced mechanical properties, desirable porosity, controlled degradation rates, and increasing binding sites. Producing polymer fibers acts to mimic the fibrous nature of the tissue ECM. Accurately creating these synthetic polymer fibers is done through methods such as electrospinning where critical characteristics such as fiber diameter and orientation can be completely controlled. The need for composite scaffolds arises in situations such as thrombus formation in the vasculature of decellularized tissues. Because the ECM is highly thrombogenic, it is critical that ECM in the engineered graft is not exposed to blood. In a study to address this, a polyester elastomer, poly (1,8 octanediol citrate) (POC) was incorporated to link heparin to the ECM based scaffold; as a result, the increased interaction of heparin and the ECM allowed for a decrease in thrombosis [77]. It was also seen that the presence of heparin immobilization after incorporating POC within the decellularized tissue, lead to increased proliferation of HUVECs which in a way moderated the interaction between ECM proteins and blood. In addition to cell proliferation and heparin binding, POC also offers mechanical integrity to the vasculature of the tissue thus providing mechanical support in heart valve tissue engineering. As decellularization removed some ECM components along with cells from the porcine aortic valve, the mechanical characteristics of the valve were also compromised. In efforts to address this, biodegradable polymers such as polyhydroxybutyrate (P3HB) and poly-4-hydroxybutyrate (P4HB) were introduced to a dehydrated decellularized tissue followed by rehydration. The addition of polymers led to increased biomechanics in suture retention and tensile tissue strengths of the dECM constructs [78]. Results showed increased proliferation of mouse fibroblasts on the composite graft in vitro using dECM-polymer composite grafts. In vivo studies conducted using a rabbit abdominal aorta patch implantation model, resulted in early inflammatory cell infiltration which steadily reduced in the following weeks. Also, there was no calcification or wall thickening present that would compromise the vessel's functionality.
Although a degree of degradation is desirable for the composite grafts, it is crucial that the integrity of the graft is not compromised during both decellularization and recellularization. For the best results, degradation rates of grafts should be at a slow enough rate where it can maintain cell proliferation while also eventually allowing for the production of the new ECM to create de novo tissue. In a study, three polymer compositions of different degrees of degradation were used to test the composite grafts for mechanical performance and degradation characteristics. For this reason, different structural compositions of polymers were used to determine a proper degradation rate. Polymers with high degradation rates lost a significant amount of mechanical integrity and became very brittle by the end of the cellularization-decell-recell cycle. It was found that the polymer with medium degradation rate allowed for the best ECM deposition while also maintaining the structure of the ECM. Polymers can also be incorporated as fiber mats through electrospinning tyrosine-derived polycarbonates. Another study utilizing different extracellular matrices with polymeric structures showed that chondrocytes cultured in these fiber mats displayed higher differentiation tendencies. The pDTEC fiber mat in the form of template structure provided mechanical basis and stiffness for in vitro testing of cell proliferation and differentiation [91]. When introduced, ECM covered the fiber mat surface while also retaining pore structure leading to increased cell attachment.
There are several advantages of incorporating synthetic and natural polymers in ECM grafts including their biocompatibility, biofunctionality, and having control over physio-chemical properties. Composite scaffolds also allow for the incorporation of tethered or immobilized peptides and GFs to further enhance bioactivity and performance of dECM. In addition to incorporating polymeric fibers, osteoinductive components such as hydroxyapatites and calcium phosphates can be introduced to synergistically improve osteogenic performance of the composite grafts for bone tissue engineering. In a study where chitosan and nano-hydroxyapatite particles were incorporated onto a decellularized goat-lung matrix, factors such as osteoblast attachment and proliferation were enhanced. The increased degree of crosslinking between the collagen-based tissue and CS/nHAp composite allowed for better stiffness and attachment of cells. The incorporation of the CS/nHAp provided a bone-like environment which led to enhanced cellular attachment and increased proliferation of the seeded osteoblasts. Not only did the additive CS/nHAp provide the desired environment for cells, the resulting surface roughness on the composite ECM graft also increased the tendency for cell attachment.
4.4. Whole organ
Whole organ decellularization serves as a method to provide an organ template to be recellularized and implanted in order to address donor organ shortage. When preparing for whole-organ decellularization, it is critical to consider the resulting decellularized organ composition and potential host response post-implantation. In addition, the vascular and neural network of the decellularized organ to be intact to allow for re-cellularization.
Antegrade or retrograde perfusion is a common method to decellularize whole organs without disrupting the organ's structure [92,93]. This technique delivers decellularizing agents through the vasculature of the organ. Retrograde perfusion has been performed on hearts where agents such as Triton X-100 and SDS are delivered through a cannulated aorta followed by perfusion of deionized water and PBS. Perfusion was again performed to ensure that the vasculature of the heart remained intact after delivering decellularizing agents. An advantage to decellularization by perfusion is the ability to control the time of decellularization by adjusting the pressure at which the perfusate is pumped. Progressively increasing the perfusion pressure causes the vessels to dilate; as a result, flow rate increases allowing for quicker cell removal. Because of the progressive increase of pressure, the vessels are not damaged from the dilation.
In addition to hearts, perfusion is also an advantageous technique in decellularizing lungs as they contain two systems of entry for perfusion: the vasculature and airway and alveolar system. Because of the additional structure of the airway compartment, decellularization perfusion can be adequately performed strictly through the vasculature as it is done with hearts, or through the airway compartment and alveolar structures to quicken the process. To further remove cellular content, organs can be repeatedly incubated with NaCl and DNase to discard any remaining nuclei and DNA [94]. Another way to remove cell residue is distributing supercritical carbon dioxide through the tissue. This process is commonly used as it provides an inert gas and allows for the maintenance of the organ basic structure and mechanical integrity.
To tackle liver disease and shortage of donor organs, Uygun et al. presented a potential solution in the form of generating decellularized livers for transplantation [95]. The rat livers were decellularized through portal vein perfusion with special attention given to maintaining the vascular network, which would be reconnected to the body's circulation after implantation. To ensure that the vasculature remained intact, Allura Red dye was delivered through perfusion, which clearly displayed the vascular network within the translucent matrix. Although the study displayed an efficient decellularization process for rat livers, some of the vascular integrity was lost due to the removal of nonparenchymal cells, such as the liver sinusoidal endothelium. As a result, the recellularization process could be improved to incorporate nonparenchymal cells to restore vascular network and their integration with the body's circulatory system.
5. Decellularized ECM: tissue engineering applications
Developments in techniques for decellularized ECM-based biological scaffolds have come a long way and have since been increasingly considered for tissue engineering and regenerative medicine strategies [92]. Initial and current advancements in whole organ decellularization focus on perfusion-based techniques, taking advantage of the large native vascular network. Perfusion decellularization is based on the pressure induced perfusion of various detergents, chemicals and enzymatic treatment through the vasculature network for the removal of cellular material with minimal damage to vital ECM components and 3D architecture. Perfusion based decellularization treatments largely depend on the mechanical, thickness/density characteristics and type of the whole organ. For instance, complex organs often require higher concentrations and longer exposure times of agents for complete decellularization without compromising the native ECM. The acellular organ constructs are then recellularized with relevant autologous or stem cells and cultured in a bioreactor for the development of functional tissue engineered organs to alleviate the shortage of available organs for transplantation. Many organs have been decellularized for this purpose including heart, liver, lung, and kidney.
5.1. Heart
In 2008, Ott et al. first described decellularization of rat hearts through coronary perfusion of four different detergents [96]. The resultant decellularized tissue was visibly translucent, allowing for reperfusion following treatment. Upon recellularization with neonatal cardiomyocytes, heart tissue showed electric and contractile responses to single pace electrical stimulation. In efforts to optimize decellularization methods for preservation of ECM properties, Seo et al. developed a detergent free method using supercritical carbon dioxide and ethanol co-solvent treatment [97]. Decellularized heart tissue retained more ECM components such as collagen, GAGs, laminin, fibronectin and angiogenic factors, compared with the detergent treated tissue.
Several recent studies have also focused on developing decellularized heart valves with anti-calcification properties. Efforts include the formation of a balanced charged network that prevent the transport of Ca2+ ions and enzymes, use of VEGF encapsulated within PCL nanoparticles and the use of osteoprotegerin [[98], [99], [100], [101]]. In order to enhance in vitro recellularization of heart valves, VeDepo et al. investigated the effects of bioreactor conditioning parameters [102]. Reseeded ovine aortic heart valves were exposed to varying conditions of hypoxia/normoxia and high/negative cyclic pressures. Results demonstrated that hypoxic conditioning led to increased cellular infiltration into the valve leaflet tissue compared to normoxic conditioning. In another study, a porous MMP degradable PEG hydrogel incorporated with SDF-1α combined with decellularized porcine aortic valves were fabricated [103]. The hydrogel inclusion led to enhanced BMSC adhesion, viability and proliferation, as well promoted MSC recruitment while facilitating M2 macrophage phenotype polarization in a rat subdermal model.
To overcome the major obstacles in the field, research has been focused on the development of cardiac patches with functional vascularization for the repair of malformed/damaged myocardium and valve reconstruction [[104], [105], [106]]. In one study Jang et al. developed a hdECM-based bio-ink that was 3D printed for fabrication of multiple cell-laden patches (cardiac progenitor cells [CPC] and MSCs) [105]. The precise dual patterning of the cells and use of tissue-specific bio-ink with VEGFs promoted vascularization with enhanced cardiac function when implanted subcutaneously in nude mice, compared with a CPC patch. In another study, decellularized myocardium slices (dPMS) were reseeded with hMSCs with the goal of assembling prevascularized tissue. These dPMS supported cell attachment and survival, demonstrated thickness dependent cell seeding efficiency, and induced endothelial differentiation of hMSCs. This study noted limitations with static seeding and efforts have been made by other researchers using perfusion bioreactors to enhance recellularization cell density and subsequent vasculature formation of thick constructs [107].
5.2. Liver
Perfusion based decellularization and recellularization via the vasculature of the construct has also been explored for other organs with differences in vasculature mode of entry/routes available for decellularization and redelivery of cells for functional repair. Liver decellularization has been performed through perfusion via the portal vein, inferior and superior vena cava or the hepatic artery [108]. In 2010, Uygun et al. first utilized SDS for the decellularization of liver tissue via the portal vein over the span of 72 h [95]. Immunological staining revealed retention of ECM proteins namely collagen type I and IV, fibronectin and laminin-β1 and approximately ∼50% of GAGs.
Efforts in the recent years have been to accelerate and improve the efficacy of the decellularization procedure [[109], [110], [111], [112]]. In a study by Willemse et al., porcine livers were decellularized using Triton X-100 or Triton X-100 in combinations with SDS, maintained under constant pressure perfusion (120 mm Hg) [113]. Analysis post decellularization revealed effective cell and DNA removal with the Triton X-100 only protocol retaining 1.5 and 2.5 times more collagen and sGAG, respectively compared with treatment with Triton X-100 and SDS. When applied to human livers, the Triton X-100 only decellularization protocol with pressure-controlled perfusion showed semi-transparent liver within 20 h, which was otherwise not achieved without pressure-controlled perfusion within a span of 64–96 h. Another study decellularized whole porcine livers using milder agents, namely through perfusion of saponin, sodium deoxycholate and deionized water. Using this method, authors produced an acellular scaffold with intact vasculature and preserved ECM (including collagen I and IV and laminin) in less than 24 h, reducing the time even further [108]. A study by Watanabe et al. sought the formation of hierarchical vascular network in recellularized livers to overcome the challenge of damaged vascular network during decellularization [114]. By subjecting fibronectin coated decellularized livers to perfusion culture at 4.7 ml/min, authors demonstrated the formation of sinusoid-scale micro-vessels via angiogenesis, which was otherwise not observed in static culture. These studies suggest that mechanical and adhesion factors may play a role in construct of vascular networks. Sustained perfusion for up to 15 days has also been recently demonstrated in immunosuppressed pigs [115]. The recellularization of livers and the re-establishment of the biliary system and vasculature are of utmost importance for the restoration of bile flow and proper hepatic function.
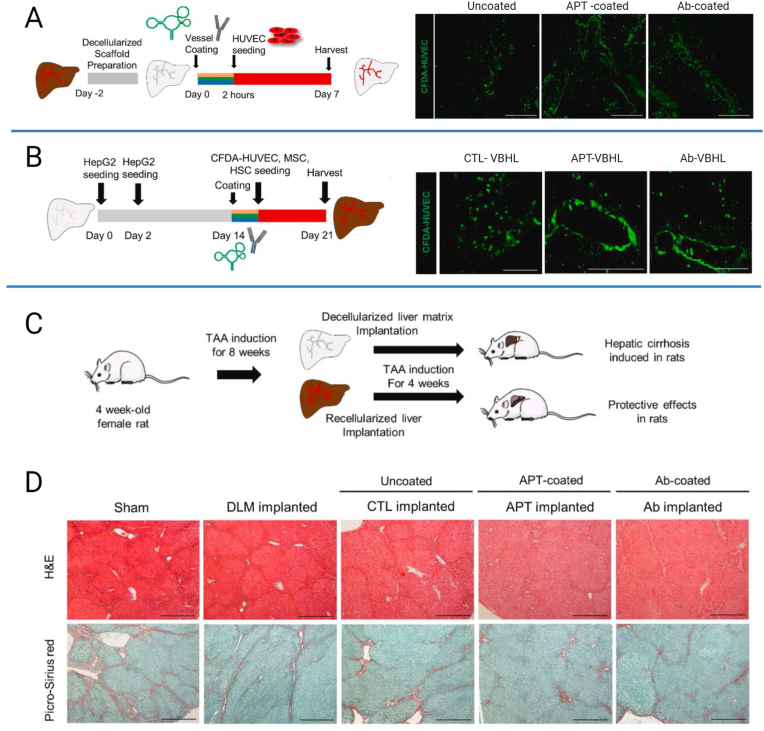
Vascularized bioengineered human livers (VBHL) were also fabricated using decellularized liver [116]. Decellularized liver constructs were coated with anti-CD31 aptamer (APT-coated) or anti-CD31 antibodies (Ab-coated), seeded with HUVEC cells and subjected to bioreactor culture for 7 days. The HUVECs in the presence of the anti-CD31 aptamer coating formed continuous endothelium along the vascular lumens, promoting more efficient endothelization through the liver construct than the anti-CD31 antibody (Fig. 3A). To repopulate the liver scaffolds with the parenchymal and non-parenchymal cells, different delivery routes and seedings were employed at different times by cannulating both the bile duct and portal vein. After additional 7 days of culture, the αSMA-positive cells, regarded as integrated MSCs, were distributed around the perivascular region of the anti-CD31 aptamer-coated vessels and interconnected with ECs, unlike the anti-CD31 antibody coated vessel which was unable to form complete vascular-like structures (Fig. 3B). In vivo evaluation of the VBHL scaffolds was also investigated in a rat hepatic cirrhosis model induced by TAA administration and implantation into the interlobular space of the liver (Fig. 3C). H&E and picrosirius red staining showed that the APT-VBHL constructs attenuated hepatic fibrosis compared to the decellularized liver matrix (DLM) and sham groups which showed eosinophilic changes and interlobular septal thickening (Fig. 3D). The study showed that reconstruction of a vascularized liver construct using anti-CD31 aptamer coating that promotes the re-endothelization supported liver functions in a rat model of liver fibrosis.
Fig. 3.
Generation of VBHL constructs for vascularized liver reconstruction. (A) Schematic diagram illustrating the strategy for re-endothelialization of the decellularized rat liver constructs with HUVECs. Representative immunofluorescence images of endothelialized vessels with CFDA-labeled HUVECs in the scaffolds without the coating agent (Uncoated) or with the following coating agents; anti-CD31 aptamer (APT-coated) and anti-CD31 antibody (Ab-coated). Three constructs were fabricated for each group and the scaffolds were cultured within a bioreactor for 7 days. Scale bar, 200 μm. (B) Schematic diagram of the strategy illustrating the recellularization of the decellularized rat liver constructs with HepG2 cells, LX2 cells, HUVECs and MSCs. Representative immunohistochemical images of CTL-VBHL, APT-VBHL and Ab-VBHL constructs stained with α-SMA (red). CFDA-labeled HUVECs (green) and nuclei (blue) were also detected. Scale bar, 100 μm. (C) Schematic diagram of the strategy used to implant VBHL constructs into the TAA-induced cirrhotic rats. After 8 weeks of TAA induction, the rats received heterotopic implantation of decellularized liver matrix (DLM implanted) or VBHL constructs (CTL-VBHL; CTL implanted, APT-VBHL; APT implanted, Ab-VBHL; Ab implanted). (D) Representative H&E and picrosirius red staining images of harvested host liver tissue sections after 4 weeks of implantation. Scale bar, 40 μm. Adapted from the study by Kim et al. with permission of Biomaterials, copyright 2023 [116].
5.3. Lung
Lung decellularization routes include both the airways and vasculature and are focused on the restoration of proper gas exchange for function and regeneration. Several groups have explored lung decellularization by means of perfusion of various decellularization agents including SDS, Triton X-100, and CHAPs [[117], [118], [119], [120]]. In 2010, Ott et al., performed pulmonary arterial perfusion of 0.1% SDS and 1% Triton X-100 over the span of 72 h [121]. Whole lungs repopulated with HUVECs and rat fetal lung cells demonstrated repopulation of entire pulmonary scaffolds; however, ultimately presented with pulmonary edema when tested in vivo. Since then, many of the early studies demonstrated ability to support elementary organ function, but caused microstructural damage to both the airways and vascular network leading to pulmonary leakage and limited function [122,123].
One recent study by Young et al. investigated the need for dlECM enhancement with basement membrane proteins following decellularization for proper epithelial and alveolar barrier formation [124]. In vitro coating of dlECM supplemented with laminin or fibronectin demonstrated superior alveolar epithelium barrier function. This increased barrier resistance was associated with an up-regulation of junction proteins, including Claudin-18, which may play a role in the stabilization of the alveolar barrier. In another study, Obata et al. investigated the use of a natural fatty acid, soap potassium laurate (PL) as an alternative to the harsh detergent SDS [125]. Rat lung decellularized with PL demonstrated cellular removal with improved preservation of architecture (elastin microfibrils, sGAG and ECM proteins) compared to SDS decellularization. PL-decellularized scaffolds also showed increased uniform distribution of rat epithelial cells. Furthermore, few recent studies also demonstrate the effect of Epac agonist in improved endothelial barrier function with increased junction proteins, which can be useful for improved vascular barrier formation [124,126].
Further research is needed to advance decellularization and recellularization techniques to minimize lung ECM component loss and ECM damage. This is to allow for appropriate cell distribution upon recellularization of the airways and vasculature for gas exchange, compliance, and proper in vivo lung function.
5.4. Kidney
Like whole lung decellularization, kidney decellularization is commonly achieved via the vasculature, namely the renal artery as well as the ureter. In 2009, Ross et al. reported the first decellularization of whole rat kidneys in which decellularization was achieved through arterial perfusion of various agents including Triton X-100, SDS, sodium deoxycholate and DNase [127]. Post-recellularization with murine embryonic cells, cells exhibited apoptosis forming lumens and progressive loss of embryonic features suggesting differentiation. Recently, research has been focused on minimizing damage to the renal microstructure and vascular for improved re-endothelization and creatinine/urine production for functional kidney development [[128], [129], [130], [131]].
For maintenance of vascular integrity of acellular kidney scaffolds, two different treatment protocols (SDS + DNase or Triton X-100 + SDS) were employed for the decellularization of pig kidneys for a total of 97 or 144 h, respectively [132]. Triton X-100 + SDS treated kidneys demonstrated improved intact microvascular architectures with vascular patency in comparison to the other protocol which presented disrupted vascular morphology and led to blood extravasation. However, for re-endothelization with MS1 endothelial cells, both protocols showed decreased platelet adhesion resulting in blood vessel thrombosis. In a recent study, decellularization of sheep kidneys was performed by perfusion of Triton X-100 in combination with SDS or SDS only [133]. Treatment of SDS only led to extravasation and blood leakage in vitro and in vivo in a sheep model due to poor vascular integrity that was otherwise not detected in the other treatment and demonstrated vascular integrity and function for up to 12 h. In another study, rat kidney grafts were decellularized by renal artery perfusion of SDS for 6 h. Decellularized grafts exhibited intact scaffold microarchitecture of the glomeruli and tubules with an intact blood vessel integrity [134,135]. When infused with human induced pluripotent stem cell-derived endothelial cells, histological examination demonstrated recellularization within the cortical region of the kidney, distributed in the arterial structure and glomerular capillaries with cell maintained expression of endothelial marker CD144 [135].
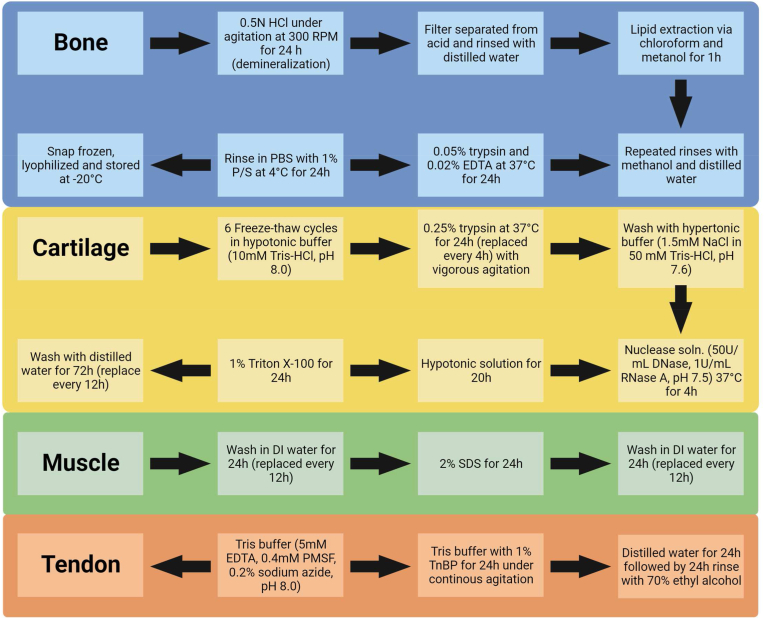
Although most initial and current studies focused on perfusion-based treatment for whole organ decellularization, other tissues do not contain a vascular network to allow for these methods, therefore other decellularization approaches and methods were developed. The most common methods developed utilized agitation, but immersion, pressure gradient systems, and supercritical fluids were employed as well [136]. Using these methods, decellularization of different tissues was employed including cartilage, bone, muscle and tendon. Commonly employed decellularization techniques (combination of physical, chemical and enzymatic) for bone, cartilage, muscle and tendon tissues are shown in Fig. 4.
Fig. 4.
Musculoskeletal tissue decellularization protocols. Commonly employed decellularization techniques/protocols for bone, cartilage, muscle, and tendon tissues. These protocols utilize the combination of physical, chemical and enzymatic decellularization methods to achieve better outcomes in terms of DNA removal while preserving tissue mechanical properties and bio-chemical composition.
5.5. Bone
As an alternative to bone grafting, decellularized bone derived scaffolds have been investigated for bone repair strategies. Early studies focused on the demineralization of bone using an acidic treatment for the removal of mineral components whilst leaving proteins, calcium-based solids, inorganic phosphates and some trace cell debris [137,138]. Later studies also focused on the removal of cellular constituents from the source, known as decellularization [139,140]. Since then researchers have investigated the osteoconductive potential of decellularized bone ECM (dbECM) to be used as a natural bioactive biomaterial [141]. Numerous studies have demonstrated the osteoinductive ability of the dECM matrices which can induce osteogenic differentiation and bone formation in vitro and in vivo [[142], [143], [144]].
Additionally, decellularized bECM is often used in combination with collagen, hydroxyapatite (HA), BMP and other relevant GFs for enhanced osteogenesis and bone formation [[145], [146], [147]] [[145], [146], [147]]. In one study, decellularized bone scaffolds were coated with a collagen/HA mixture and loaded with SDF-1α [148]. The results of the study demonstrated enhanced osteogenesis of MSCs in vitro and evidence of recruitment of endogenous stem cells when subcutaneously implanted. In another study, Rindon et al. developed heparin conjugated dbECM particles tethered with PDGF-BB (HP-DCB-PDGF) in order to promote sustained release of the growth factor and provide synergistic osteogenic cues [149]. Compared to grafts without PDGF-BB, all grafts with the growth factor exhibited increased osteogenic differentiation with HP-DCB-PDGF presenting significantly greater calcium deposition compared to grafts containing only PDGF by ASCs in vitro.
Furthermore, hydrogels fabricated from decellularized bones have also been investigated through solubilization of dbECM [140,143,150]. Alom et al. studied the osteogenic potential of dbECM in the presence and absence of osteogenic medium [150]. Immunocytochemistry staining revealed higher levels of osteogenesis specific proteins, OPN and OCN expressed by mouse primary calvaria cells when cultured on dbECM hydrogels in both osteogenic and basal medium. However, when cultured on collagen type I hydrogels or TCP, cells only expressed OPN and OCN when cultured in osteogenic medium, indicating the osteogenic potential of dbECM without osteogenic supplements. Similar results were also demonstrated by Paduano et al., in which cells cultured on dbECM hydrogels had a significant up-regulation of RUNX-2 and BSP in the absence of osteogenic inducers compared to when cultured on Col-I hydrogels [143]. The bone regeneration capacity of non-tissue specific ECM was also evaluated. In this study, injectable hydrogels were developed from decellularized porcine skin incorporated with biphasic calcium phosphate powder (BCP) [151]. Biochemical analysis of these hydrogels revealed retention of collagen and GAG content after solubilization along with trace amounts of VEGF and BMP-2. In vivo evaluation demonstrated increased bone formation at 8 weeks in hydrogels containing BCP (ECM-BCP) compared to those that did not. Moreover, the hydrogels showed evidence of bone formation by endochondral ossification by bridging and connecting the fracture ends with collagen cluster and bone formation colocalized with osteoblasts/osteoclasts after 4 and 8 weeks, respectively. As hydrogel use in bone tissue repair is often limited due to their low mechanical properties, hydrogel reinforcements must be considered for their use in load-bearing settings.
5.6. Cartilage
In 2010, Yang et al. describe the decellularization of cartilage powder by chronological treatment with trypsin, nucleases, hypotonic buffer and Triton X-100 [152]. The acellular powder was then crosslinked with UV irritation and when seeded with MSCs showed good biocompatibility. Evaluation of the tissue demonstrated cell fragment and DNA removal, however, witnessed some level of disruption in terms of cartilaginous structure and mechanics. Since then efforts have been focused on improvements in decellularization methods/protocols for limited disruption to the native architecture/loss of cartilaginous ECM proteins for improved chondrogenesis and mechanical function [[153], [154], [155]] [[153], [154], [155]] [[153], [154], [155]].
Recent research has focused on improvements in the treatment methods needed to successfully decellularize the intrinsic dense and compact structure of cartilage. In one study, cartilage sheet samples were decellularized using two different treatments of SDS or Triton X-100 [25]. By preparing thin sheets, the tissue was decellularized using gentle treatment compared to traditional treatments and even displayed presence of growth factors including TGF-β1, IGF-1 and BMP-2. However, depending on the application, these sheets may not contain adequate mechanical properties. The application of ultrasonic bath was also explored to improve the penetration of decellularization reagents, however requires further advancements to improve the decellularization efficacy [156].
Various studies focused on scaffold structure development and approaches for mechanical enhancement of dcECM including different crosslinking methods, composite scaffolds, or electrospun and thermoplastic reinforced scaffolds [[157], [158], [159]] [[157], [158], [159]] [[157], [158], [159]]. Browe et al. developed decellularized cartilage ECM-derived (dcECM) scaffolds that were crosslinked using glyoxal and dehydrothermal treatment [160]. These scaffolds supported cartilage ECM synthesis when seeded with fat pad derived stromal cells (FPSCs) and displayed high elastic properties when evaluated by compression tests. However, the mechanical properties were still inadequate compared to native AC and noted the need for additional mechanical support. One strategy developed electrospun gelatin-polycaprolactone nanofibers and dcECM composite scaffolds and showed that the integration of the nanofibers significantly increased the mechanical properties while the dcECM led to increased secretion of cartilage-specific proteins [161]. While another study fabricated scaffolds by combining PLGA and decellularized cartilage powder via solvent casting/salt-leaching technique followed by EDC/NHS mediated crosslinking [162]. Scaffolds produced by this method revealed increased compressive strength (0.89 MPa), which is comparable to native cartilage.
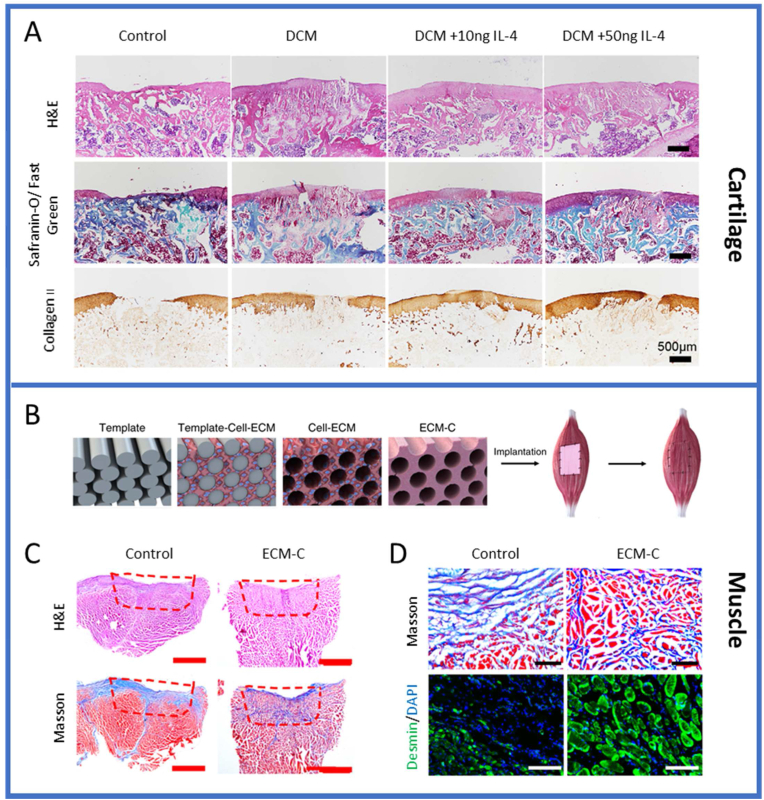
Meanwhile there are a few attempts to process cartilage as a whole tissue. Luo et al. investigated the decellularization of whole cartilage explants by introduction of channels to allow for infiltration of decellularization agents and subsequent recellularization [154]. Results showed ∼90% DNA reduction with near complete sGAG removal, little increase in porosity of the tissue and decreased mechanical properties. When reseeded with FPSCs, the channels supported cell viability however, demonstrated limited cell migration into the explant ECM and thus inadequate recellularization. In efforts to improve this, another study utilized laser surface engineering for creation of micropores onto the surface of cartilage implants [163]. After 8 weeks of culture, these laser-modified scaffolds exhibited improved cell attachment and evidence of ECM deposition on the surface and within the micropores by rabbit chondrocytes in vitro, which is attributed to enhanced porosity and surface roughness. Recently, Golebiowska et al., developed a rapid protocol to decellularize articular cartilage while retaining ECM and biochemical components including GAGs, Collagen II and some of the growth factors native to articular cartilage [164]. Decellularized cartilage matrix (DCM) was developed and combined with intra-articular injection of M2-polarizing cytokine IL-4 and assessed in an in vivo osteochondral rat defect model [165]. Evaluation of the osteochondral defect regeneration showed that compared with the control group (no implant), partial hyaline cartilage regeneration was achieved in the DCM group after 8 weeks, and a specific dose of IL-4 (10 ng) achieved a better repair effect (Fig. 5A). The Safranin O and fast green staining showed that the DCM +10 ng IL-4 group promoted regeneration of hyaline-like tissue at 8 weeks and the effect of subchondral bone reconstruction was better than that of the other groups. Immunohistochemical results also showed that collagen type II deposition increased in all the DCM groups, with DCM+ 10 ng IL-4 outperforming the other groups. The study demonstrated that the immunomodulatory effects of the cell-free DCM scaffold using IL-4 could achieve cartilage regeneration in a rat knee osteochondral defect model.
Fig. 5.
In vivo evaluation of the decellularized tissue biomaterials/grafts for tissue repair and regeneration. (A) Histological evaluation of in vivo cartilage regeneration after 8 weeks. Safranin O- Fast green and immunohistochemical staining of COL II of repaired cartilage in different groups after 8 weeks. Adapted from the study by Tian et al. with permission of Acta Biomaterialia, copyright 2023 [165]. Muscle regeneration of the rat tibialis anterior (TA) muscle defects treated with ECM-C or control scaffolds, (B) Fabrication of ECM-C and control scaffolds. The schematic diagram of the PCL fiber template, template-cell-ECM, cell-ECM, ECM-C and control group during the preparation process and implantation. (C) Macroscopic view of regenerated TA muscle defects by staining the cross-sections with Masson trichrome and H&E. (D) Microscopic images of the cross-sections of regenerated TA muscle at 1 month, stained with Masson trichrome and immunofluorescently stained for desmin. Adapted from the study by Zhu et al., open access, https://creativecommons.org/licenses/ [166].
5.7. Muscle
Decellularized ECM has also been applied to skeletal muscle, specifically in cases of irreversible volumetric muscle loss (VML). Many current studies have made developments in micro-/nano-architecture fabricated constructs to mimic the native myofiber alignment and network structure [167,168]. In one study, Choi et al. developed sinusoidal wavy polystyrene surfaces (wavelengths 20, 40 and 80 μm) coated with decellularized muscle ECM [169]. The combination of the two led to the formation of multinucleated myotubes that were well aligned as well as exhibited enhanced myogenic differentiation compared to collagen-coated and non-coated substrates. In another study, electrospun scaffolds were developed of aligned nanofibers of PCL and decellularized muscle tissue [170]. Unidirectional alignment of nanofibers supported primary satellite cell proliferation and differentiation in vitro. When tested in a murine model, increased myofiber regeneration was observed at day 7 and 28, however, with limited improvements in muscle force production, possibly owning to the need of longer time-points for evaluation [171]. Results from these studies indicate the influence of topographical and biochemical cues on cellular behavior and promoting myogenic activity.
Further studies have utilized exogenous GFs to enhance cellular recruitment and infiltration and aid in functional muscle regeneration. In one study, macroporous sponges were developed from decellularized mECM with chemically immobilized SDF-1α within the constructs [172]. These constructs showed significantly higher infiltration of muscle-derived stem cells with better distribution compared to no SDF-1α. In vivo results showed increased infiltration of CXCR4 + cells as well as significantly increased number of vessels, indicating higher induction of angiogenesis. In another study, a biofunctionalized scaffolding system was developed consisting of decellularized mECM and IGF-1 [173]. In vitro testing showed that these scaffolds led to higher cellular infiltration of C2C12 cells as well as higher myosin heavy chain expression and myotube formation compared to the control groups [173,174]. Moreover, these cell-free scaffolding systems were also tested in rabbit tibial anterior muscle defect models and demonstrated higher host cell infiltration and greater number of muscle fiber formation compared to collagen and dECM groups [174].
In order to improve the homogenous decellularization and preservation of architecture and bioactive cues/functional capacity, perfusion-based methods have also been adopted for large and thick tissues, such as muscle tissue. Through perfusion of enzymatic and detergent treatments, Zhang et al. decellularized porcine rectus abdominal muscles [175]. The obtained tissue retained the intricate architecture and internal vasculature while also preserving bioactive components and mechanical properties compared to native tissue. ECM scaffolds with aligned microchannels were also developed and assessed in vivo for skeletal muscle defect regeneration [166]. ECM scaffolds were engineered with parallel microchannels (ECM-C) by subcutaneous implantation of sacrificial templates, followed by template removal and decellularization (Fig. 5B). Histological staining showed a large number of cells infiltrated the interior of the scaffold within the ECM-C group with the deposition of large amount of new ECM (Fig. 5C). Selected regions stained either with Masson trichrome or for desmin revealed the presence of a high density of neo-muscle fibers within ECM-C, while only sparse neo-muscle fibers were seen around the control scaffolds-treated defects (Fig. 5D). These studies demonstrate the potential use of decellularized ECM-based scaffolds for various tissue repair and regeneration strategies.
5.8. Tendon
The burdens of tendon injuries have also caused researchers to create bio-functional and bio-mechanical tendons for injury repair to which decellularized grafts represent promising alternatives [176]. Due to the dense compact structure of tendons, thin sheets or slices have largely been used and developed for enhanced decellularization and subsequent recellularization/infiltration efficacy [177,178]. In one study, book-shaped scaffolds were prepared by stacking multi-layers of decellularized tendon slices and BMSC sheets [179]. In vitro studies revealed homogenous distribution and alignment of cells as well as an up-regulation of tendon-related genes, including tenomodulin and Alpha-1 collagen type I, compared to the control. In another study, multilayer decellularized tendon slices were prepared for reconstructing large rotator cuffs in rabbit models [180]. Results showed that these grafts promoted host cell ingrowth along with fibrocartilage and bone formation at the tendon-bone interface that led to improvements in the mechanical properties.
In order to improve homogenous cellular distribution (whilst reseeding or cellular infiltration), dynamic culture/mechanical stimulation has been explored recently [181,182]. For the purpose of replicating mechanical properties of the native ACL, Lee et al. subjected tendons to simultaneous tension and torsion in a bioreactor through biaxial cyclic loading [183]. The use of the bioreactor led to significantly increased expression of tendon-specific genes and ultimate tensile load and stiffness of the recellularized tendons. In a recent study, tendons were seeded with MSCs and subjected to bidirectional perfusion and stretch cycling [184]. The use of this bioreactor demonstrated similar results with homogenous distribution of cells with superior production and organization of newly formed collagen compared to static culturing.
Similar to decellularized muscle applications, decellularized tendon scaffolds have also been incorporated with exogenous GFs for enhanced tenogensis including TGF-β3 and BMP-12 [185,186]. Few decellularized triphasic scaffolds have also been developed for tendon to bone ruptures for enthesis repair [187,188]. In these studies, tri-phasic tendon-fibrocartilage-bone interfacial tissue constucts were developed to facilitate tendon-to-bone healing. In a recent study, a gradient book-type triphasic scaffold was developed and showed superior osteogenic, chondrogenic and tenogenic inducibility in the respective regions [189]. When implanted in a rabbit BTI injury model, the scaffolds showed accelerated healing with attained triple biomimetic structure and cellular distribution.
6. Improved decellularization methods
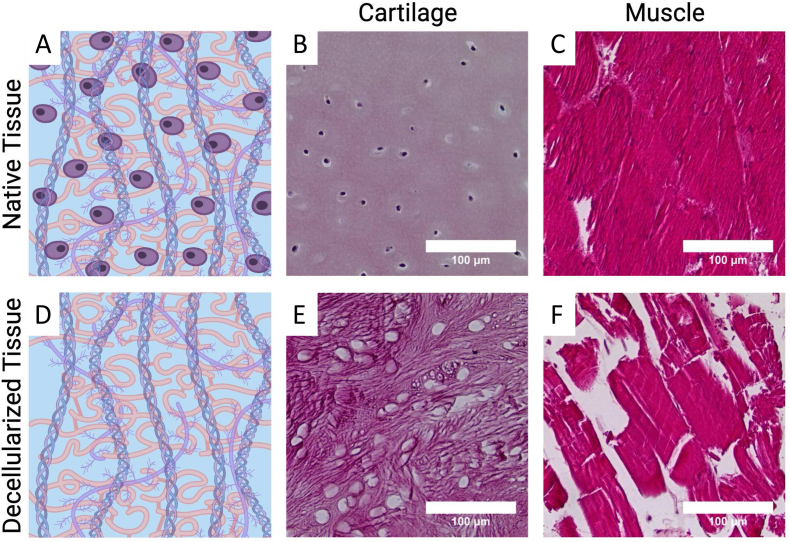
Due to the large variety of tissues/organ sources and protocols investigated thus far, standard criteria for establishing decellularized tissues have been proposed [92,190,191]. In general, these include the absence of visible cell nuclei and removal of DNA content which is critical to minimize the potential adverse host responses to ECM products [192]. Along with assessing the removal of cellular and genetic material, the perseveration of proteins and other ECM components such as collagen, glycosaminoglycans and GFs as well as mechanical properties is also required (Fig. 6) [190]. Maintaining the native milieu of biochemical and biomechanical cues is essential for providing proper signaling that are required for governing cell behavior and cell-cell/cell-ECM interactions for recellularization and restoration of functional tissues/organs.
Fig. 6.
Schematic of native tissue containing various ECM components and cells (A) and decellularized tissue void of cells (D). Histological H&E stained images of native cartilage tissue (B), native muscle tissue (C), decellularized cartilage tissue (E) and decellularized muscle tissue (F). Native tissue demonstrating intact extracellular matrix with presence of cells while decellularized tissue demonstrating lack of cell nuclei presence with some disruption in the matrix structure. Part of this figure is adapted from the study by Golebiowska et al. with permission of Annals of Biomedical Engineering, copyright 2023 [164].
Long exposure times to harsh decellularization reagents cause significant reduction in ECM components [193,194]. Recent efforts have been in the area of improving decellularization outcomes via use of milder detergents and other reagents (e.g. salt solutions and enzymes), pressure-/agitation-assisted and advanced decellularization techniques [195]. Mazza et al., first described decellularization of liver left lobe in 2015 [196]. Decellularization was a perfusion-based regime consisting of different reagent treatments including: Trypsin-EDTA, SDS, Triton X-100, peracetic acid and ethanol. Decellularized liver scaffolds showed significant DNA reduction and no evidence of cellular debris as well as architecturally preserved tissue. This protocol however took 2–6 weeks to complete and thus is not ideal for clinical applications. Efforts by Mazza et al. continued again in which they describe rapid decellularization of liver cubes (125 mm3), conducted by employing different agitation speeds (g-force intensities) [197]. At high g-force values (45g), liver tissue cubes turned translucent in 3 h with removal of cellular material and preservation of ECM components confirmed by H&E staining and SR and Elastin Van Gieson staining, respectively. These studies demonstrate rapid protocol employment through utilization of high shear stress for liver ECM preservation and minimization of detergent/reagent exposure and processing times.
Similarly, Golebiowska et al., developed a rapid decellularization for articular cartilage tissue decellularization (cite). Decellularization of cartilage tissue was performed through a series of physical, chemical and enzymatic treatments. Modifications in the exposure to harsh treatments, specifically trypsin and Triton X-100, led to the rapid decellularization of cartilage tissue to nearly ¼ of the total time to implement. H&E staining and dsDNA quantification confirmed the removal of cellular components. Additionally, the modifications to the protocol led to a higher retention of biochemical and ECM components confirmed by sGAG and hydroxyproline quantification. The authors speculate that the rapid protocol developed by reducing the exposure to enzymatic and chemical treatment may aid in the retention of GFs and other signaling molecules important for repair and regeneration. These can serve as guiding protocols for future work where existing decellularization protocols can be revisited with the goal of preserving native structural and biochemical components.
Numerous decellularization procedures have been investigated, however, there is currently no consensus regarding optimal methods, as this is often tissue-/source- and application specific. While many decellularization procedures often use harsh treatment methods that result in the successful removal of cellular remnants, they also lead to varying effects on the dECM constructs. These include damage to/reduction of structural and signaling proteins, significantly compromising the integrity and performance of dECM constructs [198]. Given that native tissues contain the necessary tissue-specific factors, proteins, and 3D ultrastructure for residing cells, there is a need for improved and optimized decellularization techniques that minimizes the impairment of the ECM tissue and components during processing.
As improvements in decellularization processing techniques continues, some of the fundamental/basic questions are still unclear. Decellularized ECM products have varying relevant physical and chemical cues post-processing, with no criteria present based on the retention of those cues/properties that might led to better regeneration. These products are often repopulated with cells or rely on cellular infiltration, acting not only as a cell carrier but also as a complex biologically relevant scaffold for regulating cellular behavior and tissue regeneration [199]. Still, there is limited knowledge on the specific components and their concentration that is associated with and required for tissue regeneration.
7. Decellularized ECM-induced chemotaxis
An in situ regeneration approach that utilizes the body's own regenerative capacity by mobilizing host endogenous stem cells or tissue-specific progenitor cells to recellularize grafts is a promising cell-free approach for TE. The mobilization of endogenous cells relied on in these approaches is through a process known as chemotaxis. Chemotaxis is the directional migration of cells in response to a gradient of soluble chemoattractants [200]. The migration is induced by homing signals that are released and is driven by a number of growth factors and chemokines. TE approaches that rely on cellular recruitment or chemotaxis can allow for the development of cell-free strategies and overcome the limitations of using exogenous cells including reduced FDA approval difficulties/hurdles associated.
Decellularized ECM has the ability to retain native counterpart constituents, including structural components and biochemical signaling molecules such as growth factors [201,202]. Demineralized bone matrix has long been used as bone graft substitutes due to their osteoinductivity that has been attributed to the presence of growth factors that are detected including BMP, IGF-1 and TGF-β [203]. More recently studies have investigated the instructive ECM elements that are preserved in dECM. Detergent decellularized human kidney demonstrated the presence of a number of heparin-binding growth factors, including FGF2, VEGF, BMP-2, HGF, EGF, PDGF-bb and TGF-β, although these were at reduced levels compared to native tissue [201,204,205]. Other studies have demonstrated similar results with the preservation of GFs in decellularized porcine decellularized mesothelium including VEGF, FGF and TGF-β, which stimulated human fibroblasts to produce more VEGF compared to fibroblasts grown on TCP [206].
The bio-instructive signaling cues present in the dECM-based grafts may provide tissue-specific cues for directing cellular behavior and orchestrating cellular chemotaxis (Fig. 7) [207]. Towards these efforts, reinforced composite scaffolds consisting of decellularized ECM microparticles in a hyaluronic acid-based hydrogel were developed for cellular signaling [208]. Results showed that within 48 h, primary chondrocytes receullarized the particles and maintained chondrogenic phenotype via gene expression analysis. Others have developed cell-derived matrices (CDMs) in the form of cell sheets decellularized with different concentrations of SDS. Treatment with lowest SDS (0.5%) led to preserved bioactive components of the cell-laid ECM, increased the recruitment of MSCs and improved regeneration of osteochondral defects in rabbits [209]. Additionally, zebrafish cardiac ECM was decellularized, lyophilized and resuspended in normal saline [210]. When used for cell migration studies, the dECM demonstrated prominent migration of human cardiac stem cells and human heart perivascular MSCs when cultured under nutrient-deprived culture conditions (25% complete media and 2.5% FBS). These studies suggest that the preservation of ECM exhibits bioactivity post-decellularization and chemotactic effects; however, limited studies are available regarding chemotactic ability of decellularized ECM [211].
Fig. 7.
Schematic demonstrating chemotactic ability of the decellularized tissue or cell-derived extracellular matrix. Directed or oriented movement of cells in response to a chemical stimulus or chemoattractant is referred to as chemotaxis. Utilization of dECM biomaterial or grafts can provide native tissue-specific bioactivity and offer biomimetic framework consisting of the major ECM components to mimic an in vivo microenvironment as well as can contribute to endogenous recruitment through their release of bioactive cues. Such strategies can stimulate an in situ tissue regeneration response as a cell-/growth factor-free tissue engineering strategy for the repair and regeneration of various tissues. The inner circle shows cellular recruitment by the implanted decellularized cartilage tissue, while the outer circle is depicting various tissues and organs for which the same concept can be applied to achieve cellular migration (or chemotaxis) needed for repair/regeneration.
Our own laboratory utilized 2D and 3D chemotaxis assays and live cell imaging to track the cellular migration in response to decellularized cartilage ECM. Articular cartilage was decellularized, solubilized and the chemotactic activity of the tissue-derived gel was investigated [164]. The results showed that the tissue-derived dECM retained biochemical cues of the native tissue. Moreover, the results demonstrated the ability of the decellularized cartilage ECM to stimulate migration of hBMSCs in both 2D and 3D model systems which was at similar levels compared to a known MSC chemoattractant (Fig. 8). Furthermore, the antagonist studies also demonstrated that the directed migration of the hBMSCs was likely attributed to the GFs presence/SDF-1α induced.
Fig. 8.
MSC Chemotaxis induced by the decellularized cartilage gel. Representative time course images of collagen gel-embedded cells in response to decellularized cartilage ECM immediately after seeding (A), at 12 h (B) and 24 h (C) with overlaid cell migration trajectories. (D) 3D chemotaxis assays with live cell imaging. Migration tracks of human MSCs in response to (E–L) SDF-1alpha, (M–P) dcECM P2. Forward migration index (FMI) parallel to the gradient of hMSCs migrating in response to SDF-1alpha and dcECM (Q) (n = 40) and the center of mass of cellular endpoints from trajectory plots (R). Adapted from the study by Golebiowska et al. with permission of Annals of Biomedical Engineering, copyright 2023 [164].
These studies demonstrate the ability of decellularized tissues to induce cellular migration and affect cellular behavior. These grafts have the potential to be used as a strategy for guided tissue regeneration (GTR) by providing bioactive cues within the scaffold to induce or allow for cellular recruitment to drive tissue regeneration. These regenerative approaches allow for the design of ECM-based grafts to trigger chemotaxis without the need for cellular transplantation to drive regeneration. However, further studies are needed to investigate the retained growth factor types and amounts and their bioactivity assessment. With little or no externally added growth factors or signaling molecules, decellularized tissue biomaterials can be clinically used for in situ tissue engineering, where the repair and regeneration is guided by the dECM biomaterials/grafts. This can lead to a new paradigm in tissue engineering.
8. Decellularized tissue/graft mechanical performance
In addition to improved retention of compositional components of decellularized ECM based biomaterials, the mechanical properties are critical. Differences/mismatches in mechanical properties between TE grafts and native tissue can lead to stress concentrations at the implant-tissue interface and mechanical failure. Maintaining the mechanical integrity of the ECM is important to ensure its proper functionality, often evaluated as elastic modulus, ultimate tensile/compressive strength, and yield strength. These properties are largely provided by the structural ECM proteins such as collagen, elastin, fibronectin and laminin [212].
The decellularization process has an impact on these proteins/ECM structures, affecting the mechanical properties of these materials and thus can have negative impacts/limited success. Minimizing the damage to the microstructure and mechanical integrity of the remaining dECM products is therefore necessary for structural applications. Table 3 summarizes the effect of decellularization on mechanical properties. For example, Partington et al. decellularized tracheas by multiple cycles of detergent-enzyme treatments. The resulting decellularized tissues had reduced collagen and GAG content levels and mechanical testing evaluation showed decreased tensile strength [213]. The retention levels of these proteins must be optimized based upon the tissue mechanics necessary for function.
Table 3.
Decellularized tissue/graft mechanical properties in comparison with the native tissue.
| Tissue | Mechanical Testing | Property Assessed | Native Tissue | dECM | Ref |
|---|---|---|---|---|---|
| Trachea | |||||
| Tensile Testing | Elastic Modulus | 2.68 MPa | 2 MPa | [283] | |
| Cartilage | |||||
| Compression Testing | Instantaneous Compressive Modulus | 4 MPa | 0.6 MPa | [284] | |
| Compressive Strength Testing | Elastic Modulus | 0.36 ± 0.07 MPa | 0.55 ± 0.15 MPa | [285] | |
| Tension-Torsion Compression Testing | Ultimate Tensile Strength | 2.10 ± 0.29 MPa | 1.90 ± 0.31 MPa | [286] | |
| Elastic Modulus | 18.40 ± 4.02 MPa | 16.49 ± 4.79 MPa | |||
| Stress-Relaxation Testing | Equilibrium Modulus | ∼148 kPa | ∼20 kPa | [154] | |
| Bone | |||||
| Unconfined Compression Testing | Elastic Modulus | 198.2 ± 159.5 MPa | 144.9 ± 101.3 MPa | [287] | |
| Compression Testing Three-Point Bending Testing |
Elastic Modulus | Radius: 7 ± 1 GPa Ulna: 6 ± 1 GPa |
Radius: 7 ± 3 GPa Ulna: 9 ± 3 GPa |
[288] | |
| Compressive Strength | Radius: 173 ± 4 MPa Ulna: 125 ± 24 MPa |
Radius: 116 ± 42 MPa Ulna: 129 ± 39 MPa |
|||
| Yield Strength | Radius: 168 ± 5 MPa Ulna: 120 ± 26 MPa |
Radius: 113 ± 41 MPa Ulna: 124 ± 40 MPa |
|||
| Bone-Fibrocartilage-Tendon (BFT) | Uniaxial Tensile Testing | Elastic Modulus | 501.48 ± 91.56 MPa | 384.97 ± 86.37 MPa | [188] |
| Ultimate Tensile Strength | 53.27 ± 11.84 MPa | 45.41 ± 7.05 MPa | |||
| Failure Strain at UTS | 0.143 ± 0.009 | 0.129 ± 0.014 | |||
| Muscle | |||||
| Stress-Relaxation Testing | Tangent Modulus | 308 ± 51.1 kPa | 218.24.5 kPa | [289] | |
| Uniaxial Tensile Testing | Elastic Modulus | 0.19 ± 0.07 MPa | 0.41 ± 0.32 MPa | [290] | |
| Ultimate Tensile Strength | 0.21 ± 0.08 MPa | 1.32 ± 0.85 MPa | |||
| Strain at Failure | 1.24 ± 0.27 | 160 ± 0.30 | |||
| Uniaxial Tensile Testing | Elastic Modulus | 2.04 ± 0.54 MPa | 1.74 ± 0.46 MPa | [291] | |
| Ultimate Tensile Strength | 0.19 ± 0.03 MPa | 0.23 ± 0.04 MPa | |||
| Strain at UTS | 1.23 ± 0.44 | 1.38 ± 0.27 | |||
| Tendon | |||||
| Tensile Testing | Elastic Modulus | 299.71 ± 41.67 MPa | 210.68 ± 46.43 MPa | [292] | |
| Ultimate Tensile Strength | 34.67 ± 3.47 MPa | 29.69 ± 6.73 MPa | |||
| Strain at UTS | 15.50 ± 3.12% | 19.16 ± 4.58% | |||
| Stiffness | 25.50 ± 3.71 N/mm | 27.40 ± 8.66 N/mm | |||
| Ultimate Tensile Stress Testing | Yield Strain | 8.337 ± 0.142% | 7.829 ± 0.442% | [293] | |
| Failure Strain | 8.712 ± 0.510% | 8.851 ± 0.216% | |||
| Yield Stress | 5.774 ± 0.44 MPa | 5.782 ± 0.775 MPa | |||
| Failure Stress | 6.029 ± 0.414 MPa | 6.045 ± 0.759 MPa | |||
| Elastic Modulus | 76.13 ± 4.12 MPa | 70.31 ± 5.91 MPa | |||
| Ultimate Load to Failure Testing | Ultimate Load | 200.39 ± 22.11 N | 185.95 ± 7.91 N | [294] | |
| Stiffness | 44.26 ± 2.96 N/mm | 45.99 ± 5.4 N/mm | |||
| Aorta | |||||
| Tensile Testing | Elastic Modulus | 551.1 ± 152.2 kPa | 416.5 ± 72.6 kPa | [77] | |
Additionally, the most challenging dECM products are hydrogels as mechanical strength is typically much lower than that of their native counterparts [140,214]. To overcome the inadequate mechanical properties of decellularized ECM-based hydrogels, many are often supplemented with synthetic biomaterials or crosslinked to tune and boost mechanical properties that were compromised during processing [87,215,216]. Understanding how processing techniques affects the tissue/graft structure physically, and how the structural changes can lead to differences in terms of mechanical properties post-decellularization must be addressed. Further efforts that preserve decellularized tissue/graft mechanical properties are warranted.
9. Decellularized tissue-based bio-inks (tissue inks) for 3D-printing
Biofabrication allows the ability to generate tissue analogues achieving precise 3D architectural placement for controlled pre-determined deposition of materials to attain complex geometries, pore size, etc. This technology unites cells, biomaterials, and bioactive molecules and assembles them into 3D constructs in a layer-by-layer fashion. Although numerous materials in the form of bio-inks have been developed for these purposes, many are unable to recapitulate the complexity of natural ECM for which they are designed to replace, creating less than favorable microenvironments for encapsulated cells. Thus, the use of decellularized tissue as a bio-ink or Tissue-Ink has attracted much attention in their capacity to retain biochemical cues/features of the native ECM, allowing for their ability to mimic native cell-ECM interactions through inductive cues, leading to in vivo tissue ingrowth for functional repair and regeneration. In other words, the use of Tissue-Ink with the above stated bioactive features can lead to Guided Tissue Regeneration (GTR), a much-desired aspect and hard to achieve with conventional biomaterials in TE.
dECM bio-inks have been shown to provide cells with the appropriate biochemical/physical cues needed to modulate cellular fate and for this reason, several types of tissue- and organ-based decellularized ECM bio-inks have been developed as tissue substitutes, including muscle, liver, heart, kidney, cartilage, tendon and skin [48,106,[217], [218], [219], [220], [221]]. The utilization of bio-fabrication technology using decellularized ECM-based bio-inks has been summarized below in Table 4. For example, Won et al. developed a printable Tissue-Ink through decellularization and subsequent pepsin digestion, solubilization and pH adjustment of porcine skin ECM [221]. Printed cell-laden constructs with human dermal fibroblasts showed 90% viability and proliferation as well as increased genes related to epidermis formation, which was attributed to the presence of bio-reactive molecules and growth factors present in the bio-ink.
Table 4.
Biofabricated/3D printed constructs using decellularized ECM bio-inks/cell-derived matrices (CDMs).
| Animal Source | Tissue/Organ | Gelation Mechanism/Crosslinking | Bio-ink Components | In vitro/in vivo assessment | Ref. |
|---|---|---|---|---|---|
| Porcine | Skin | Thermal gelation | Skin dECM Human dermal fibroblasts (hDF) |
Supported viability and proliferation Increased epidermis formation-related genes. |
[221] |
| Porcine | Heart | Vitamin B2-induced UVA crosslinking + thermal gelation | Heart dECM Cardiac progenitor cells (CPCs) |
Supported high cell viability and proliferation with increased cardiomyogenic differentiation | [222] |
| Porcine | Skeletal muscles | Methacrylation | Skeletal muscle dECM C2C12 myoblasts |
Supported differentiation and aligned myotube formation with increase gene expression and basement membrane component secretion | [223] |
| Porcine | Cartilage | Genipin | Cartilage dECM | PLGA gradient structure led to graded pore/fiber orientation structure and enhanced mechanical properties | [225] |
| Porcine | Tibialis anterior muscle and descending aorta | Thermal gelation | Skeletal muscle dECM + human muscle cells Vascular dECM + HUVECs |
Supported high cell viability Improved vascularization, neural integration and functional recovery in VML rat model |
[226] |
| Porcine | Hyaline Cartilage | Alginate and CaCl2 | Cartilage dECM Human adipose-derived stem cells Alginate |
Observed increased expression of chondrogenic markers. In vivo shape and structure maintenance with cartilaginous tissue formation after 12 weeks |
[227] |
| Porcine | Heart | Thermal gelation | Heart dECM Primary cardiomyocytes |
Enhanced cardiomyocyte maturation with aligned structural organization under dynamic culture and enhanced expression of cell adhesion molecules, integrin-based proteins, basement membrane proteins, and matrix remodeling metallopeptidases | [228] |
| Murine | Osteoblast/osteocyte-like cells (MLO-A5s) | – | MLO-A5 CDMs Human embryonic stem cell-derived mesenchymal progenitor cells |
Improved attachment and significantly higher cell proliferation and osteogenic activity with higher angiogenic potential | [249] |
Biofabricated constructs must retain its shape post-printing, degrade in a timely manner to allow for in vivo tissue ingrowth and contain similar mechanical properties of the surrounding native tissue. Thus, Tissue-Inks have also been modified to allow for crosslinking, which increased stability and mechanical properties. In particular, for the purpose of stabilizing and enhancing/tailoring mechanical properties of printed dECM bio-ink, authors combined vitamin B2 and UVA light and thermal crosslinking, which showed to have supported high cell viability and proliferation of cardiac progenitor cells with increased cardiomyogenic differentiation [222]. dECM bio-ink mechanical stability has also been enhanced using methacrylate photo-crosslinking process [223]. The authors combined methacrylated dECM bio-inks and PVA as a sacrificial fibrillated component to produce a uniaxially aligned micro-topographical structure with mechanically stable struts. These constructs led to the formation of a stable structure with highly aligned cell/ECM and myotube formation by C2C12 cells. Aside from the need for mechanically stable bio-inks during/immediately after the printing process, biofabricated constructs may require additional mechanical strength for certain tissues/applications, such as those requiring load-bearing properties. These mechanically enhanced constructs can be fabricated through incorporation of degradable polymers including PLA, PCL and PLGA. We used PLLA as a template structure and introduced a gel in between the polymeric filaments through selective printing, infusion, and gel printing on the template structure and its settlement into the porous structure. All three strategies supported cell placement via the gel component and their survival post-printing [9]. One strategy used PCL as a framework followed by the alternating deposition of cell-laden dECM bio-ink with varied line width of the synthetic polymer for stiffness adjustment [224]. Another used PLGA to fabricate gradient structures of low, medium and high-density grids [225]. These were thens injected with dECM + genipin crosslinker and underwent directional freezing to produce gradient oriented dECM with mechanical strength similar to that of articular cartilage.
In addition to mechanical stability, vascularization of biofabricated constructs is important for sufficient tissue ingrowth and integration with the surrounding tissue. For this purpose, Choi et al. combined muscle and vascular dECM encapsulating human skeletal muscle cells and HUVECs, respectively for the development of pre-vascularized muscle constructs for VML treatment [226]. Using co-axial printing, authors printed a compartmentalized core-shell structure, in which the vdECM served as the outer shell and the mdECM as the inner core. It was observed that these constructs led to endothelial network and myotube formation throughout the construct, which was otherwise not observed when the two cell-laden bio-inks were mixed. In vivo studies showed the generation of thick and densely packed newly formed muscle fibers and formation of functional blood vessels integrated with host vasculature.
Furthermore, advancements in biofabrication using tissue-specific dECM bio-ink has enabled fabrication of customizable and anatomically correct constructs for patient-specific tailored applications. Using computer-aided design and 3D fabrication, Yi et al. generated 3D models of customized nasal implants to generate the 3D exterior and interior architecture shape [227]. Afterwards, the model was injected with a bio-ink based on dcECM containing ADSCs. It was seen that tissue-derived bio-ink supported high cell viability, with higher expression of chondrogenic markers (SOX9, ACAN and COL21A) and GAG presence compared to an alginate hydrogel.
More recently, other advancements in the field include the combination of dECM biofabricated constructs with external stimulation. Heart derived ECM printed constructs underwent dynamic culture to compare the effects of culture conditioning on rat primary cardiomyocytes [228]. The hdECM and dynamic culture showed cardiomyocytes with a unidirectional, elongated and aligned structural organization with enhanced structural maturation compared to static culture or collagen encapsulated cells.
10. Cell-derived matrices
Cell-derived matrices (CDMs) emerge as promising alternatives to conventional decellularized tissue and organ sources. They came in existence to mainly overcome the possible inflammatory/pathogenic risk associated with dECM tissues/matrices. Unlike tissue- and organ-derived ECM, CDMs are produced in vitro through cell-assembled ECM deposition of a fibrillar network constituted of a variety of proteins including collagen, proteoglycans, and polysaccharides. The three common methods of obtaining CDMs include monolayer cell sheet culture, template culture and pellet culture (Fig. 9). These CDMs generally require milder methods for accelerated decellularization with greater preservation of ECM components and bioactivity with effective removal of cellular debris [229,230]. Decellularized CDMs can be used as an in vitro model for studying cellular behavior and allow for application-specific tailoring through cell type and culture method (2D/3D, static/dynamic, culture medium, etc.). The resultant CDMs therefore contain different properties and characteristics such as protein composition, stiffness etc. depending on the culture conditions used. The utilization of cell-derived matrix technology has been summarized below in Table 5.
Fig. 9.
Methodologies to form cell-derived ECM. (A) Monolayer culture of cells to allow for ECM deposition, followed by decellularization to obtain cell sheets. Cell sheets can be then stacked to construct 3D tissue-like structures. (B) Cells cultured on a 3D template structure to allow for ECM deposition, followed by cell and template removal for 3D ECM scaffold. (C) Cell pellet culture and decellularization for the formation of pellet ECM scaffolds.
Table 5.
Cell-derived ECM biomaterials/grafts developed using different cell sources.
| Cells | Decellularization Method | Application | In vitro Recellularization | In vitro/in vivo assessment | Reference |
|---|---|---|---|---|---|
| Bone marrow mesenchymal stem cells (BMSCs) MC3T3 osteoblasts L929 fibroblasts |
Five freeze-thaw cycles (liquid nitrogen and 37 °C water bath) Rinsed with sterile PBS, double distilled water and hypotonic solution DNase I treatment |
Bone Tissue | Bone marrow mesenchymal stem cells (BMSCs) | Supported BMSCs proliferation and osteogenic differentiation to different extents Ectopic osteogenesis after subcutaneous implantation |
[232] |
| Bone marrow (BM)- and adipose (AD)-derived stromal cells | PBS containing 0.5% Triton X-100 and 20 mM NH4OH at 37 °C Washed with PBS and sterile distilled water |
Tissue specific culture system development for replicating in vivo niche | BM- and AD-mesenchymal stem cell (MSC) | Promoted proliferation and differentiation of MSCs, enhanced when cell origin matched CDM ECM | [233] |
| Coculture of Mesenchymal stem/stromal cells (MSCs) and Human umbilical vein endothelial cells (HUVECs) | 0.5% Triton X‐100 containing 20 mM NH4OH in PBS for 5 min Washed using PBS 5 times and air-dried |
Bone Tissue | Bone marrow mesenchymal stem cells | Enhanced osteogenic differentiation and angiogenic response | [234] |
| Adipose tissue-derived stem cells (ASCs) cultured in growth and adipogenic medium | Triton X-100 containing 20 mM (NH4OH) in 0.1 M glycine in PBS Washed with PBS and distilled water DNase and RNAse treatment Freeze-thaw cycles Fixed in 0.1% glutaraldehyde and treated with 0.1 M glycine in PBS |
Stepwise regulation of stem cell function in adipose tissue engineering | Adipose tissue-derived stem cells | Greater migration ability cultured on growth CDM and adipogenic differentiation on adipogenic CDM | [235] |
| C2C12 Myoblasts at various stages of differentiation | PBS containing 0.5% Triton X-100 and 20 mM NH4OH PBS containing 10 mM MgCl2, DNase I and RNase A Treatment with 0.1% glutaraldehyde in PBS for 6 h |
Muscle Tissue | C2C12 Myoblasts | Promoted myogenic differentiation in myogenic culture with early and late myogenic markers with varying levels of myogenic culture | [236] |
| Human lung fibroblasts | Treatment with 0.2% Triton X-100 and 10 mM NH4OH DNase I and RNase A treatment Washed with PBS |
Expansion culture of MSCs with maintained “stemness” | Umbilical cord blood-derived MSCs (UCB-MSCs) | Enhanced cell proliferation with elongated morphology. Improved cell motility with up-regulated CXCR4 cell migration marker. Retained differentiation capacity into osteogenic lineage |
[238] |
| Human articular chondrocytes (AC) Bone marrow stromal cells (BM) |
PBS containing 0.5% Triton X-100 with 20 mM NH4OH Washed with PBS and deionized water |
In vitro chondrocyte expansion with decreased dedifferentiation | Human articular chondrocytes | Faster proliferation and highest ratio of COL2A1/COL1A1 cultured on AC-CDMs compared to BM-CDMs | [242] |
| Synovium-derived stem cells (SDSCs) Adipose-derived stem cells Dermal fibroblasts |
0.5% Triton X-100 containing 20 mM NH4OH | Cartilage tissue | Synovium-derived stem cells | Increased cell proliferation and increased chondrogenic markers when cultured on SDSC-CDM with lower hypertrophy potential | [243] |
| Human adipose-derived stem cells (hASCs) | Six freeze-thaw cycles (−80 °C and RT) | Wound dressings | L929 Fibroblasts | Supported survival and proliferation of cells in vitro. Improved wound healing of full-thickness skin excision in mouse model |
[246] |
| Embryonic stem cells (ESCs) | 1% Triton X-100 for 30 min DNAse I treatment Rinsed with PBS |
Neural Tissue | ESC-derived neural progenitor cells | Enhanced proliferation and neural differentiation | [247] |
The use of CDMs emphasizes the ability of studying cellular behavior namely migration, proliferation and differentiation as well as cellular response to ECM [231]. In one study, cell sheets were prepared by seeding BMSCs, MC3T3 pre-osteoblasts and fibroblasts on fiber mats followed by decellularization [232]. CDMs re-seeded with BMSCs promoted superior proliferation compared to TCP. Differentiation studies of the CDMs revealed that BMSC-ECM promoted stronger osteogenic differentiation as well as expressed higher presence of osteogenesis related factors VEGF and BMP-2. In another study, ECMs secreted by both BMSCs and ADSCs were used to study the effect of each culture substrate on BMSC and ADSC behavior [233]. ECM secreted from the two cell types promoted enhanced proliferation and differentiation of MSCs when cultured on its respective ECM (BMSCs on BMSC-ECM and ADSCs on ADSC-ECM), indicating the tissue-specific ECM influence on cell behavior. Furthermore, analysis of the ECM demonstrated that although the structural proteins were at similar levels, there existed unique differences in the architecture of the ECM (fiber orientation and compactness). More recently, Carvalho et al. investigated CDMs prepared from the co-culture of MSCs and HUVECs [234]. These co-culture derived matrices (Co-CDMs) supported enhanced osteogenic differentiation of hBMSCs and production of a more mature mineralized matrix as well as better angiogenic response compared to culture on CDMs derived from MSCs only, HUVECs only or TCP, suggesting the synergistic effects of Co-CDMs for facilitating ECM-like environment. These studies indicate the ability of cell-derived ECMs to guide tissue-/lineage-specific regeneration based on the matrix composition and bio-chemical cue presence, these together regulate cell behavior.
CDMs have also shown to have a conditioning effect on cells, demonstrating contact communication between CDMs and stem cells. In a recent study, Zhang et al. investigated the effects of stepwise ECM secreted components from ADSCs cultured in two different medium, namely growth and adipogenic [235]. After reseeding ADSCs on the decellularized constructs, the results of the study revealed that CDMs from ADSCs cultured in growth medium exhibited greater migration ability whereas CDMs from ADSCs cultured in adipogenic medium underwent adipogenic differentiation. Similar studies were also conducted to investigate the effects of myogenic stages of C2C12 myoblasts secreted ECM on myogenesis of stem cells [236]. Other studies investigated the influence of various stages of differentiation in regulating cellular differentiation, revealing that different stages resulted in ECM compositional differences and led to changes in cellular fate [237]. Together, these results suggest the regulatory effects of the varied secreted dECM components and can shed light on mechanisms involved in CDM mediated cellular behavior.
Numerous studies additionally support the suitability of CDMs as novel cell culture substrates for maintenance of MSC stemness and phenotypic retention/reduced dedifferentiation of chondrocytes during ex vivo expansion purposes with large clinical relevance in comparison to traditional culture methods [[238], [239], [240], [241]]. Chondrocyte- and BMSC-derived ECM were prepared by Zhang et al. to compare the effects of each ECM on in vitro expansion of human articular chondrocytes (HAC) [242]. When cultured on chondrocyte CDMs, HACs showed faster proliferation and higher maintenance of COL2A1/COL1A1 ratio. Pellet culture studies also showed cartilage-like ECM production of HAC expanded on both ECMs with better-maintained chondrocyte phenotype on chondrocyte-CDMs. In another study, decellularized CDMs derived from synovium-derived stem cells (SDSCs) demonstrated increased cellular proliferation and chondrogenic marker expression [243]. Additionally, these CDMs also showed decreased potential for hypertrophy compared to CDMs derived from ADSCs and dermal fibroblasts. As an alternative expansion substrate, human fibroblast-derived ECM (hFDM) was used for expansion of hMSCs [238]. It was seen that when cultured on these substrates, cellular proliferation and migration was significantly improved with a notable up-regulation of cell migration marker CXCR4. Differentiation studies also showed the retained differentiation capacity via gene expression and alkaline phosphatase activity.
Additionally, immortalized stem cells were used to prepare dECM and showed the ability to modulate stem cell lineage proliferation and differentiation capabilities [244]. Decellularized extracellular matrix (dECM) deposited by simian virus 40 large T antigen (SV40LT) transduced autologous infrapatellar fat pad stem cells (IPFSCs) demonstrated the ability to rejuvenate high-passage IPFSCs in proliferation and chondrogenic differentiation. Cells cultured on dECM deposited by passage 5 IPFSCs showed increased proliferation and chondrogenic differentiation capacity, however, this was reduced if cultured on dECM deposited by passage 15 IPFSCs, suggesting that passage number plays a critical role in cellular behavior when re-seeded on CDMs. CDMs were also evaluated in a rabbit osteochondral defect model. Rabbit IPFSCs were expanded on dECM deposited by human urine-derived stem cells (UDSCs) to prepare 10-day premature tissue constructs and implanted for 26 weeks [245]. The study demonstrated that UECM-expanded cells exhibited robust resurfacing effect through histological and mechanical assessment. Additionally, RNA-Seq analysis indicated that inflammation-mediated macrophage activation and polarization are potentially involved in the CDM-mediated promotion of IPFSC's chondrogenic capacity.
Although promising, CDMs with architectural, compositional, and mechanical properties similar to native counterparts are often difficult to achieve. Therefore, in order to produce mechanically relevant CDMs, composite scaffolds with biodegradable polymers have been fabricated [91]. One strategy used PLGA electrospun nanofibrous template and hADSCs to fabricate a nanofibrous dressing for wound healing [246]. Decellularized CDMs preserved type I collagen and laminin, were hydrophilic and had appropriate mechanical strength suitable for wound healing. In addition, when seeded with fibroblasts, these CDMs supported cell survival and proliferation. Crosslinking of CDMs has also been investigated for improvements in stability and resistance to rapid degradation [247]. Alternatively, biphasic calcium phosphate scaffolds with rat BMSC derived ECM were developed for a mechanically supportive and biofunctionalized scaffold [248]. The scaffolds showed increased osteoblastic differentiation of pre-osteoblasts with up-regulated osteoblastic genes: osteopontin, alkaline phosphates and BMP-2. 3D printing has also been integrated with CDMs. 3D-printed porous PCL scaffolds were populated with bone cells and cultured to allow for deposition for development of CDMs and subsequently decellularized [249]. The addition of the CDMs as part of the scaffold led to increased cellular attachment, proliferation and osteogenic activity of mesenchymal progenitor cells compared to PCL-only scaffolds. The use of CDMs is also promising for autologously derived ECMs for various applications within tissue engineering such as those mentioned previously as well as for providing a reservoir of signaling molecules/GFs [250]. In a way, a tissue biopsy or bone-marrow derived from a patient can be used as a cell source to develop a CDM that can become a graft to be implanted in the same patient. This process has merits as the patient-derived graft is being used for implantation. Patient-derived CDMs may improve favorable modulation of cellular behavior as the matrix is laid out by the same cells and limit any adverse effects that are associated with tissue-/organ-derived ECM.
Still there are differences between CDMs and tissue/organ-dECM based grafts for tissue regeneration strategies [251]. The differences lie in the key factors found within the dECM, involving the structural architecture and mechanical properties as well as matrix components such as binding motifs for cellular adhesion and signaling molecules to direct cellular behavior (Table 6). CDMs can be generated using different cellular sources, including fibroblasts, MSCs and pluripotent cells, to form a microenvironment that mimics native tissue microenvironment, however, the generation of CDMs to mimic complex tissues that require multiple cell types and their proper orientation/organization and function may be more challenging, for which tissue derived ECM may be more suited for these applications. Furthermore, the decellularization methods of CDMs and tissue-derived ECMs differ in that CDMs are prepared through in vitro culturing of cells for a period of time and require milder decellularization methods, whereas tissue derived ECM require more involved decellularization process, often requiring a combination of different methods. The decellularization process removes many of the foreign cellular material and antigens that may elicit immune response in order to minimize the immunogenicity risk of tissue derived ECM. CDMs, on the other hand, have the potential of being generated from autologous cells in order to produce autologous ECM scaffolds and graft systems to avoid the undesired host response altogether. However, the requirement of isolation/expansion of patient-specific cells and generation of the autologous ECM grafts is a time-consuming process compared to easily available xenogeneic donor tissues.
Table 6.
Systematic comparison of decellularized ECM tissue with engineered matrices and cell-derived ECM.
| Properties | Engineered Matrices | Cell-derived ECM | Tissue-derived ECM |
|---|---|---|---|
| Structural | ☆☆ | ☆☆☆ | ☆☆☆☆☆ |
| Biochemical/Compositional | ☆☆ | ☆☆☆ | ☆☆☆☆ |
| Binding Motifs | ☆ | ☆☆☆ | ☆☆☆☆☆ |
| Signaling Molecules | ☆ | ☆ | ☆☆☆ |
11. Conclusions
Decellularized extracellular matrix (dECM) biomaterials have gained significant interest and research attention in tissue engineering and regenerative medicine over the past decade. The therapeutic potential of these biomaterials – cell, tissue and organ derived - has been realized, as evidenced by the extensive publication of over 5000 articles. FDA-approved grafts, including de-mineralized bone, skin, and ligament, further demonstrate their clinical utility as tissue substitutes. Ongoing research has led to improved methods and protocols for developing cell- and tissue-derived nonimmune biomaterials with reproducible structure and biological function. The versatility of dECM allows for the development of various biomaterial forms, such as powders, gels, sheets, 3D structures through additive manufacturing, and even tissues and organ structures with intact blood vessel structure and nerve innervation. These non-immune and bioactive biomaterials and structures outperform engineered matrices made from natural or synthetic biomaterials due to their native structural and compositional properties, including binding motifs, and biochemical signaling cues that play crucial role in host-cell interaction and guided tissue regeneration. Despite significant progress, several challenges remain. These include the need for improved cellularization methods for effective product design, a deeper understanding of how dECM influences cell behavior, and the achievement of mechanical properties that resemble those of native tissues. Standardized sterilization and preservation methods and characterization techniques need to be developed to advance decellularized tissue biomaterials and grafts for clinical translation. Additionally, the development of chemotactic dECM biomaterials that can actively promote tissue repair and regeneration without the need of respective cells and growth factors.
Ethics approval and consent to participate
Not applicable.
Conflict of interest statement
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge support from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (#R01EB030060 & #R01EB020640). Dr. Nukavarapu also acknowledges funding from NSF EFMA (#1640008 & 1908454).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Tissue Regeneration and Organ Repair,” Medscape. https://www.medscape.com/viewarticle/457173 (accessed May 20, 2023).
- 2.Vacanti J.P., Vacanti C.A. In: Principles of Tissue Engineering. fourth ed. Lanza R., Langer R., Vacanti J., editors. Academic Press; Boston: 2014. Chapter 1 - the history and scope of tissue engineering; pp. 3–8. [DOI] [Google Scholar]
- 3.Francois E., Dorcemus D., Nukavarapu S. In: Regenerative Engineering of Musculoskeletal Tissues and Interfaces. Nukavarapu S.P., Freeman J.W., Laurencin C.T., editors. Woodhead Publishing; 2015. 1 - biomaterials and scaffolds for musculoskeletal tissue engineering; pp. 3–23. [DOI] [Google Scholar]
- 4.Amini A.R., Adams D.J., Laurencin C.T., Nukavarapu S.P. Optimally porous and biomechanically compatible scaffolds for large-area bone regeneration. Tissue Eng. Jul. 2012;18(13–14):1376–1388. doi: 10.1089/ten.tea.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikael P.E., Golebiowska A.A., Kumbar S.G., Nukavarapu S.P. Evaluation of autologously derived biomaterials and stem cells for bone tissue engineering. Tissue Eng. Oct. 2020;26(19–20):1052–1063. doi: 10.1089/ten.tea.2020.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikael P.E., Golebiowska A.A., Xin X., Rowe D.W., Nukavarapu S.P. Evaluation of an engineered hybrid matrix for bone regeneration via endochondral ossification. Ann. Biomed. Eng. Mar. 2020;48(3):992–1005. doi: 10.1007/s10439-019-02279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorcemus D.L., Kim H.S., Nukavarapu S.P. Gradient scaffold with spatial growth factor profile for osteochondral interface engineering. Biomed. Mater. Bristol Engl. Dec. 2020 doi: 10.1088/1748-605X/abd1ba. [DOI] [PubMed] [Google Scholar]
- 8.Kumbar S.G., Nukavarapu S.P., James R., Nair L.S., Laurencin C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials. Oct. 2008;29(30):4100–4107. doi: 10.1016/j.biomaterials.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golebiowska A.A., Nukavarapu S.P. Bio-inspired zonal-structured matrices for bone-cartilage interface engineering. Biofabrication. Feb. 2022;14(2) doi: 10.1088/1758-5090/ac5413. [DOI] [PubMed] [Google Scholar]
- 10.Amini A.R., Xu T.O., Chidambaram R.M., Nukavarapu S.P. Oxygen tension-controlled matrices with osteogenic and vasculogenic cells for vascularized bone regeneration in vivo. Tissue Eng. Apr. 2016;22(7–8):610–620. doi: 10.1089/ten.tea.2015.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amini A., Nukavarapu S. Oxygen-tension controlled matrices for enhanced osteogenic cell survival and performance. Ann. Biomed. Eng. Jun. 2014;42(6):1261–1270. doi: 10.1007/s10439-014-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nukavarapu S.P., Laurencin C.T., Amini A.R., Dorcemus D.L. US9707322B2; Jul. 18, 2017. Gradient Porous Scaffolds.https://patents.google.com/patent/US9707322B2/en?inventor=nukavarapu&oq=nukavarapu [Online]. Available: [Google Scholar]
- 13.Bae W.-G., et al. Bio-inspired configurable multiscale extracellular matrix-like structures for functional alignment and guided orientation of cells. Biomaterials. Nov. 2015;69:158–164. doi: 10.1016/j.biomaterials.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Young J.L., Holle A.W., Spatz J.P. Nanoscale and mechanical properties of the physiological cell–ECM microenvironment. Exp. Cell Res. Apr. 2016;343(1):3–6. doi: 10.1016/j.yexcr.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Lutolf M.P., Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. Jan. 2005;23(1) doi: 10.1038/nbt1055. Art. no. 1. [DOI] [PubMed] [Google Scholar]
- 16.Unal A.Z., West J.L. Synthetic ECM: bioactive synthetic hydrogels for 3D tissue engineering. Bioconjugate Chem. Oct. 2020;31(10):2253–2271. doi: 10.1021/acs.bioconjchem.0c00270. [DOI] [PubMed] [Google Scholar]
- 17.Ligorio C., Mata A. Synthetic extracellular matrices with function-encoding peptides. Nat. Rev. Bioeng. Apr. 2023:1–19. doi: 10.1038/s44222-023-00055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolas J., Magli S., Rabbachin L., Sampaolesi S., Nicotra F., Russo L. 3D extracellular matrix mimics: fundamental concepts and role of materials chemistry to influence stem cell fate. Biomacromolecules. Jun. 2020;21(6):1968–1994. doi: 10.1021/acs.biomac.0c00045. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura N., Kimura T., Kishida A. Overview of the development, applications, and future perspectives of decellularized tissues and organs. ACS Biomater. Sci. Eng. Jul. 2017;3(7):1236–1244. doi: 10.1021/acsbiomaterials.6b00506. [DOI] [PubMed] [Google Scholar]
- 20.Bejleri D., Davis M.E. Decellularized extracellular matrix materials for cardiac repair and regeneration. Adv. Healthcare Mater. 2019;8(5) doi: 10.1002/adhm.201801217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusindarta D.L., Wihadmadyatami H. The role of extracellular matrix in tissue regeneration. Tissue Regen. Mar. 2018 doi: 10.5772/intechopen.75728. [DOI] [Google Scholar]
- 22.Chen F.-M., Liu X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. Feb. 2016;53:86–168. doi: 10.1016/j.progpolymsci.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. Dec. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caralt M., et al. Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. Jan. 2015;15(1):64–75. doi: 10.1111/ajt.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Z., et al. Comparison of various reagents for preparing a decellularized porcine cartilage scaffold. Am. J. Transl. Res. Mar. 2019;11(3):1417–1427. [PMC free article] [PubMed] [Google Scholar]
- 26.Rosario D.J., Reilly G.C., Ali Salah E., Glover M., Bullock A.J., MacNeil S. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen. Med. Mar. 2008;3(2):145–156. doi: 10.2217/17460751.3.2.145. [DOI] [PubMed] [Google Scholar]
- 27.Elder B.D., Eleswarapu S.V., Athanasiou K.A. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials. Aug. 2009;30(22):3749–3756. doi: 10.1016/j.biomaterials.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama K.H., Batchelder C.A., Lee C.I., Tarantal A.F. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng. Jul. 2010;16(7):2207–2216. doi: 10.1089/ten.tea.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng C.W., Solorio L.D., Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol. Adv. 2014;32(2):462–484. doi: 10.1016/j.biotechadv.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangold S., et al. Evaluation of decellularized human umbilical vein (HUV) for vascular tissue engineering – comparison with endothelium-denuded HUV. J. Tissue Eng. Regen. Med. 2015;9(1):13–23. doi: 10.1002/term.1603. [DOI] [PubMed] [Google Scholar]
- 31.Sabetkish S., et al. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix liver scaffolds. J. Biomed. Mater. Res. 2015;103(4):1498–1508. doi: 10.1002/jbm.a.35291. [DOI] [PubMed] [Google Scholar]
- 32.Hoshiba T., Lu H., Kawazoe N., Chen G. Decellularized matrices for tissue engineering. Expet Opin. Biol. Ther. Dec. 2010;10(12):1717–1728. doi: 10.1517/14712598.2010.534079. [DOI] [PubMed] [Google Scholar]
- 33.Batchelder C.A., Martinez M.L., Tarantal A.F. Natural scaffolds for renal differentiation of human embryonic stem cells for kidney tissue engineering. PLoS One. Dec. 2015;10(12) doi: 10.1371/journal.pone.0143849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Neill J.D., et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann. Thorac. Surg. Sep. 2013;96(3):1046–1056. doi: 10.1016/j.athoracsur.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price A.P., et al. Automated decellularization of intact, human-sized lungs for tissue engineering. Tissue Eng. C Methods. Jan. 2015;21(1):94–103. doi: 10.1089/ten.tec.2013.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz S., et al. Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications. Tissue Eng. Jun. 2012;18(21–22):2195–2209. doi: 10.1089/ten.tea.2011.0705. [DOI] [PubMed] [Google Scholar]
- 37.Chen K., et al. Decellularized periosteum as a potential biologic scaffold for bone tissue engineering. Acta Biomater. Jun. 2015;19:46–55. doi: 10.1016/j.actbio.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Rana D., Zreiqat H., Benkirane-Jessel N., Ramakrishna S., Ramalingam M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017;11(4):942–965. doi: 10.1002/term.2061. [DOI] [PubMed] [Google Scholar]
- 39.Ghazanfari S., Alberti K.A., Xu Q., Khademhosseini A. Evaluation of an elastic decellularized tendon-derived scaffold for the vascular tissue engineering application. J. Biomed. Mater. Res. 2019;107(6):1225–1234. doi: 10.1002/jbm.a.36622. [DOI] [PubMed] [Google Scholar]
- 40.Ventura R.D., Padalhin A.R., Park C.M., Lee B.T. Enhanced decellularization technique of porcine dermal ECM for tissue engineering applications. Mater. Sci. Eng. C. Nov. 2019;104 doi: 10.1016/j.msec.2019.109841. [DOI] [PubMed] [Google Scholar]
- 41.Kim Y.S., Majid M., Melchiorri A.J., Mikos A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019;4(1):83–95. doi: 10.1002/btm2.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahendiran B., et al. Decellularized natural 3D cellulose scaffold derived from Borassus flabellifer (Linn.) as extracellular matrix for tissue engineering applications. Carbohydr. Polym. Nov. 2021;272 doi: 10.1016/j.carbpol.2021.118494. [DOI] [PubMed] [Google Scholar]
- 43.McInnes A.D., Moser M.A.J., Chen X. Preparation and use of decellularized extracellular matrix for tissue engineering. J. Funct. Biomater. Dec. 2022;13(4) doi: 10.3390/jfb13040240. Art. no. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao M., et al. Sterilization and disinfection methods for decellularized matrix materials: review, consideration and proposal. Bioact. Mater. Feb. 2021;6(9):2927–2945. doi: 10.1016/j.bioactmat.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J.S., et al. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules. Jan. 2014;15(1):206–218. doi: 10.1021/bm4015039. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Nicolas C.T., Chen H.S., Ross J.J., De Lorenzo S.B., Nyberg S.L. Recent advances in decellularization and recellularization for tissue-engineered liver grafts. Cells Tissues Organs. Oct. 2017;204(3–4):125–136. doi: 10.1159/000479597. [DOI] [PubMed] [Google Scholar]
- 47.Mazza G., Al-Akkad W., Rombouts K., Pinzani M. Liver tissue engineering: from implantable tissue to whole organ engineering. Hepatol. Commun. 2018;2(2):131–141. doi: 10.1002/hep4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H., et al. Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules. Apr. 2017;18(4):1229–1237. doi: 10.1021/acs.biomac.6b01908. [DOI] [PubMed] [Google Scholar]
- 49.Basara G., Ozcebe S.G., Ellis B.W., Zorlutuna P. Tunable human myocardium derived decellularized extracellular matrix for 3D bioprinting and cardiac tissue engineering. Gels. Jun. 2021;7(2) doi: 10.3390/gels7020070. Art. no. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X., et al. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater. Sci. Eng. C. Jan. 2021;118 doi: 10.1016/j.msec.2020.111388. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X., Chen X., Hong H., Hu R., Liu J., Liu C. Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Bioact. Mater. Apr. 2022;10:15–31. doi: 10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu J., et al. A biological functional hybrid scaffold based on decellularized extracellular matrix/gelatin/chitosan with high biocompatibility and antibacterial activity for skin tissue engineering. Int. J. Biol. Macromol. Sep. 2021;187:840–849. doi: 10.1016/j.ijbiomac.2021.07.162. [DOI] [PubMed] [Google Scholar]
- 53.Khoshnood N., Zamanian A. Decellularized extracellular matrix bioinks and their application in skin tissue engineering. Bioprinting. Dec. 2020;20 doi: 10.1016/j.bprint.2020.e00095. [DOI] [Google Scholar]
- 54.Baiguera S., Del Gaudio C., Di Nardo P., Manzari V., Carotenuto F., Teodori L. 3D printing decellularized extracellular matrix to design biomimetic scaffolds for skeletal muscle tissue engineering. BioMed Res. Int. Nov. 2020;2020 doi: 10.1155/2020/2689701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong M.L., Griffiths L.G. Immunogenicity in xenogeneic scaffold generation: antigen removal versus decellularization. Acta Biomater. May 2014;10(5):1806–1816. doi: 10.1016/j.actbio.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu K., et al. Efficient decellularization for tissue engineering of the tendon-bone interface with preservation of biomechanics. PLoS One. Feb. 2017;12(2) doi: 10.1371/journal.pone.0171577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kajbafzadeh A.-M., Javan-Farazmand N., Monajemzadeh M., Baghayee A. Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human-sized liver tissue. Tissue Eng. C Methods. Dec. 2012;19(8):642–651. doi: 10.1089/ten.tec.2012.0334. [DOI] [PubMed] [Google Scholar]
- 58.Dai Z., Ronholm J., Tian Y., Sethi B., Cao X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. May 2016;7 doi: 10.1177/2041731416648810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chou J.W., Skornicki M., Cohen J.T. Unintended consequences of the potential phase-out of gamma irradiation. F1000Research. Mar. 2018;7 doi: 10.12688/f1000research.14090.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mendes G.C.C., Brandão T.R.S., Silva C.L.M. Ethylene oxide sterilization of medical devices: a review. Am. J. Infect. Control. Nov. 2007;35(9):574–581. doi: 10.1016/j.ajic.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Balestrini J.L., et al. Sterilization of lung matrices by supercritical carbon dioxide. Tissue Eng. C Methods. Dec. 2015;22(3):260–269. doi: 10.1089/ten.tec.2015.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Y.-H., et al. Preparation of acellular scaffold for corneal tissue engineering by supercritical carbon dioxide extraction technology. Acta Biomater. Aug. 2017;58:238–243. doi: 10.1016/j.actbio.2017.05.060. [DOI] [PubMed] [Google Scholar]
- 63.Qiu Q.-Q., Leamy P., Brittingham J., Pomerleau J., Kabaria N., Connor J. Inactivation of bacterial spores and viruses in biological material using supercritical carbon dioxide with sterilant. J. Biomed. Mater. Res. B Appl. Biomater. 2009;91B(2):572–578. doi: 10.1002/jbm.b.31431. [DOI] [PubMed] [Google Scholar]
- 64.Zouhair S., et al. Preservation strategies for decellularized pericardial scaffolds for off-the-shelf availability. Acta Biomater. Jan. 2019;84:208–221. doi: 10.1016/j.actbio.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 65.Sheridan W.S., Duffy G.P., Murphy B.P. Optimum parameters for freeze-drying decellularized arterial scaffolds. Tissue Eng. C Methods. Dec. 2013;19(12):981–990. doi: 10.1089/ten.tec.2012.0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alshaikh A.B., et al. Decellularization of the mouse ovary: comparison of different scaffold generation protocols for future ovarian bioengineering. J. Ovarian Res. Jun. 2019;12(1):58. doi: 10.1186/s13048-019-0531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaetani R., et al. Evaluation of different decellularization protocols on the generation of pancreas-derived hydrogels. Tissue Eng. C Methods. Dec. 2018;24(12):697–708. doi: 10.1089/ten.tec.2018.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Urbani L., et al. Long-term cryopreservation of decellularised oesophagi for tissue engineering clinical application. PLoS One. Jun. 2017;12(6) doi: 10.1371/journal.pone.0179341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonenfant N.R., et al. The effects of storage and sterilization on de-cellularized and Re-cellularized whole lung. Biomaterials. Apr. 2013;34(13):3231–3245. doi: 10.1016/j.biomaterials.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tapias L.F., Ott H.C. Decellularized scaffolds as a platform for bioengineered organs. Curr. Opin. Organ Transplant. Apr. 2014;19(2):145–152. doi: 10.1097/MOT.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schaner P.J., et al. Decellularized vein as a potential scaffold for vascular tissue engineering. J. Vasc. Surg. Jul. 2004;40(1):146–153. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 72.Edgar L., et al. Utility of extracellular matrix powders in tissue engineering. Organogenesis. Sep. 2018;14(4):172–186. doi: 10.1080/15476278.2018.1503771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jakus A.E., et al. ‘Tissue papers’ from organ-specific decellularized extracellular matrices. Adv. Funct. Mater. 2017;27(34) doi: 10.1002/adfm.201700992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mazzitelli S., et al. Production and characterization of engineered alginate-based microparticles containing ECM powder for cell/tissue engineering applications. Acta Biomater. Mar. 2011;7(3):1050–1062. doi: 10.1016/j.actbio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Wu K., et al. Injectable decellularized extracellular matrix hydrogel containing stromal cell-derived factor 1 promotes transplanted cardiomyocyte engraftment and functional regeneration after myocardial infarction. ACS Appl. Mater. Interfaces. 2022 doi: 10.1021/acsami.2c16682. [DOI] [PubMed] [Google Scholar]
- 76.Yeleswarapu S., Chameettachal S., Bera A.K., Pati F. Smooth muscle matrix bioink promotes myogenic differentiation of encapsulated adipose-derived stem cells. J. Biomed. Mater. Res., Part A. 2022;110(11):1761–1773. doi: 10.1002/jbm.a.37433. [DOI] [PubMed] [Google Scholar]
- 77.Jiang B., Akgun B., Lam R.C., Ameer G.A., Wertheim J.A. A polymer–extracellular matrix composite with improved thromboresistance and recellularization properties. Acta Biomater. May 2015;18:50–58. doi: 10.1016/j.actbio.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goyal R., Guvendiren M., Freeman O., Mao Y., Kohn J. Optimization of polymer-ECM composite scaffolds for tissue engineering: effect of cells and culture conditions on polymeric nanofiber mats. J. Funct. Biomater. Jan. 2017;8(1) doi: 10.3390/jfb8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vriend L., et al. Limited efficacy of adipose stromal cell secretome-loaded skin-derived hydrogels to augment skin flap regeneration in rats. Stem Cell. Dev. 2022;31(19–20):630–640. doi: 10.1089/scd.2022.0003. [DOI] [PubMed] [Google Scholar]
- 80.Wu J., Xu J., Huang Y., Tang L., Hong Y. Regional-specific meniscal extracellular matrix hydrogels and their effects on cell–matrix interactions of fibrochondrocytes. Biomed. Mater. Dec. 2021;17(1) doi: 10.1088/1748-605X/ac4178. [DOI] [PubMed] [Google Scholar]
- 81.Allbritton-King J.D., Kimicata M., Fisher J.P. Incorporating a structural extracellular matrix gradient into a porcine urinary bladder matrix-based hydrogel dermal scaffold. J. Biomed. Mater. Res., Part A. 2021;109(10):1893–1904. doi: 10.1002/jbm.a.37181. [DOI] [PubMed] [Google Scholar]
- 82.Parthiban S.P., Athirasala A., Tahayeri A., Abdelmoniem R., George A., Bertassoni L.E. BoneMA-synthesis and characterization of a methacrylated bone-derived hydrogel for bioprinting of in-vitro vascularized tissue constructs. Biofabrication. 2021;13(3) doi: 10.1088/1758-5090/abb11f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ravichandran A., Murekatete B., Moedder D., Meinert C., Bray L.J. Photocrosslinkable liver extracellular matrix hydrogels for the generation of 3D liver microenvironment models. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-94990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei Q., et al. TGF-β1-supplemented decellularized annulus fibrosus matrix hydrogels promote annulus fibrosus repair. Bioact. Mater. 2023;19:581–593. doi: 10.1016/j.bioactmat.2022.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin Y.H., et al. Effect of glutaraldehyde-crosslinked cartilage acellular matrix film on anti-adhesion and nerve regeneration in a rat sciatic nerve injury model. J. Tissue Eng. Regen. Med. 2021;15(11):1023–1036. doi: 10.1002/term.3249. [DOI] [PubMed] [Google Scholar]
- 86.Fernández-Pérez J., Ahearne M. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci. Rep. Oct. 2019;9(1) doi: 10.1038/s41598-019-49575-2. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Výborný K., et al. Genipin and EDC crosslinking of extracellular matrix hydrogel derived from human umbilical cord for neural tissue repair. Sci. Rep. Jul. 2019;9(1):1–15. doi: 10.1038/s41598-019-47059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saldin L.T., Cramer M.C., Velankar S.S., White L.J., Badylak S.F. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. Feb. 2017;49:1–15. doi: 10.1016/j.actbio.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu L., et al. Genipin cross-linked decellularized nucleus pulposus hydrogel-like cell delivery system induces differentiation of ADSCs and retards intervertebral disc degeneration. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.807883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishiguchi A., Taguchi T. A pH-driven genipin gelator to engineer decellularized extracellular matrix-based tissue adhesives. Acta Biomater. 2021;131:211–221. doi: 10.1016/j.actbio.2021.06.033. [DOI] [PubMed] [Google Scholar]
- 91.Mao Y., Hoffman T., Wu A., Goyal R., Kohn J. Cell type–specific extracellular matrix guided the differentiation of human mesenchymal stem cells in 3D polymeric scaffolds. J. Mater. Sci. Mater. Med. 2017;28(7) doi: 10.1007/s10856-017-5912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crapo P.M., Gilbert T.W., Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. Apr. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petersen T.H., et al. Tissue-engineered lungs for in vivo implantation. Science. Jul. 2010;329(5991):538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Price A.P., England K.A., Matson A.M., Blazar B.R., Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng. Aug. 2010;16(8):2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uygun B.E., et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. Jul. 2010;16(7):814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ott H.C., et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. Feb. 2008;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 97.Seo Y., Jung Y., Kim S.H. Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater. Feb. 2018;67:270–281. doi: 10.1016/j.actbio.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 98.Zhou J., et al. Surface biofunctionalization of the decellularized porcine aortic valve with VEGF-loaded nanoparticles for accelerating endothelialization. Mater. Sci. Eng., C. Apr. 2019;97:632–643. doi: 10.1016/j.msec.2018.12.079. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Q., et al. Coupled OPG-fc on decellularized aortic valves by EDC/NHS attenuates rat MSCs calcification in vitro. ASAIO J. Am. Soc. Artif. Intern. Organs 1992. 2019;65(2):197–204. doi: 10.1097/MAT.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 100.Li Y., et al. Biofunctionalization of decellularized porcine aortic valve with OPG-loaded PCL nanoparticles for anti-calcification. RSC Adv. Apr. 2019;9(21):11882–11893. doi: 10.1039/C9RA00408D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guo F., Liu Y., Jiao K., Yang R., Hou M., Zhang X. Artificial heart valves with balanced charged networks exhibiting anti-calcification properties. ACS Appl. Bio Mater. Feb. 2020;3(2):838–847. doi: 10.1021/acsabm.9b00902. [DOI] [PubMed] [Google Scholar]
- 102.VeDepo M.C., Buse E.E., Paul A., Converse G.L., Hopkins R.A. Non-physiologic bioreactor processing conditions for heart valve tissue engineering. Cardiovasc. Eng. Technol. Dec. 2019;10(4):628–637. doi: 10.1007/s13239-019-00438-x. [DOI] [PubMed] [Google Scholar]
- 103.Dai J., Qiao W., Shi J., Liu C., Hu X., Dong N. Modifying decellularized aortic valve scaffolds with stromal cell-derived factor-1α loaded proteolytically degradable hydrogel for recellularization and remodeling. Acta Biomater. Apr. 2019;88:280–292. doi: 10.1016/j.actbio.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 104.Wan L., et al. Human heart valve-derived scaffold improves cardiac repair in a murine model of myocardial infarction. Sci. Rep. 2017;7(Jan) doi: 10.1038/srep39988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jang J., et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. Jan. 2017;112:264–274. doi: 10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 106.Bejleri D., et al. Bioprinted cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair. Adv. Healthcare Mater. Dec. 2018;7(23) doi: 10.1002/adhm.201800672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarig U., et al. Pushing the envelope in tissue engineering: ex vivo production of thick vascularized cardiac extracellular matrix constructs. Tissue Eng. May 2015;21(9–10):1507–1519. doi: 10.1089/ten.tea.2014.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ansari T., et al. Development and characterization of a porcine liver scaffold. Stem Cell. Dev. Dec. 2019;29(5):314–326. doi: 10.1089/scd.2019.0069. [DOI] [PubMed] [Google Scholar]
- 109.E.A. Rossi, L.F. Quintanilha, C.K.V. Nonaka, B.S. de F. Souza, Advances in hepatic tissue bioengineering with decellularized liver bioscaffold, Stem Cell. Int. vol. 2019, p. 2693189, (2019). [DOI] [PMC free article] [PubMed]
- 110.Hillebrandt K., et al. Procedure for decellularization of rat livers in an oscillating-pressure perfusion device. J. Vis. Exp. Aug. 2015;102 doi: 10.3791/53029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maghsoudlou P., et al. Optimization of liver decellularization maintains extracellular matrix micro-architecture and composition predisposing to effective cell seeding. PLoS One. May 2016;11(5) doi: 10.1371/journal.pone.0155324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Verstegen M.M.A., et al. Decellularization of whole human liver grafts using controlled perfusion for transplantable organ bioscaffolds. Stem Cell. Dev. 2017;26(18):1304–1315. doi: 10.1089/scd.2017.0095. 15. [DOI] [PubMed] [Google Scholar]
- 113.Willemse J., et al. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion. Mater. Sci. Eng. C. Mar. 2020;108 doi: 10.1016/j.msec.2019.110200. [DOI] [PubMed] [Google Scholar]
- 114.Watanabe M., et al. Construction of sinusoid-scale microvessels in perfusion culture of a decellularized liver. Acta Biomater. Sep. 2019;95:307–318. doi: 10.1016/j.actbio.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 115.Shaheen M.F., et al. Sustained perfusion of revascularized bioengineered livers heterotopically transplanted into immunosuppressed pigs. Nat. Biomed. Eng. Apr. 2020;4(4) doi: 10.1038/s41551-019-0460-x. Art. no. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim D.-H., et al. Development of highly functional bioengineered human liver with perfusable vasculature. Biomaterials. Jan. 2021;265 doi: 10.1016/j.biomaterials.2020.120417. [DOI] [PubMed] [Google Scholar]
- 117.Song J.J., et al. Enhanced in vivo function of bioartificial lungs in rats. Ann. Thorac. Surg. Sep. 2011;92(3):998–1006. doi: 10.1016/j.athoracsur.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 118.Gilpin S.E., et al. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J. Heart Lung Transplant. Mar. 2014;33(3):298–308. doi: 10.1016/j.healun.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 119.Petersen T.H., Petersen T.H., Calle E.A., Colehour M.B., Niklason L.E. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs. 2012;195(3):222–231. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nichols J.E., et al. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng. Sep. 2013;19(17–18):2045–2062. doi: 10.1089/ten.tea.2012.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ott H.C., et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. Aug. 2010;16(8) doi: 10.1038/nm.2193. Art. no. 8. [DOI] [PubMed] [Google Scholar]
- 122.Tsuchiya T., Sivarapatna A., Rocco K., Nanashima A., Nagayasu T., Niklason L.E. Future prospects for tissue engineered lung transplantation. Organogenesis. Apr. 2014;10(2):196–207. doi: 10.4161/org.27846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gilpin S.E., et al. Regenerative potential of human airway stem cells in lung epithelial engineering. Biomaterials. Nov. 2016;108:111–119. doi: 10.1016/j.biomaterials.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Young B.M., Shankar K., Tho C.K., Pellegrino A.R., Heise R.L. Laminin-driven Epac/Rap1 regulation of epithelial barriers on decellularized matrix. Acta Biomater. 2019;100:223–234. doi: 10.1016/j.actbio.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Obata T., et al. Utilization of natural detergent potassium laurate for decellularization in lung bioengineering. Tissue Eng. C Methods. Jul. 2019;25(8):459–471. doi: 10.1089/ten.tec.2019.0016. [DOI] [PubMed] [Google Scholar]
- 126.Yuan Y., et al. Epac agonist improves barrier function in iPSC-derived endothelial colony forming cells for whole organ tissue engineering. Biomaterials. 2019;200:25–34. doi: 10.1016/j.biomaterials.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 127.Ross E.A., et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J. Am. Soc. Nephrol. JASN. Nov. 2009;20(11):2338–2347. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Poornejad N., et al. The impact of decellularization agents on renal tissue extracellular matrix. J. Biomater. Appl. Oct. 2016;31(4):521–533. doi: 10.1177/0885328216656099. [DOI] [PubMed] [Google Scholar]
- 129.Poornejad N., et al. Efficient decellularization of whole porcine kidneys improves reseeded cell behavior. Biomed. Mater. Mar. 2016;11(2) doi: 10.1088/1748-6041/11/2/025003. [DOI] [PubMed] [Google Scholar]
- 130.He M., Callanan A., Lagaras K., Steele J.a.M., Stevens M.M. Optimization of SDS exposure on preservation of ECM characteristics in whole organ decellularization of rat kidneys. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105(6):1352–1360. doi: 10.1002/jbm.b.33668. [DOI] [PubMed] [Google Scholar]
- 131.Schmitt A., et al. Optimized protocol for whole organ decellularization. Eur. J. Med. Res. Sep. 2017;22(1):31. doi: 10.1186/s40001-017-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zambon J.P., et al. Comparative analysis of two porcine kidney decellularization methods for maintenance of functional vascular architectures. Acta Biomater. Jul. 2018;75:226–234. doi: 10.1016/j.actbio.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 133.Kajbafzadeh A.-M., et al. Whole organ sheep kidney tissue engineering and in vivo transplantation: effects of perfusion-based decellularization on vascular integrity. Mater. Sci. Eng. C. May 2019;98:392–400. doi: 10.1016/j.msec.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 134.Remuzzi A., et al. Experimental evaluation of kidney regeneration by organ scaffold recellularization. Sci. Rep. Mar. 2017;7 doi: 10.1038/srep43502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ciampi O., et al. Engineering the vasculature of decellularized rat kidney scaffolds using human induced pluripotent stem cell-derived endothelial cells. Sci. Rep. May 2019;9(1) doi: 10.1038/s41598-019-44393-y. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Taylor D.A., Sampaio L.C., Ferdous Z., Gobin A.S., Taite L.J. Decellularized matrices in regenerative medicine. Acta Biomater. Jul. 2018;74:74–89. doi: 10.1016/j.actbio.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 137.Zhang H., et al. Demineralized bone matrix carriers and their clinical applications: an overview. Orthop. Surg. 2019;11(5):725–737. doi: 10.1111/os.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gruskin E., Doll B.A., Futrell F.W., Schmitz J.P., Hollinger J.O. Demineralized bone matrix in bone repair: history and use. Adv. Drug Deliv. Rev. Sep. 2012;64(12):1063–1077. doi: 10.1016/j.addr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen I.-C., Su C.-Y., Lai C.-C., Tsou Y.-S., Zheng Y., Fang H.-W. Preparation and characterization of moldable demineralized bone matrix/calcium sulfate composite bone graft materials. J. Funct. Biomater. Dec. 2021;12(4) doi: 10.3390/jfb12040056. Art. no. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sawkins M.J., et al. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. Aug. 2013;9(8):7865–7873. doi: 10.1016/j.actbio.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hashimoto Y., Funamoto S., Kimura T., Nam K., Fujisato T., Kishida A. The effect of decellularized bone/bone marrow produced by high-hydrostatic pressurization on the osteogenic differentiation of mesenchymal stem cells. Biomaterials. Oct. 2011;32(29):7060–7067. doi: 10.1016/j.biomaterials.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 142.Lee D.J., et al. Decellularized bone matrix grafts for calvaria regeneration. J. Tissue Eng. 2016;7(Dec) doi: 10.1177/2041731416680306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Paduano F., et al. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2017;28(8):730–748. doi: 10.1080/09205063.2017.1301770. [DOI] [PubMed] [Google Scholar]
- 144.Hung B.P., et al. Three-dimensional printing of bone extracellular matrix for craniofacial regeneration. ACS Biomater. Sci. Eng. Oct. 2016;2(10):1806–1816. doi: 10.1021/acsbiomaterials.6b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ko E., et al. Nanostructured tendon-derived scaffolds for enhanced bone regeneration by human adipose-derived stem cells. ACS Appl. Mater. Interfaces. Sep. 2016;8(35):22819–22829. doi: 10.1021/acsami.6b05358. [DOI] [PubMed] [Google Scholar]
- 146.Shridhar A., Amsden B.G., Gillies E.R., Flynn L.E. Investigating the effects of tissue-specific extracellular matrix on the adipogenic and osteogenic differentiation of human adipose-derived stromal cells within composite hydrogel scaffolds. Front. Bioeng. Biotechnol. 2019;7(Dec) doi: 10.3389/fbioe.2019.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim J.-Y., et al. Synergistic effects of beta tri-calcium phosphate and porcine-derived decellularized bone extracellular matrix in 3D-printed polycaprolactone scaffold on bone regeneration. Macromol. Biosci. 2018;18(6) doi: 10.1002/mabi.201800025. [DOI] [PubMed] [Google Scholar]
- 148.Chen G., Lv Y. Matrix elasticity-modified scaffold loaded with SDF-1α improves the in situ regeneration of segmental bone defect in rabbit radius. Sci. Rep. May 2017;7 doi: 10.1038/s41598-017-01938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rindone A.N., et al. Heparin-conjugated decellularized bone particles promote enhanced osteogenic signaling of PDGF-BB to adipose-derived stem cells in tissue engineered bone grafts. Adv. Healthcare Mater. 2019;8(10) doi: 10.1002/adhm.201801565. [DOI] [PubMed] [Google Scholar]
- 150.Alom N., Peto H., Kirkham G.R., Shakesheff K.M., White L.J. Bone extracellular matrix hydrogel enhances osteogenic differentiation of C2C12 myoblasts and mouse primary calvarial cells. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(2):900–908. doi: 10.1002/jbm.b.33894. [DOI] [PubMed] [Google Scholar]
- 151.Ventura R.D., Padalhin A.R., Kim B., Park M., Lee B.T. Evaluation of bone regeneration potential of injectable extracellular matrix (ECM) from porcine dermis loaded with biphasic calcium phosphate (BCP) powder. Mater. Sci. Eng. C. May 2020;110 doi: 10.1016/j.msec.2020.110663. [DOI] [PubMed] [Google Scholar]
- 152.Yang Z., et al. Fabrication and repair of cartilage defects with a novel acellular cartilage matrix scaffold. Tissue Eng. C Methods. Nov. 2009;16(5):865–876. doi: 10.1089/ten.tec.2009.0444. [DOI] [PubMed] [Google Scholar]
- 153.Bautista C.A., Park H.J., Mazur C.M., Aaron R.K., Bilgen B. Effects of chondroitinase ABC-mediated proteoglycan digestion on decellularization and recellularization of articular cartilage. PLoS One. Jul. 2016;11(7) doi: 10.1371/journal.pone.0158976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Luo L., Eswaramoorthy R., Mulhall K.J., Kelly D.J. Decellularization of porcine articular cartilage explants and their subsequent repopulation with human chondroprogenitor cells. J. Mech. Behav. Biomed. Mater. Mar. 2016;55:21–31. doi: 10.1016/j.jmbbm.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 155.Lin L., et al. Nanofibrous Wharton's jelly scaffold in combination with adipose-derived stem cells for cartilage engineering. Mater. Des. Jan. 2020;186 doi: 10.1016/j.matdes.2019.108216. [DOI] [Google Scholar]
- 156.Forouzesh F., Rabbani M., Bonakdar S. A comparison between ultrasonic bath and direct sonicator on osteochondral tissue decellularization. J. Med. Signals Sens. Oct. 2019;9(4):227–233. doi: 10.4103/jmss.JMSS_64_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang Q., et al. Silk fibroin/cartilage extracellular matrix scaffolds with sequential delivery of TGF-β3 for chondrogenic differentiation of adipose-derived stem cells. Int. J. Nanomed. Sep. 2017;12:6721–6733. doi: 10.2147/IJN.S141888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sivandzade F., Mashayekhan S. Design and fabrication of injectable microcarriers composed of acellular cartilage matrix and chitosan. J. Biomater. Sci. Polym. Ed. 2018;29(6):683–700. doi: 10.1080/09205063.2018.1433422. [DOI] [PubMed] [Google Scholar]
- 159.Wiggenhauser P.S., Schwarz S., Koerber L., Hoffmann T.K., Rotter N. Addition of decellularized extracellular matrix of porcine nasal cartilage improves cartilage regenerative capacities of PCL-based scaffolds in vitro. J. Mater. Sci. Mater. Med. Oct. 2019;30(11):121. doi: 10.1007/s10856-019-6323-x. [DOI] [PubMed] [Google Scholar]
- 160.Browe D.C., et al. Glyoxal cross-linking of solubilized extracellular matrix to produce highly porous, elastic, and chondro-permissive scaffolds for orthopedic tissue engineering. J. Biomed. Mater. Res. 2019;107(10):2222–2234. doi: 10.1002/jbm.a.36731. [DOI] [PubMed] [Google Scholar]
- 161.Li Y., Liu Y., Xun X., Zhang W., Xu Y., Gu D. Three-dimensional porous scaffolds with biomimetic microarchitecture and bioactivity for cartilage tissue engineering. ACS Appl. Mater. Interfaces. Oct. 2019;11(40):36359–36370. doi: 10.1021/acsami.9b12206. [DOI] [PubMed] [Google Scholar]
- 162.Oh H.J., Kim S.H., Cho J.-H., Park S.-H., Min B.-H. Mechanically reinforced extracellular matrix scaffold for application of cartilage tissue engineering. Tissue Eng. Regen. Med. Feb. 2018;15(3):287–299. doi: 10.1007/s13770-018-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li Y., et al. Decellularized cartilage matrix scaffolds with laser-machined micropores for cartilage regeneration and articular cartilage repair. Mater. Sci. Eng. C. Dec. 2019;105 doi: 10.1016/j.msec.2019.110139. [DOI] [PubMed] [Google Scholar]
- 164.Golebiowska A.A., Jala V.R., Nukavarapu S.P. Decellularized tissue-induced cellular recruitment for tissue engineering and regenerative medicine. Ann. Biomed. Eng. Mar. 2023 doi: 10.1007/s10439-023-03182-5. [DOI] [PubMed] [Google Scholar]
- 165.Tian G., et al. Cell-free decellularized cartilage extracellular matrix scaffolds combined with interleukin 4 promote osteochondral repair through immunomodulatory macrophages: in vitro and in vivo preclinical study. Acta Biomater. Jun. 2021;127:131–145. doi: 10.1016/j.actbio.2021.03.054. [DOI] [PubMed] [Google Scholar]
- 166.Zhu M., et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. Oct. 2019;10(1) doi: 10.1038/s41467-019-12545-3. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smoak M.M., et al. Fabrication and characterization of electrospun decellularized muscle-derived scaffolds. Tissue Eng. C Methods. Mar. 2019;25(5):276–287. doi: 10.1089/ten.tec.2018.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee H., Kim W., Lee J., Yoo J.J., Kim G.H., Lee S.J. Effect of hierarchical scaffold consisting of aligned dECM nanofibers and poly(lactide-co-glycolide) struts on the orientation and maturation of human muscle progenitor cells. ACS Appl. Mater. Interfaces. Oct. 2019;11(43):39449–39458. doi: 10.1021/acsami.9b12639. [DOI] [PubMed] [Google Scholar]
- 169.Choi Y.-J., Park S.J., Yi H.-G., Lee H., Kim D.S., Cho D.-W. Muscle-derived extracellular matrix on sinusoidal wavy surfaces synergistically promotes myogenic differentiation and maturation. J. Mater. Chem. B. Sep. 2018;6(35):5530–5539. doi: 10.1039/C8TB01475B. [DOI] [PubMed] [Google Scholar]
- 170.Patel K.H., et al. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro. Biomed. Mater. Apr. 2019;14(3) doi: 10.1088/1748-605X/ab0b06. [DOI] [PubMed] [Google Scholar]
- 171.K. H. Patel et al., “Aligned nanofibers of decellularized muscle extracellular matrix for volumetric muscle loss,” J. Biomed. Mater. Res. B Appl. Biomater., vol. n/a, no. n/a, doi: 10.1002/jbm.b.34584. [DOI] [PubMed]
- 172.Rajabi S., Jalili‐Firoozinezhad S., Ashtiani M.K., Carrou G.L., Tajbakhsh S., Baharvand H. Effect of chemical immobilization of SDF-1α into muscle-derived scaffolds on angiogenesis and muscle progenitor recruitment. J. Tissue Eng. Regen. Med. 2018;12(1):e438–e450. doi: 10.1002/term.2479. [DOI] [PubMed] [Google Scholar]
- 173.Shapiro L., et al. In vitro evaluation of functionalized decellularized muscle scaffold for in situ skeletal muscle regeneration. Biomed. Mater. Jun. 2019;14(4) doi: 10.1088/1748-605X/ab229d. [DOI] [PubMed] [Google Scholar]
- 174.Lee H., et al. A novel decellularized skeletal muscle-derived ECM scaffolding system for in situ muscle regeneration. Methods. Jan. 2020;171:77–85. doi: 10.1016/j.ymeth.2019.06.027. [DOI] [PubMed] [Google Scholar]
- 175.Zhang J., et al. Perfusion-decellularized skeletal muscle as a three-dimensional scaffold with a vascular network template. Biomaterials. May 2016;89:114–126. doi: 10.1016/j.biomaterials.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 176.A.B. Lovati, M. Bottagisio, M. Moretti, Decellularized and engineered tendons as biological substitutes: a critical review, Stem Cell. Int. vol. 2016, p. 7276150, (2016). [DOI] [PMC free article] [PubMed]
- 177.Yao X., et al. Stem cell extracellular matrix-modified decellularized tendon slices facilitate the migration of bone marrow mesenchymal stem cells. ACS Biomater. Sci. Eng. Sep. 2019;5(9):4485–4495. doi: 10.1021/acsbiomaterials.9b00064. [DOI] [PubMed] [Google Scholar]
- 178.Zhang C.-H., et al. Evaluation of decellularized bovine tendon sheets for achilles tendon defect reconstruction in a rabbit model. Am. J. Sports Med. Sep. 2018;46(11):2687–2699. doi: 10.1177/0363546518787515. [DOI] [PubMed] [Google Scholar]
- 179.Xie S., et al. Book-shaped decellularized tendon matrix scaffold combined with bone marrow mesenchymal stem cells-sheets for repair of achilles tendon defect in rabbit. J. Orthop. Res. 2019;37(4):887–897. doi: 10.1002/jor.24255. [DOI] [PubMed] [Google Scholar]
- 180.Liu G.-M., et al. Bridging repair of large rotator cuff tears using a multilayer decellularized tendon slices graft in a rabbit model. Arthrosc. J. Arthrosc. Relat. Surg. Sep. 2018;34(9):2569–2578. doi: 10.1016/j.arthro.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 181.M. Bottagisio, et al., Achilles tendon repair by decellularized and engineered xenografts in a rabbit model, Stem Cell. Int. vol. 2019, p. 5267479, (2019). [DOI] [PMC free article] [PubMed]
- 182.Liu Q., et al. Novel engineered tendon–fibrocartilage–bone composite with cyclic tension for rotator cuff repair. J. Tissue Eng. Regen. Med. 2018;12(7):1690–1701. doi: 10.1002/term.2696. [DOI] [PubMed] [Google Scholar]
- 183.Lee K.I., et al. In vitro and in vivo performance of tissue-engineered tendons for anterior cruciate ligament reconstruction. Am. J. Sports Med. Jun. 2018;46(7):1641–1649. doi: 10.1177/0363546518759729. [DOI] [PubMed] [Google Scholar]
- 184.Talò G., D'Arrigo D., Lorenzi S., Moretti M., Lovati A.B. Independent, controllable stretch-perfusion bioreactor chambers to functionalize cell-seeded decellularized tendons. Ann. Biomed. Eng. 2020;48(3):1112–1126. doi: 10.1007/s10439-019-02257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang G., Rothrauff B.B., Lin H., Yu S., Tuan R.S. Tendon-derived extracellular matrix enhances transforming growth factor-β3-induced tenogenic differentiation of human adipose-derived stem cells. Tissue Eng. Feb. 2017;23(3–4):166–176. doi: 10.1089/ten.tea.2015.0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roth S.P., Schubert S., Scheibe P., Groß C., Brehm W., Burk J. Growth factor-mediated tenogenic induction of multipotent mesenchymal stromal cells is altered by the microenvironment of tendon matrix. Cell Transplant. Oct. 2018;27(10):1434–1450. doi: 10.1177/0963689718792203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu Q., et al. Engineered tendon-fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials. Feb. 2019;192:189–198. doi: 10.1016/j.biomaterials.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 188.Su M., et al. Preparation of decellularized triphasic hierarchical bone-fibrocartilage-tendon composite extracellular matrix for enthesis regeneration. Adv. Healthcare Mater. 2019;8(19) doi: 10.1002/adhm.201900831. [DOI] [PubMed] [Google Scholar]
- 189.Tang Y., et al. Structure and ingredient-based biomimetic scaffolds combining with autologous bone marrow-derived mesenchymal stem cell sheets for bone-tendon healing. Biomaterials. May 2020;241 doi: 10.1016/j.biomaterials.2020.119837. [DOI] [PubMed] [Google Scholar]
- 190.Garreta E., et al. Tissue engineering by decellularization and 3D bioprinting. Mater. Today. May 2017;20(4):166–178. doi: 10.1016/j.mattod.2016.12.005. [DOI] [Google Scholar]
- 191.Hensley A., et al. Decellularization and characterization of a whole intervertebral disc xenograft scaffold. J. Biomed. Mater. Res. Sep. 2018;106(9):2412–2423. doi: 10.1002/jbm.a.36434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zheng M.H., Chen J., Kirilak Y., Willers C., Xu J., Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J. Biomed. Mater. Res. B Appl. Biomater. 2005;73B(1):61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 193.Simsa R., et al. Systematic in vitro comparison of decellularization protocols for blood vessels. PLoS One. Dec. 2018;13(12) doi: 10.1371/journal.pone.0209269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schneider C., et al. Systematic comparison of protocols for the preparation of human articular cartilage for use as scaffold material in cartilage tissue engineering. Tissue Eng. C Methods. Nov. 2016;22(12):1095–1107. doi: 10.1089/ten.tec.2016.0380. [DOI] [PubMed] [Google Scholar]
- 195.Song Y.H., et al. Development of novel apoptosis-assisted lung tissue decellularization methods. Biomater. Sci. May 2021;9(9):3485–3498. doi: 10.1039/D1BM00032B. [DOI] [PubMed] [Google Scholar]
- 196.Mazza G., et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. Aug. 2015;5(1):1–15. doi: 10.1038/srep13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mazza G., et al. Rapid production of human liver scaffolds for functional tissue engineering by high shear stress oscillation-decellularization. Sci. Rep. Jul. 2017;7(1):1–14. doi: 10.1038/s41598-017-05134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huling J.C., Atala A., Yoo J.J. In: Kidney Development, Disease, Repair and Regeneration. Little M.H., editor. Academic Press; San Diego: 2016. Chapter 42 - decellularized whole organ scaffolds for the regeneration of kidneys; pp. 569–578. [DOI] [Google Scholar]
- 199.Sasikumar S., Chameettachal S., Cromer B., Pati F., Kingshott P. Decellularized extracellular matrix hydrogels—cell behavior as a function of matrix stiffness. Curr. Opin. Biomed. Eng. Jun. 2019;10:123–133. doi: 10.1016/j.cobme.2019.05.002. [DOI] [Google Scholar]
- 200.Vanden Berg-Foels W.S. In situ tissue regeneration: chemoattractants for endogenous stem cell recruitment. Tissue Eng., Part B. Feb. 2014;20(1):28–39. doi: 10.1089/ten.teb.2013.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hanai H., Jacob G., Nakagawa S., Tuan R.S., Nakamura N., Shimomura K. Potential of soluble decellularized extracellular matrix for musculoskeletal tissue engineering – comparison of various mesenchymal tissues. Front. Cell Dev. Biol. Nov. 2020;8 doi: 10.3389/fcell.2020.581972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nokhbatolfoghahaei H., et al. Fabrication of decellularized engineered extracellular matrix through bioreactor-based environment for bone tissue engineering. ACS Omega. Dec. 2020;5(49):31943–31956. doi: 10.1021/acsomega.0c04861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Blum B., Moseley J., Miller L., Richelsoph K., Haggard W. Measurement of bone morphogenetic proteins and other growth factors in demineralized bone matrix. Orthopedics. Jan. 2004;27(1):S161–S165. doi: 10.3928/0147-7447-20040102-17. [DOI] [PubMed] [Google Scholar]
- 204.Ullah I., et al. Vegf – supplemented extracellular matrix is sufficient to induce endothelial differentiation of human iPSC. Biomaterials. Sep. 2019;216 doi: 10.1016/j.biomaterials.2019.119283. [DOI] [PubMed] [Google Scholar]
- 205.Coronado R.E., Somaraki-Cormier M., Natesan S., Christy R.J., Ong J.L., Halff G.A. Decellularization and solubilization of porcine liver for use as a substrate for porcine hepatocyte culture: method optimization and comparison. Cell Transplant. Dec. 2017;26(12):1840–1854. doi: 10.1177/0963689717742157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hoganson D.M., et al. Preserved extracellular matrix components and retained biological activity in decellularized porcine mesothelium. Biomaterials. Sep. 2010;31(27):6934–6940. doi: 10.1016/j.biomaterials.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 207.Urciuolo A., et al. Decellularised skeletal muscles allow functional muscle regeneration by promoting host cell migration. Sci. Rep. May 2018;8(1) doi: 10.1038/s41598-018-26371-y. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Barthold J.E., et al. Recellularization and integration of dense extracellular matrix by percolation of tissue microparticles. Adv. Funct. Mater. 2021;31(35) doi: 10.1002/adfm.202103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang Z., et al. Extracellular matrix derived from allogenic decellularized bone marrow mesenchymal stem cell sheets for the reconstruction of osteochondral defects in rabbits. Acta Biomater. Dec. 2020;118:54–68. doi: 10.1016/j.actbio.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 210.Chen W.C.W., et al. Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Sci. Adv. Nov. 2016;2(11) doi: 10.1126/sciadv.1600844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vorotnikova E., et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. Oct. 2010;29(8):690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 212.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. Dec. 2010;123(24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Partington L., et al. Biochemical changes caused by decellularization may compromise mechanical integrity of tracheal scaffolds. Acta Biomater. Feb. 2013;9(2):5251–5261. doi: 10.1016/j.actbio.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 214.Wolf M.T., et al. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. Oct. 2012;33(29):7028–7038. doi: 10.1016/j.biomaterials.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ahearne M., Coyle A. Application of UVA-riboflavin crosslinking to enhance the mechanical properties of extracellular matrix derived hydrogels. J. Mech. Behav. Biomed. Mater. Feb. 2016;54:259–267. doi: 10.1016/j.jmbbm.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 216.Hong Y., et al. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber - extracellular matrix hydrogel biohybrid scaffold. Biomaterials. May 2011;32(13):3387–3394. doi: 10.1016/j.biomaterials.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Choi Y.-J., et al. 3D cell printing of functional skeletal muscle constructs using skeletal muscle-derived bioink. Adv. Healthcare Mater. 2016;5(20):2636–2645. doi: 10.1002/adhm.201600483. [DOI] [PubMed] [Google Scholar]
- 218.Jung C.S., Kim B.K., Lee J., Min B.-H., Park S.-H. Development of printable natural cartilage matrix bioink for 3D printing of irregular tissue shape. Tissue Eng. Regen. Med. Dec. 2017;15(2):155–162. doi: 10.1007/s13770-017-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ali M., Pr A.K., Yoo J.J., Zahran F., Atala A., Lee S.J. A photo-crosslinkable kidney ECM-derived bioink accelerates renal tissue formation. Adv. Healthcare Mater. Apr. 2019;8(7) doi: 10.1002/adhm.201800992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Toprakhisar B., Nadernezhad A., Bakirci E., Khani N., Skvortsov G.A., Koc B. Development of bioink from decellularized tendon extracellular matrix for 3D bioprinting. Macromol. Biosci. 2018;18(10) doi: 10.1002/mabi.201800024. [DOI] [PubMed] [Google Scholar]
- 221.Won J.-Y., et al. A potential dermal substitute using decellularized dermis extracellular matrix derived bio-ink. Artif. Cell Nanomed. Biotechnol. Dec. 2019;47(1):644–649. doi: 10.1080/21691401.2019.1575842. [DOI] [PubMed] [Google Scholar]
- 222.Jang J., Kim T.G., Kim B.S., Kim S.-W., Kwon S.-M., Cho D.-W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. Mar. 2016;33:88–95. doi: 10.1016/j.actbio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 223.Kim W., et al. Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials. Feb. 2020;230 doi: 10.1016/j.biomaterials.2019.119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pati F., et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. Jun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Xu Y., et al. Construction of bionic tissue engineering cartilage scaffold based on three-dimensional printing and oriented frozen technology. J. Biomed. Mater. Res. 2018;106(6):1664–1676. doi: 10.1002/jbm.a.36368. [DOI] [PubMed] [Google Scholar]
- 226.Choi Y.-J., et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials. Jun. 2019;206:160–169. doi: 10.1016/j.biomaterials.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 227.Yi H.-G., et al. Three-dimensional printing of a patient-specific engineered nasal cartilage for augmentative rhinoplasty. J. Tissue Eng. Jan. 2019;10 doi: 10.1177/2041731418824797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Das S., et al. Decellularized extracellular matrix bioinks and the external stimuli to enhance cardiac tissue development in vitro. Acta Biomater. Sep. 2019;95:188–200. doi: 10.1016/j.actbio.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 229.Noh Y.K., Du P., Dos Santos Da Costa A., Park K. Induction of chondrogenesis of human placenta-derived mesenchymal stem cells via heparin-grafted human fibroblast derived matrix. Biomater. Res. May 2018;22 doi: 10.1186/s40824-018-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fitzpatrick L.E., McDevitt T.C. Cell-derived matrices for tissue engineering and regenerative medicine applications1. Biomater. Sci. Jan. 2015;3(1):12–24. doi: 10.1039/C4BM00246F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Li M., et al. Inhibition of osteoclastogenesis by stem cell-derived extracellular matrix through modulating the intracellular reactive oxygen species. Acta Biomater. Apr. 2018;71:118–131. doi: 10.1016/j.actbio.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gao C.-Y., et al. Directing osteogenic differentiation of BMSCs by cell-secreted decellularized extracellular matrixes from different cell types. J. Mater. Chem. B. Nov. 2018;6(45):7471–7485. doi: 10.1039/C8TB01785A. [DOI] [PubMed] [Google Scholar]
- 233.Marinkovic M., et al. One size does not fit all: developing a cell-specific niche for in vitro study of cell behavior. Matrix Biol. J. Int. Soc. Matrix Biol. 2016;52(54):426–441. doi: 10.1016/j.matbio.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Carvalho M.S., Silva J.C., Cabral J.M.S., da Silva C.L., Vashishth D. Cultured cell-derived extracellular matrices to enhance the osteogenic differentiation and angiogenic properties of human mesenchymal stem/stromal cells. J. Tissue Eng. Regen. Med. 2019;13(9):1544–1558. doi: 10.1002/term.2907. [DOI] [PubMed] [Google Scholar]
- 235.Z. Zhang, R. Qu, T. Fan, J. Ouyang, F. Lu, J. Dai, Stepwise adipogenesis of decellularized cellular extracellular matrix regulates adipose tissue-derived stem cell migration and differentiation, Stem Cell. Int. Vol. 2019, p. 1845926, (2019). [DOI] [PMC free article] [PubMed]
- 236.Hoshiba T., Yokoyama N. Decellularized extracellular matrices derived from cultured cells at stepwise myogenic stages for the regulation of myotube formation. Biochim. Biophys. Acta BBA - Mol. Cell Res. Apr. 2020;1867(4) doi: 10.1016/j.bbamcr.2020.118658. [DOI] [PubMed] [Google Scholar]
- 237.Guneta V., Zhou Z., Tan N.S., Sugii S., Wong M.T.C., Choong C. Recellularization of decellularized adipose tissue-derived stem cells: role of the cell-secreted extracellular matrix in cellular differentiation. Biomater. Sci. Dec. 2017;6(1):168–178. doi: 10.1039/C7BM00695K. [DOI] [PubMed] [Google Scholar]
- 238.Van S.Y., Noh Y.K., Kim S.W., Oh Y.M., Kim I.H., Park K. Human umbilical cord blood mesenchymal stem cells expansion via human fibroblast-derived matrix and their potentials toward regenerative application. Cell Tissue Res. May 2019;376(2):233–245. doi: 10.1007/s00441-018-2971-2. [DOI] [PubMed] [Google Scholar]
- 239.Liu X., et al. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells. Mater. Sci. Eng. C. Apr. 2016;61:437–448. doi: 10.1016/j.msec.2015.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yang Y., Lin H., Shen H., Wang B., Lei G., Tuan R.S. Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater. Mar. 2018;69:71–82. doi: 10.1016/j.actbio.2017.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Anasiz Y., Ozgul R.K., Uckan-Cetinkaya D. A new chapter for mesenchymal stem cells: decellularized extracellular matrices. Stem Cell Rev. Rep. Oct. 2017;13(5):587–597. doi: 10.1007/s12015-017-9757-x. [DOI] [PubMed] [Google Scholar]
- 242.Mao Y., et al. Extracellular matrix derived from chondrocytes promotes rapid expansion of human primary chondrocytes in vitro with reduced dedifferentiation. Acta Biomater. Feb. 2019;85:75–83. doi: 10.1016/j.actbio.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 243.Li J., et al. Role of lineage-specific matrix in stem cell chondrogenesis. Biomaterials. Feb. 2020;231 doi: 10.1016/j.biomaterials.2019.119681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang Y., et al. Matrix reverses immortalization-mediated stem cell fate determination. Biomaterials. Jan. 2021;265 doi: 10.1016/j.biomaterials.2020.120387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pei M., et al. Matrix from urine stem cells boosts tissue-specific stem cell mediated functional cartilage reconstruction. Bioact. Mater. Nov. 2022;23:353–367. doi: 10.1016/j.bioactmat.2022.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tang K.-C., et al. Human adipose-derived stem cell secreted extracellular matrix incorporated into electrospun poly(lactic-co-glycolic acid) nanofibrous dressing for enhancing wound healing. Polymers. Oct. 2019;11(10) doi: 10.3390/polym11101609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sart S., et al. Crosslinking of extracellular matrix scaffolds derived from pluripotent stem cell aggregates modulates neural differentiation. Acta Biomater. Jan. 2016;30:222–232. doi: 10.1016/j.actbio.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 248.Kim B., Ventura R., Lee B.-T. Functionalization of porous BCP scaffold by generating cell-derived extracellular matrix from rat bone marrow stem cells culture for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018;12(2):e1256–e1267. doi: 10.1002/term.2529. [DOI] [PubMed] [Google Scholar]
- 249.Aldemir Dikici B., Reilly G.C., Claeyssens F. Boosting the osteogenic and angiogenic performance of multiscale porous polycaprolactone scaffolds by in vitro generated extracellular matrix decoration. ACS Appl. Mater. Interfaces. Mar. 2020;12(11):12510–12524. doi: 10.1021/acsami.9b23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Teng Y., et al. Extracellular matrix powder from cultured cartilage-like tissue as cell carrier for cartilage repair. J. Mater. Chem. B. May 2017;5(18):3283–3292. doi: 10.1039/C7TB00640C. [DOI] [PubMed] [Google Scholar]
- 251.Sun Y., Yan L., Chen S., Pei M. Functionality of decellularized matrix in cartilage regeneration: a comparison of tissue versus cell sources. Acta Biomater. Jul. 2018;74:56–73. doi: 10.1016/j.actbio.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yamanaka H., Morimoto N., Yamaoka T. Decellularization of submillimeter-diameter vascular scaffolds using peracetic acid. J. Artif. Organs. Jun. 2020;23(2):156–162. doi: 10.1007/s10047-019-01152-0. [DOI] [PubMed] [Google Scholar]
- 253.Huh M.-I., Lee K.-P., Kim J., Yi S., Park B.-U., Kim H.K. Generation of femtosecond laser-cut decellularized corneal lenticule using hypotonic trypsin-EDTA solution for corneal tissue engineering. J. Ophthalmol. Apr. 2018;2018 doi: 10.1155/2018/2590536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bera A.K., Sriya Y., Pati F. Formulation of dermal tissue matrix bioink by a facile decellularization method and process optimization for 3D bioprinting toward translation research. Macromol. Biosci. 2022;22(8) doi: 10.1002/mabi.202200109. [DOI] [PubMed] [Google Scholar]
- 255.Li Y., Wu Q., Li L., Chen F., Bao J., Li W. Decellularization of porcine whole lung to obtain a clinical-scale bioengineered scaffold. J. Biomed. Mater. Res. 2021;109(9):1623–1632. doi: 10.1002/jbm.a.37158. [DOI] [PubMed] [Google Scholar]
- 256.Lopera Higuita M., Griffiths L.G. Antigen removal process preserves function of small diameter venous valved conduits, whereas SDS-decellularization results in significant valvular insufficiency. Acta Biomater. Apr. 2020;107:115–128. doi: 10.1016/j.actbio.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bongolan T., et al. Decellularization of porcine kidney with submicellar concentrations of SDS results in the retention of ECM proteins required for the adhesion and maintenance of human adult renal epithelial cells. Biomater. Sci. 2022;10(11):2972–2990. doi: 10.1039/D1BM01017D. [DOI] [PubMed] [Google Scholar]
- 258.Mora-Navarro C., et al. Monitoring decellularization via absorbance spectroscopy during the derivation of extracellular matrix scaffolds. Biomed. Mater. Nov. 2021;17(1) doi: 10.1088/1748-605X/ac361f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rahman S., Griffin M., Naik A., Szarko M., Butler P.E.M. Optimising the decellularization of human elastic cartilage with trypsin for future use in ear reconstruction. Sci. Rep. Feb. 2018;8(1) doi: 10.1038/s41598-018-20592-x. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Giraldo-Gomez D.M., et al. Trypsin as enhancement in cyclical tracheal decellularization: morphological and biophysical characterization. Mater. Sci. Eng. C. Feb. 2016;59:930–937. doi: 10.1016/j.msec.2015.10.094. [DOI] [PubMed] [Google Scholar]
- 261.Pu W., Han Y., Yang M. Human decellularized adipose tissue hydrogels as a culture platform for human adipose-derived stem cell delivery. J. Appl. Biomater. Funct. Mater. Jan. 2021;19 doi: 10.1177/2280800020988141. [DOI] [PubMed] [Google Scholar]
- 262.Sackett S.D., et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep. Jul. 2018;8(1) doi: 10.1038/s41598-018-28857-1. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gzik-Zroska B., et al. Assessment of the impact of decellularization methods on mechanical properties of biocomposites used as skin substitute. Materials. Aug. 2021;14(17):4785. doi: 10.3390/ma14174785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sarmin A.M., Connelly J.T. Fabrication of human skin equivalents using decellularized extracellular matrix. Curr. Protoc. 2022;2(3):e393. doi: 10.1002/cpz1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Haupt J., et al. Detergent-based decellularization strategy preserves macro- and microstructure of heart valves. Interact. Cardiovasc. Thorac. Surg. Feb. 2018;26(2):230–236. doi: 10.1093/icvts/ivx316. [DOI] [PubMed] [Google Scholar]
- 266.Tuan-Mu H.-Y., Chang Y.-H., Hu J.-J. Removal of an abluminal lining improves decellularization of human umbilical arteries. Sci. Rep. Jun. 2020;10(1) doi: 10.1038/s41598-020-67417-4. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gilpin A., Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wu L.-C., et al. Optimized decellularization protocol including α-Gal epitope reduction for fabrication of an acellular porcine annulus fibrosus scaffold. Cell Tissue Bank. Sep. 2017;18(3):383–396. doi: 10.1007/s10561-017-9619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Burk J., et al. Freeze-thaw cycles enhance decellularization of large tendons. Tissue Eng. C Methods. Apr. 2014;20(4):276–284. doi: 10.1089/ten.tec.2012.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Nonaka P.N., et al. Effects of freezing/thawing on the mechanical properties of decellularized lungs. J. Biomed. Mater. Res. 2014;102(2):413–419. doi: 10.1002/jbm.a.34708. [DOI] [PubMed] [Google Scholar]
- 271.Szarko M., Muldrew K., Bertram J.E. Freeze-thaw treatment effects on the dynamic mechanical properties of articular cartilage. BMC Muscoskel. Disord. Oct. 2010;11(1):231. doi: 10.1186/1471-2474-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Funamoto S., et al. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials. May 2010;31(13):3590–3595. doi: 10.1016/j.biomaterials.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 273.Santoso E.G., et al. Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PLoS One. Jul. 2014;9(7) doi: 10.1371/journal.pone.0103201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Negishi J., Funamoto S., Kimura T., Nam K., Higami T., Kishida A. Effect of treatment temperature on collagen structures of the decellularized carotid artery using high hydrostatic pressure. J. Artif. Organs. May 2011;14(3):223. doi: 10.1007/s10047-011-0570-z. [DOI] [PubMed] [Google Scholar]
- 275.Hiemer B., et al. Devitalisation of human cartilage by high hydrostatic pressure treatment: subsequent cultivation of chondrocytes and mesenchymal stem cells on the devitalised tissue. Sci. Rep. Sep. 2016;6(1) doi: 10.1038/srep33747. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Nakamura N., Sugano K., Nam K., Kimura T., Fujisato T., Kishida A. A basic study of osteogenesis between decellularized cortical bone pieces for bone graft construction. Adv. Biomed. Eng. 2013;2:95–100. doi: 10.14326/abe.2.95. [DOI] [Google Scholar]
- 277.Sloff M., et al. The impact of γ-irradiation and EtO degassing on tissue remodeling of collagen-based hybrid tubular templates. ACS Biomater. Sci. Eng. Sep. 2018;4(9):3282–3290. doi: 10.1021/acsbiomaterials.8b00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Dearth C.L., et al. The effect of terminal sterilization on the material properties and in vivo remodeling of a porcine dermal biologic scaffold. Acta Biomater. Mar. 2016;33:78–87. doi: 10.1016/j.actbio.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 279.Hennessy R.S., et al. Supercritical carbon dioxide–based sterilization of decellularized heart valves. JACC Basic Transl. Sci. Feb. 2017;2(1):71–84. doi: 10.1016/j.jacbts.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.de Wit R.J.J., et al. Scaffold-based tissue engineering: supercritical carbon dioxide as an alternative method for decellularization and sterilization of dense materials. Acta Biomater. Jan. 2023;155:323–332. doi: 10.1016/j.actbio.2022.11.028. [DOI] [PubMed] [Google Scholar]
- 281.Hunt C.J. Technical considerations in the freezing, low-temperature storage and thawing of stem cells for cellular therapies. Transfus. Med. Hemotherapy. 2019;46(3):134–150. doi: 10.1159/000497289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Deering T.F., Chang C., Snyder C., Natarajan S.K., Matheny R. Enhanced antimicrobial effects of decellularized extracellular matrix (CorMatrix) with added vancomycin and gentamicin for device implant protection. Pacing Clin. Electrophysiol. 2017;40(6):615–623. doi: 10.1111/pace.13061. [DOI] [PubMed] [Google Scholar]
- 283.Zhang F., et al. Biocompatibility and cellular compatibility of decellularized tracheal matrix derived from rabbits. Int. J. Artif. Organs. Sep. 2019;42(9):500–507. doi: 10.1177/0391398819847216. [DOI] [PubMed] [Google Scholar]
- 284.Elder S., Pinheiro A., Young C., Smith P., Wright E. Evaluation of genipin for stabilization of decellularized porcine cartilage. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. Sep. 2017;35(9):1949–1957. doi: 10.1002/jor.23483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ghassemi T., Saghatoleslami N., Mahdavi‐Shahri N., Matin M.M., Gheshlaghi R., Moradi A. A comparison study of different decellularization treatments on bovine articular cartilage. J. Tissue Eng. Regen. Med. 2019;13(10):1861–1871. doi: 10.1002/term.2936. [DOI] [PubMed] [Google Scholar]
- 286.Graham M.E., Gratzer P.F., Bezuhly M., Hong P. Development and characterization of decellularized human nasoseptal cartilage matrix for use in tissue engineering. Laryngoscope. 2016;126(10):2226–2231. doi: 10.1002/lary.25884. [DOI] [PubMed] [Google Scholar]
- 287.Elder S., et al. Effects of antigen removal on a porcine osteochondral xenograft for articular cartilage repair. J. Biomed. Mater. Res. 2018;106(8):2251–2260. doi: 10.1002/jbm.a.36411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Anisimova N.Y., Kiselevsky M.V., Sukhorukova I.V., Shvindina N.V., Shtansky D.V. Fabrication method, structure, mechanical, and biological properties of decellularized extracellular matrix for replacement of wide bone tissue defects. J. Mech. Behav. Biomed. Mater. Sep. 2015;49:255–268. doi: 10.1016/j.jmbbm.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 289.Gillies A.R., Smith L.R., Lieber R.L., Varghese S. Method for decellularizing skeletal muscle without detergents or proteolytic enzymes. Tissue Eng. C Methods. Apr. 2011;17(4):383–389. doi: 10.1089/ten.tec.2010.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wang L., Johnson J.A., Chang D.W., Zhang Q. Decellularized musculofascial extracellular matrix for tissue engineering. Biomaterials. Apr. 2013;34(11):2641–2654. doi: 10.1016/j.biomaterials.2012.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zhao C., et al. Preparation of decellularized biphasic hierarchical myotendinous junction extracellular matrix for muscle regeneration. Acta Biomater. Mar. 2018;68:15–28. doi: 10.1016/j.actbio.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 292.Ning L.-J., et al. Preparation and characterization of decellularized tendon slices for tendon tissue engineering. J. Biomed. Mater. Res. Jun. 2012;100A(6):1448–1456. doi: 10.1002/jbm.a.34083. [DOI] [PubMed] [Google Scholar]
- 293.Youngstrom D.W., Barrett J.G., Jose R.R., Kaplan D.L. Functional characterization of detergent-decellularized equine tendon extracellular matrix for tissue engineering applications. PLoS One. May 2013;8(5) doi: 10.1371/journal.pone.0064151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Dong S., et al. Decellularized versus fresh-frozen allografts in anterior cruciate ligament reconstruction: an in vitro study in a rabbit model. Am. J. Sports Med. Aug. 2015;43(8):1924–1934. doi: 10.1177/0363546515585314. [DOI] [PubMed] [Google Scholar]