Abstract
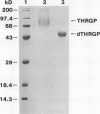
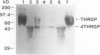
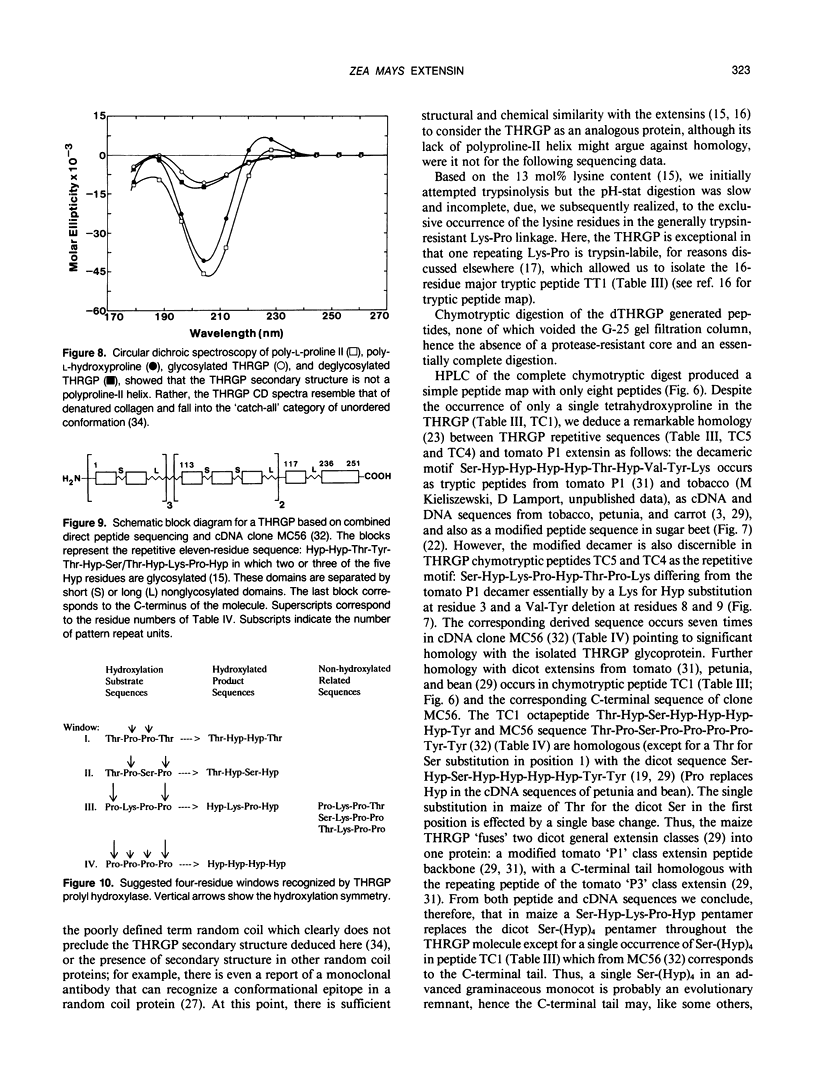
Chymotryptic digestion of a threonine-rich hydroxyproline-rich glycoprotein (THRGP) purified from the cell surface of a Zea mays cell suspension culture gave a peptide map dominated by the hexadecapeptide TC5: Thr-Hyp-Ser-Hyp-Lys-Pro-Hyp-Thr-Pro-Lys-Pro-Thr-Hyp-Hyp-Thr-Tyr, in which the repetitive motif Ser-Hyp-Lys-Pro-Hyp-Thr-Pro-Lys is homologous with the dominant decamer of P1-type dicot extensins: Ser-Hyp-Hyp-Hyp-Hyp-Thr-Hyp-Val-Tyr-Lys, modified by a Lys for Hyp substitution at residue 3, a Val-Tyr deletion at residues 8 and 9, and incomplete post-translational modification of proline residues. One of the minor peptides (TC1) contained the 8-residue sequence: Thr-Hyp-Ser-Hyp-Hyp-Hyp-Hyp-Tyr corresponding to the C-terminal tail (judging from the recently isolated maize cDNA clone MC56) which is homologous with the major repetitive motif of the `P3' class of dicot extensins. Direct peptide sequencing defined potential glycosylated regions on the THRGP corresponding to clone MC56 and showing that glycosylated and nonglycosylated domains alternate with high regularity. The THRGP is not in the polyproline-II conformation, judging from circular dichroic spectra, but nevertheless is an extended rod, from electron microscopic data. HF-solvolysis of cell walls from maize coleoptile, root, and root tip released deglycosylated THRGP detected on sodium dodecyl sulfate-polyacrylamide gel electrophoresis immunoblots with high titer rabbit polyclonal antibodies raised against the intact THRGP. In a quantitative enzyme-linked immunosorbent assay, these antibodies cross-reacted 20% with tomato P1 extensin, and 18% with anhydrous hydrogen fluoride-deglycosylated P1. These results, together with other previously published data, show that maize THRGP is homologous with the dicot P1 extensins and, as such, is the first extensin isolated from a graminaceous monocot.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolwell G. P., Robbins M. P., Dixon R. A. Elicitor-induced prolyl hydroxylase from French bean (Phaseolus vulgaris). Localization, purification and properties. Biochem J. 1985 Aug 1;229(3):693–699. doi: 10.1042/bj2290693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Kaufman P., McNeil M., Albersheim P. The Structure of Plant Cell Walls: VI. A Survey of the Walls of Suspension-cultured Monocots. Plant Physiol. 1974 Jul;54(1):109–115. doi: 10.1104/pp.54.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T. Cell Surfaces in Plant-Microorganism Interactions: I. A Structural Investigation of Cell Wall Hydroxyproline-rich Glycoproteins Which Accumulate in Fungus-infected Plants. Plant Physiol. 1979 Aug;64(2):314–319. doi: 10.1104/pp.64.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T., Lafitte C., Mazau D., Toppan A., Touzé A. Cell Surfaces in Plant-Microorganism Interactions: II. Evidence for the Accumulation of Hydroxyproline-rich Glycoproteins in the Cell Wall of Diseased Plants as a Defense Mechanism. Plant Physiol. 1979 Aug;64(2):320–326. doi: 10.1104/pp.64.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H. J., Nap J. P., Gloudemans T., Stiekema W., Van Dam H., Govers F., Louwerse J., Van Kammen A., Bisseling T. Characterization of cDNA for nodulin-75 of soybean: A gene product involved in early stages of root nodule development. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4495–4499. doi: 10.1073/pnas.84.13.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. W., Terhune B. T., Lamport D. T. Characterization of native and modified extensin monomers and oligomers by electron microscopy and gel filtration. Plant Physiol. 1988 Mar;86(3):848–856. doi: 10.1104/pp.86.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. C., Nagao R. T., Key J. L. Characterization and sequence analysis of a developmentally regulated putative cell wall protein gene isolated from soybean. J Biol Chem. 1987 Jun 15;262(17):8367–8376. [PubMed] [Google Scholar]
- Hood E. E., Shen Q. X., Varner J. E. A developmentally regulated hydroxyproline-rich glycoprotein in maize pericarp cell walls. Plant Physiol. 1988 May;87(1):138–142. doi: 10.1104/pp.87.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis M. C., Forsyth W., Duncan H. J. A survey of the pectic content of nonlignified monocot cell walls. Plant Physiol. 1988 Oct;88(2):309–314. doi: 10.1104/pp.88.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszewski M. J., Leykam J. F., Lamport D. T. Trypsin cleaves lysylproline in a hydroxyproline-rich glycoprotein from Zea mays. Pept Res. 1989 May-Jun;2(3):246–248. [PubMed] [Google Scholar]
- Kieliszewski M., Lamport D. T. Purification and Partial Characterization of a Hydroxyproline-Rich Glycoprotein in a Graminaceous Monocot, Zea mays. Plant Physiol. 1987 Nov;85(3):823–827. doi: 10.1104/pp.85.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li X. B., Kieliszewski M., Lamport D. T. A chenopod extensin lacks repetitive tetrahydroxyproline blocks. Plant Physiol. 1990 Feb;92(2):327–333. doi: 10.1104/pp.92.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabayashi H., Isemura T., Sakakibara S. Steric structure of L-proline oligopeptides. II. Far-ultraviolet absorption spectra and optical rotations of L-proline oligopeptides. Biopolymers. 1968;6(3):323–330. doi: 10.1002/bip.1968.360060307. [DOI] [PubMed] [Google Scholar]
- Saad B., Corradin G., Bosshard H. R. Monoclonal antibody recognizes a conformational epitope in a random coil protein. Eur J Biochem. 1988 Dec 1;178(1):219–224. doi: 10.1111/j.1432-1033.1988.tb14446.x. [DOI] [PubMed] [Google Scholar]
- Sanger M. P., Lamport D. T. A microapparatus for liquid hydrogen fluoride solvolysis: sugar and amino sugar composition of Erysiphe graminis and Triticum aestivum cell walls. Anal Biochem. 1983 Jan;128(1):66–70. doi: 10.1016/0003-2697(83)90345-7. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Sato K., Uchida T. Plant prolyl hydroxylase recognizes poly(L-proline) II helix. J Biol Chem. 1981 Nov 25;256(22):11397–11400. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst G. J., Varner J. E. Reinforced Polyproline II Conformation in a Hydroxyproline-Rich Cell Wall Glycoprotein from Carrot Root. Plant Physiol. 1984 Feb;74(2):247–251. doi: 10.1104/pp.74.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]