Significance
Lack of behavioral flexibility is a sign of cognitive decline with age. We modeled behavioral flexibility in rats using an olfactory threat extinction learning paradigm. Rats underwent classical threat conditioning in which an odor was paired with a foot shock, followed by extinction training with repeated presentations of the odor stimulus alone. Adult rats succeeded, while aged rats failed to extinguish the olfactory threat memory. N-methyl-D-aspartate receptor (NMDAR)-dependent synaptic plasticity in the olfactory sensory cortex enabled olfactory threat extinction learning in adult rats and was impaired in aged rats. Hence, NMDARs in the sensory cortex are not only critical for optimal learning but also ensure behavioral flexibility. This has important implications for improving aging-associated cognitive decline.
Keywords: long-term depression, extinction learning, piriform cortex, NMDA receptor, aging
Abstract
Extinction of threat memory is a measure of behavioral flexibility. In the absence of additional reinforcement, the extinction of learned behaviors allows animals and humans to adapt to their changing environment. Extinction mechanisms and their therapeutic implications for maladaptive learning have been extensively studied. However, how aging affects extinction learning is much less understood. Using a rat model of olfactory threat extinction, we show that the extinction of olfactory threat memory is impaired in aged Sprague-Darley rats. Following extinction training, long-term depression (LTD) in the piriform cortex (PC) was inducible ex vivo in aged rats and was NMDA receptor (NMDAR)-independent. On the other hand, adult rats acquired successful olfactory threat extinction, and LTD was not inducible following extinction training. Neuronal cFos activation in the posterior PC correlated with learning and extinction performance in rats. NMDAR blockade either systemically or locally in the PC during extinction training prevented successful extinction in adult rats, following which NMDAR-dependent LTD became inducible ex vivo. This suggests that extinction learning employs NMDAR-dependent LTD mechanisms in the PC of adult rats, thus occluding further LTD induction ex vivo. The rescue of olfactory threat extinction in aged rats by D-cycloserine, a partial NMDAR agonist, suggests that the impairment in olfactory threat extinction of aged animals may relate to NMDAR hypofunctioning and a lack of NMDAR-dependent LTD. These findings are consistent with an age-related switch from NMDAR-dependent to NMDAR-independent LTD in the PC. Optimizing NMDAR function in sensory cortices may improve learning and flexible behavior in the aged population.
The flip side of growing wiser with age is cognitive decline (1, 2). In addition to impairments in long-term memory, a parallel decline in the ability to flexibly adapt to a changing environment may represent a fundamental feature of age-related cognitive dysfunction in humans. Extinction of a learned response in the absence of further reinforcement, for example, is a measure of behavioral flexibility. Animal models of threat memory extinction have been essential for our understanding of the biological mechanisms underlying behavioral flexibility (3, 4). However, despite substantial progress in our understanding of the mechanisms of threat extinction, the impact of aging on extinction has been relatively unexplored.
Previous efforts to understand threat memory extinction have focused on the roles of the amygdala, particularly the basal and lateral amygdala (BLA). Two competing theories have emerged. First, reversal of conditioned threat responses following extinction may result from a loss of synaptic potentiation underlying the associative memory of the conditioned (CS) and unconditioned stimulus (US) pairing. A depotentiation model of long-term potentiation (LTP) in principal neurons of the BLA (5–7) amounts to threat memory erasure. The finding that auditory threat extinction learning occludes ex vivo de-potentiation (5, 7) suggests that extinction and depotentiation share similar synaptic mechanisms. Another theory employs an inhibitory learning model (a new memory competing with and suppressing the original memory trace), supported by LTP of inhibitory neurons or inhibitory synaptic transmission (8–13). Potentiation of inhibitory neurons or synapses could increase feedforward inhibition of principal neurons to suppress the expression of threat conditioning-induced LTP. It has been suggested that projections from the infralimbic prefrontal cortex to the amygdala constitute a critical inhibitory pathway during auditory threat extinction (14–16). Further research suggests that anatomically distinct populations in the BLA facilitate threat learning or extinction via unique circuitry (17) and that neuronal responses are extinction context-dependent (18), a role for which the hippocampus is critical (19, 20). More recent research also implicates the involvement of the insular cortex (21) and medial preoptic circuitry (22) in auditory and contextual threat extinction, respectively.
Recent learning theories posit an active role of sensory cortices in associative threat learning (23–25). In addition to amygdala-based threat processing, a sensory-based model permits the categorization of stimulus valence at early stages of sensory processing, affording an organism the evolutionary advantage of responding with minimal delay, or with additional resources (23). Consistent with this theory, the olfactory piriform cortex (PC) is capable of encoding odor valence representations (26) and is critical for olfactory threat conditioning (27, 28), especially for long-term memory (28–30). The PC is evolutionally conserved and has critical features (sparse distributed coding, extensive autoassociative circuitry) that support neuronal representations optimal for associative memory (24, 31). Anatomically, principal PC neurons, which respond to stimuli from other sensory domains including the somatosensory system (32, 33), and have strong reciprocal connections with the BLA (34), are well suited for encoding plasticity induced by convergent stimuli. However, whether the PC is also a locus of olfactory extinction learning is not known. Here, we test the hypothesis that synaptic plasticity in the PC such as long-term depression (LTD) is a critical mechanism for the extinction of olfactory threat memory and investigate how aging may alter this process.
Results
Extinction of Olfactory Threat Memory Is Impaired in Aged Rats and Correlates with cFos Expression in the PC.
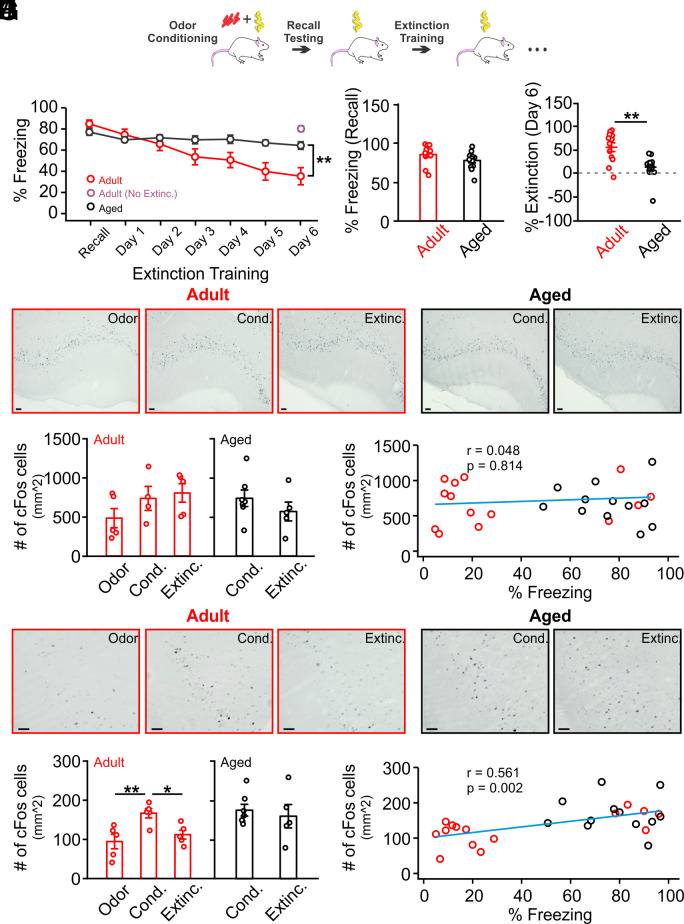
First, we compared olfactory threat extinction learning in adult (3–6 mo) and aged (20–24 mo) Sprague-Dawley rats of both sexes. Rats underwent odor and shock pairings and were tested the next day for freezing in the presence of the odor (recall test). Rats continued to be exposed to the odor daily, in the absence of shock, for the next 6 d and freezing responses were recorded (Fig. 1A). Overall, aged rats exhibited significantly less extinction learning than adult rats (Age × Time: F6,147 = 3.955, P = 0.001; Fig. 1B), and there were no sex differences (Sex: F1,147 = 2.07, P = 0.151; Sex × Age: F1,147 = 0.588, P = 0.444; SI Appendix, Fig. S1). Aged rats showed comparable freezing to adult rats on the recall day (t = 1.633, P = 0.116; Fig. 1C) but significantly less extinction of freezing responses on the last day of training (t = 3.761, P = 0.001; Fig. 1D). Freezing to odor on day 6 in another cohort of adult rats without extinction training was significantly higher compared to extinction-trained rats (Fig. 1B and SI Appendix, Fig. S2), suggesting that the reduced freezing in adult rats was not due to natural forgetting. Thus, olfactory extinction learning is impaired in aged rats while olfactory threat learning is intact.
Fig. 1.
Extinction learning of olfactory threat memory is impaired in aged rats. (A) Schematic of olfactory threat conditioning and extinction training. (B) Time course of % freezing during recall and extinction training (n = 13 adult, n = 12 aged). Adult rats showed significantly reduced freezing compared to aged rats following 6 d of extinction training. Another cohort of adult rats (n = 6) that did not undergo extinction training showed high freezing to the conditioned odor when tested 6 d following odor threat conditioning. (C) Measurement of percentage of freezing on recall day showed no difference in threat learning acquisition between adult and aged groups. (D) Measurement of percentage of extinction on the last day of extinction training (% recall freezing − % freezing on the last training day)/(% recall freezing) showed impaired extinction in the aged group. (E) Examples of cFos expression in the anterior piriform cortex of adult (n = 5 Odor; n = 4 Cond.; n = 5 Extinc.) and aged rats (n = 7 Cond. and n = 5 Extinc.). (F) Comparisons of cFos cell counts in various groups of adult and aged rats showed no difference among groups. (G) No correlation of cFos+ cell numbers in the anterior piriform cortex and % freezing in individual rats. Red and black circles indicate data from the adult and aged groups, respectively. (H) Examples of cFos expression in the posterior piriform cortex of adult (n = 5 each group) and aged rats (n = 7 Cond. and n = 5 Extinc.). (I) Comparisons of cFos cell counts in various groups of adult and aged rats showed increased cFos expression following odor threat conditioning and reduction to control levels following extinction training in adult rats only. In aged rats, no difference in cFos expression was observed between the conditioned rats and rats that underwent extinction training. (J) Positive correlation of cFos+ cell number in the posterior piriform cortex and % freezing in individual rats. Red and black circles indicate data from the adult and aged groups, respectively. Odor: odor exposure only; Cond.: olfactory threat-conditioned; Extinc.: extinction-trained (Scale bars, 50 µm). *P < 0.05, **P < 0.01.
We mapped cFos expression in the PC following olfactory threat conditioning and extinction. cFos expressions in the anterior PC (aPC) did not differ among groups in adult (F2,11 = 1.804, P = 0.210) and aged rats (t = 1.405, P = 0.320) (Fig. 1 E and F). No correlation between cFos expression and freezing behavior was observed in the aPC (r = 0.048, P = 0.814; Fig. 1G). In contrast, in adult rats, cFos expression in the posterior PC (pPC) differed among groups (F2,12 = 6.997, P = 0.010; Fig. 1 H and I). Olfactory threat conditioning increased cFos expression compared to odor exposure alone (t = 5.072, P = 0.010) and extinction training reduced cFos expression compared to threat conditioning (t = 3.839, P = 0.046; Fig. 1I). However, aged rats that underwent extinction training did not show a difference in cFos expression compared to those that underwent olfactory threat conditioning (t = 0.504, P = 0.625; Fig. 1I). We found a strong correlation between cFos expression in the pPC and freezing during testing (r = 0.561, P = 0.002; Fig. 1J).
Extinction Learning of Olfactory Threat Memory Correlates with Ex Vivo LTD in the PC.
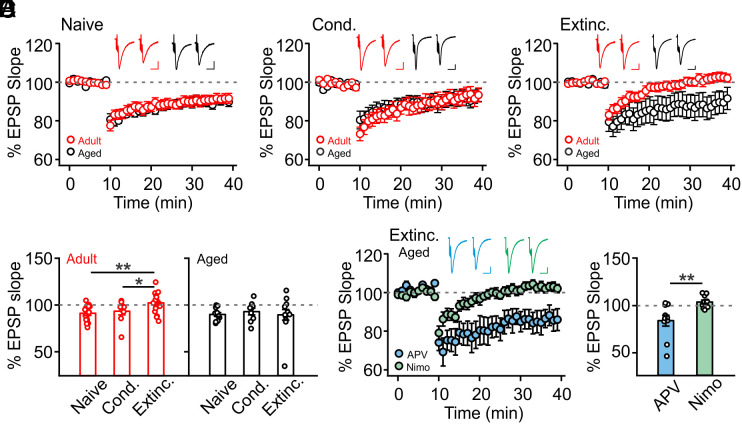
The cFos profile suggests that pPC neuronal activation levels parallel degrees of threat learning and extinction. Thus, LTP- and LTD-like synaptic changes in the PC may underlie threat learning and extinction, respectively. We investigated the relationship between olfactory threat extinction and pPC LTD recorded ex vivo. We stimulated associational fibers in layer Ib of ex vivo PC slices and recorded field excitatory postsynaptic potentials (fEPSPs) in the same layer. A standard low-frequency protocol (1 Hz, 15 min) was used for LTD induction. We compared LTD induction in naive, olfactory threat-conditioned and extinction-trained rats of both age groups. There were no differences in LTD amplitudes in naive (Fig. 2A) and threat-conditioned (Fig. 2B) rats between the two age groups. However, following extinction training, LTD was inducible in aged but not adult rats (Fig. 2C). Comparisons within the adult group showed inducible LTD in naive and threat-conditioned rats, but not rats that underwent extinction training (F2,41 = 5.838, P = 0.006; Fig. 2D). Furthermore, the amplitude of LTD ex vivo negatively correlated with the degree of extinction in individual adult rats (SI Appendix, Fig. S3). These results suggest that successful extinction in adult rats employs an LTD mechanism in the PC, which subsequently occludes ex vivo electrical stimulation-induced LTD. In contrast, aged rats from all groups showed comparable inducible LTD (F2,29 = 0.175, P = 0.840; Fig. 2D).
Fig. 2.
PC LTD is not inducible in adult rats while LTCC-dependent LTD can be induced in aged rats following extinction training. (A) LTD induction of fEPSPs in naive rats (n = 15 adult, n = 11 aged) showed no difference between adult and aged groups. (B) LTD induction of fEPSPs in olfactory threat–conditioned (Cond.) rats (n = 9 adult, n = 9 aged) showed no difference between adult and aged groups. (C) LTD induction of fEPSPs in extinction-trained rats (Extinc.) (n = 20 adult, n = 12 aged) showed that LTD was not inducible in adult rats. (D) Comparisons of % EPSP slopes in various groups of adult and aged rats. (E) LTCC-, but not NMDAR-mediation of LTD in aged rats that underwent extinction training (n = 10 APV, n = 10 Nimo). (F) Comparison of LTD induction in aged rats in E. Nimo: nimodipine. Scale bars for example fEPSP traces, 10 ms/0.2 mV. *P < 0.05, **P < 0.01.
If extinction learning is dependent on an LTD-like mechanism in the PC, then the inability of aged rats to extinguish olfactory threat memory may be related to a lack of LTD induction by extinction training. Why is LTD lacking in aged rats during extinction training, despite the aged PC being capable of generating LTD ex vivo? A previous report showed that there is a shift from NMDA receptor (NMDAR)- to L-type calcium channel (LTCC)-dependent LTD in the PC with aging (35). As such, one possibility is that olfactory extinction learning is mediated by NMDAR-dependent LTD, which is impaired in aged rats. In line with this hypothesis, PC LTD in aged rats following extinction training was NMDAR-independent, as application of the NMDAR antagonist APV did not reduce LTD. However, the LTCC blocker nimodipine fully abolished LTD (t = 3.20, P = 0.005; Fig. 2 E and F). We further predicted that 1) blocking PC NMDAR-dependent plasticity in adult rats would prevent extinction learning and that 2) enhancing NMDAR functioning in the PC of aged rats may restore their ability to extinguish threat memories.
NMDAR-dependent Plasticity in the PC Mediates Olfactory Threat Extinction in Adult Rats.
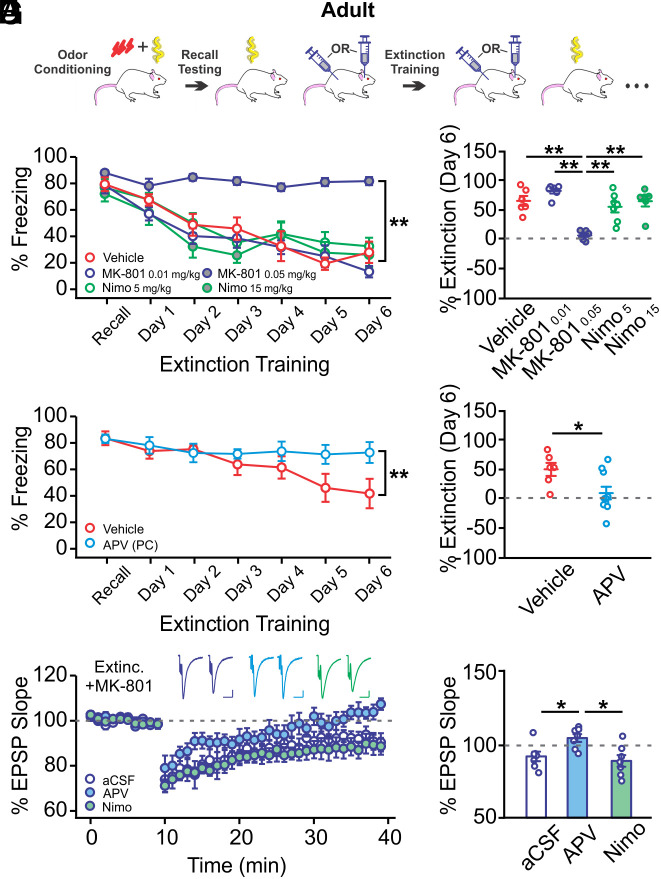
To determine whether NMDAR-dependent plasticity in the PC underlies extinction, we administered NMDAR antagonists during extinction training in adult rats (Fig. 3A). We first systemically administered either an NMDAR antagonist (MK801, 0.01 or 0.05 mg/kg, s.c.) (36), an LTCC antagonist (nimodipine, 5 or 15 mg/kg, s.c.) (37, 38), or vehicle and found differences in degree of extinction among groups (Group × Time: F24,162 = 3.545, P < 0.001; Fig. 3B). Extinction in high-dose MK-801-injected rats was impaired (F4,27 = 16.449, P < 0.001; Fig. 3C). The effect of MK-801 was not drug-state-dependent, as infusing MK-801 30 min before or immediately following each extinction trial resulted in similar freezing to the conditioned odor (SI Appendix, Fig. S4). A previous report showed that similar doses of nimodipine impaired auditory extinction learning in mice (37), which was not replicated here. Differences in species and behavioral paradigms (tone vs. odor, one-day vs. multiple-day extinction training) may account for this difference. We next infused APV in the PC (1 µg per hemisphere) (39) during extinction training to directly test the effect of PC NMDAR blockade. APV-infused rats showed impaired extinction learning compared to vehicle-infused controls (Group × Time: F6,84 = 3.195, P = 0.007; Fig. 3D). The degree of extinction in APV-infused rats was significantly lower at the end of training (t = 2.455, P = 0.028; Fig. 3E), resembling senile impairment of extinction.
Fig. 3.
NMDARs in the PC mediate olfactory threat extinction in adult rats. (A) Schematic of olfactory threat conditioning and extinction training. (B) Extinction learning curves in rats systemically injected with drugs or vehicle during extinction training (n = 6/6/7/7/6 for vehicle/MK-801 0.01 mg/kg/MK-801 0.05 mg/kg/Nimo 5 mg/kg/Nimo 15 mg/kg). MK-801 at the higher dose (0.05 mg/kg) prevented odor threat extinction learning, while nimodipine (Nimo) administration did not affect odor extinction. (C) Comparisons of extinction levels of the groups in B. (D) Extinction learning curves in rats with APV or vehicle infused to the PC during extinction training (n = 10 APV, n = 6 vehicle). NMDAR blockade in the PC prevented odor extinction. (E) Comparisons of extinction levels of the groups in D. (F) LTD recordings of MK-801-injected adult rats that underwent extinction training. LTD in these rats was mediated by NMDARs, but not LTCCs. (G) Comparisons of LTD in different groups in F. Scale bars for example fEPSP traces, 10 ms/0.2 mV. *P < 0.05, **P < 0.01.
We recorded PC LTD in a subset of MK-801-injected rats that failed to extinguish the olfactory threat memory. Compared to noninjected rats that had successful extinction (Fig. 2D Extinc.), significant LTD was induced in MK-801-injected rats following extinction training (Fig. 3 F and G aCSF) (t = 2.524, P = 0.018). Consistent with our previous report (35) and in contrast to aged rats, PC LTD in adult rats was NMDAR- but not LTCC-dependent, as APV, but not nimodipine, prevented LTD induction (F2,18 = 6.142, P = 0.009; Fig. 3 F and G). These results suggest that NMDAR-dependent LTD in the PC is employed during olfactory threat extinction training in adult rats. Prevention of extinction learning enables ex vivo induction of NMDAR-dependent LTD in these rats.
Enhancing NMDAR Activation in the PC Restores Olfactory Threat Extinction in Aged Rats.
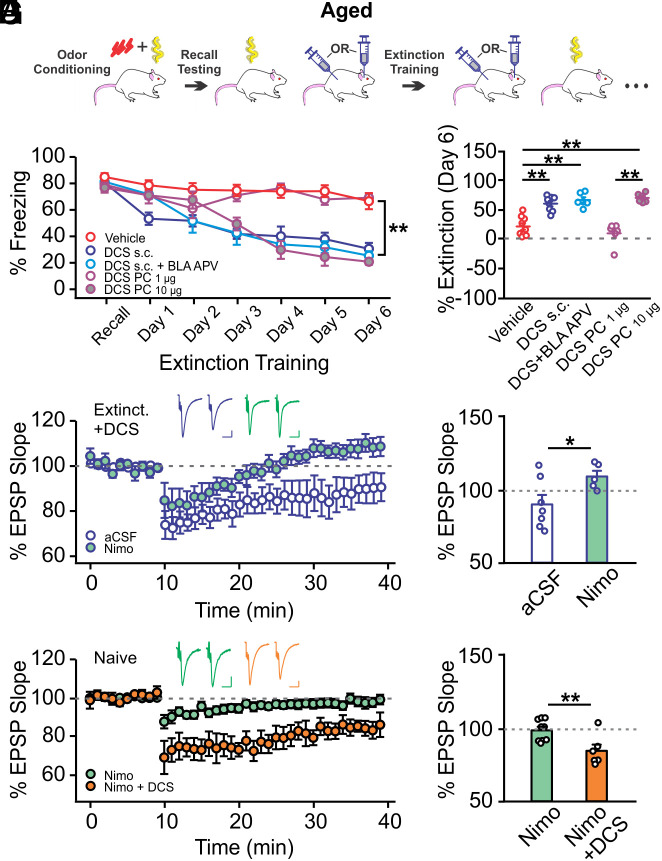
We next explored whether enhancing NMDAR activity would promote extinction learning in aged rats by administering a partial NMDAR agonist D-cycloserine (DCS) systemically (15 mg/kg, s.c.) or in the PC (1 or 10 µg infusion per hemisphere) (40, 41) (Fig. 4A). Activation of BLA NMDARs with DCS has been shown to accelerate threat extinction learning of a visual cue (40, 41). Therefore, we also included a group with s.c. DCS injections in combination with APV (1 µg per hemisphere) infusions in the BLA to isolate the effect of non-BLA NMDARs. All groups with DCS administration, except the low-dose PC DCS infusion group, succeeded in extinguishing the olfactory threat memory (Group × Time: F24,180 = 7.054, P < 0.001; Fig. 4B) and showed a higher degree of extinction at the end of training compared to vehicle controls (F4,30 = 25.818, P < 0.001; Fig. 4C).
Fig. 4.
NMDAR activation in the PC restores olfactory threat extinction in aged rats. (A) Schematic of olfactory threat conditioning and extinction training. (B) Extinction learning curves in rats injected with D-cycloserine (DCS) or vehicle during extinction training (n = 9/8/6/6/6 for vehicle/DCS s.c./DCS s.c.+BLA APV/DCS PC 1 µg/DCS PC 10 µg). Systemic injection or PC infusion (10 µg) of DCS rescued extinction learning. (C) Comparisons of extinction levels of the groups in B. (D) LTD recordings of DCS-injected aged rats that underwent extinction training (n = 7 aCSF, n = 5 nimo). LTCC-dependent LTD was inducible in these rats. Nimo, nimodipine. (E) Comparisons of LTDs in aCSF and nimodipine groups in D. (F) LTD recordings in naive aged rats in the presence of nimo or nimo+DCS (n = 9 nimo, n = 6 nimo+DCS). The addition of DCS enabled LTD induction. (G) Comparisons of LTDs in the two groups in F. Scale bars for example fEPSP traces, 10 ms/0.2 mV. *P < 0.05, **P < 0.01.
We conducted LTD recordings in a subset of aged rats that underwent extinction training with s.c. DCS injections. Interestingly, ex vivo PC LTD was still inducible in these rats despite successful extinction. However, ex vivo LTD was blocked by nimodipine application (t = 2.268, P = 0.046; Fig. 4 D and E), supporting our hypothesis that extinction learning employs NMDAR-dependent, but not LTCC-dependent LTD, which is intact ex vivo following extinction training in aged rats. This also implies that DCS administration may have enabled NMDAR-dependent LTD in the PC during olfactory threat extinction training. To test this hypothesis, we attempted ex vivo LTD induction in naive aged PC slides in the presence of nimodipine (10 µM) (38) or nimodipine plus DCS (20 µM) (42). As expected, LTD was not inducible in the presence of nimodipine but became inducible with the addition of DCS (t = 3.057, P = 0.009; Fig. 4 F and G). DCS increased the NMDAR-dependent fEPSP at the time point of LTD induction (20 min following DCS incubation; SI Appendix, Fig. S5). These results support the idea that enhancing NMDAR functioning in the PC via exogenous agonist administration during extinction training yields NMDAR-dependent LTD and associated successful extinction learning.
Discussion
Our study generates two lines of unique concepts. First, we show that sensory cortices such as the primary olfactory cortex are critically involved in the extinction of threat memories. Even though the BLA has been at the forefront of threat learning research, the idea that threat memories are encoded and stored in a distributed network of structures, including prefrontal, orbitofrontal, and sensory cortices as well as the hippocampus, is emerging (24, 43–45). While the BLA is initially critical for threat memory encoding and short-term storage, the long-term storage and expression of threat memories may be a function of the sensory cortices in both humans and animals (27–30, 46–48). The PC, especially the pPC, has extensive mutual connections with the BLA (34). Olfactory threat learning enhances network oscillations between the BLA and the pPC to promote information transfer and long-term memory formation (28). If the PC is a locus for the long-term storage of olfactory threat memories, it is pragmatic to assume that it may also play a critical role in the extinction of olfactory threat memory.
At the heart of the threat extinction debate is the question of the explanatory power of depotentiation and inhibitory learning for the behavioral manifestations of extinction. The depotentiation model does not explain some of the behavioral features associated with extinction, such as the renewal, spontaneous recovery, and reinstatement of defensive responses to the conditioned stimuli (4), which are better explained by the inhibitory learning model. However, how inhibitory plasticity is established in extinction and modulated in the recovery of defensive responses is not well understood. Both depotentiation and inhibitory mechanisms may coexist and work cooperatively (49, 50). A modeling study has proposed that extinction training leads to both the depotentiation of the principal neuron responses to CS input and the potentiation of inhibition to suppress CS-induced responses that reflect the original memory trace (51). The extent to which depotentiation and inhibition contribute to extinction has been shown to depend on the age of the animals and the stage of specific training paradigms. Juvenile rats do not exhibit spontaneous recovery and renewal following auditory extinction learning (52), suggesting that an unlearning depotentiation mechanism may dominate at this developmental stage. However, it is not known whether this phenomenon is universal across extinction learning of different sensory modalities. It has also been suggested that single-session and multiple-session extinction training regimes may engage inhibition and depotentiation mechanisms, respectively (53).
Here, we show that multiple-session olfactory threat extinction employs an LTD mechanism in the PC. Successful extinction occluded LTD induction ex vivo, while preventing extinction enabled ex vivo NMDAR-dependent LTD in adult rats. cFos activation patterns in the pPC paralleled freezing behavior, with increased activation following olfactory threat conditioning and a decrease to baseline following olfactory threat extinction. This suggests that a depotentiation process may have occurred during extinction training. However, whether the same synapses that have been potentiated undergo depression (thus de-potentiation) cannot be determined by the cFos activation patterns observed here. Modern genetic tools that enable selective labeling and recording of memory-encoding cFos+ neurons (27) can be used to address this question. Furthermore, the PC has extensive inhibitory circuitry that mediates both the feedforward and feedback inhibition of principal neurons (54). Further investigation is needed for an understanding of whether and how inhibition in the PC is modulated during extinction. Additionally, whether LTD-like synaptic changes occur in other sensory cortices following extinction of specific sensory modalities needs to be explored.
Our second observation is that a lack of NMDAR-dependent LTD in aged rats prevents extinction learning. Research studies on the mechanisms of aging-associated decline in flexible behavior have been sparse. One study reported that LTP at the site of cortical inputs to the BLA is impaired in aged rats, correlating with diminished contextual threat extinction (55). NMDARs in the amygdala are causal for the extinction of cued and contextual threat learning (56, 57). However, aging-related changes of NMDAR functioning in the BLA have not been reported. Alterations in prefrontal neuronal excitability, such as an increased and decreased excitability of the prelimbic and infralimbic cortex, respectively, have been suggested to play a role in the decline of extinction with aging (58). Here, we provide a mechanism for age-related decline of extinction learning in the sensory cortex. Our results suggest that a lack of NMDAR-dependent plasticity in the olfactory sensory cortex may be responsible for the inability of aged animals to learn olfactory extinction. Similar to the hippocampus (59, 60), there is a shift from NMDAR- to LTCC-dependent synaptic LTD in the PC with aging (35). However, NMDAR subunit expressions are not reduced in the aged rat PC (35), suggesting that functional changes of NMDARs occur with aging. Correspondingly, enhancing NMDAR functioning in the PC rescued the extinction of olfactory threat learning in aged animals, demonstrating an unemployed capacity of NMDARs to drive synaptic plasticity in neuronal networks of aged rats. Activating NMDARs in the PC alone without the involvement of the BLA was sufficient to induce olfactory threat extinction, suggesting that the PC is the more permanent repertoire for olfactory threat memory storage and serves as a sufficient route for memory unlearning.
NMDAR enhancement has been suggested as a potential therapeutic strategy to relieve maladaptive fear (61, 62), given that DCS facilitates the extinction of threat memory in animals (40, 41, 63). Animal studies including the one conducted here suggest that DCS improves the extinction of threat learning, which hints at the possibility of dampening a basic component of negative emotions such as fear and anxiety. In one study, DCS also reduced the post-extinction reinstatement of defensive responses in rats (64), the clinical implication of which would be the potential of reducing the postexposure therapy relapse of fear responses in humans. In humans, DCS has been shown to facilitate exposure-based psychotherapy (modeled by and depending on the mechanisms of extinction learning) for phobias and other anxiety disorders. For example, a clinical trial reported that DCS improved exposure therapy for fear of heights in a virtual reality environment (61). NMDAR enhancement may be a feasible approach to improve learning and learning extinction in the aging population. In fact, maladaptive fear disorders such as posttraumatic stress disorder also steepen age-related cognitive decline (65) and increase the risk of developing dementia (66). NMDAR-based therapy could alleviate both maladaptive learning and aging-associated cognitive decline.
Materials and Methods
Subjects.
Adult (3–6 mo) and aged (18–24 mo) Sprague-Dawley rats of both sexes were used for behavioral and electrophysiological experiments. For immunohistochemistry (IHC), 5- to 9- and 18- to 20-mo-old rats were used. Rats were housed on a standard 12-h light–dark cycle, with food and water ad libitum. Experimental procedures were approved by the Institutional Animal Care Committee at Memorial University of Newfoundland and followed Canadian Council’s guidelines on Animal Care.
Experimental Design.
An olfactory threat extinction paradigm was used to compare the extinction of a learned olfactory threat memory in adult and aged rats. cFos IHC was used as an index of neuronal activation following behavior in the PC and correlated with behavioral freezing responses (Fig. 1). Ex vivo LTD in PC slices was recorded and compared in naive, olfactory threat–conditioned, and extinction-trained rats of both ages. The roles of NMDARs and LTCCs in PC LTD were assessed in the presence of either an NMDAR blocker APV or an LTCC antagonist nimodipine (Fig. 2). The roles of NMDARs in olfactory threat extinction were tested with pharmacological interventions in adult (Fig. 3) and aged (Fig. 4) rats. Adult rats received NMDAR blockade via either systemic s.c. injection of MK-801 or intra-PC APV during extinction training. Systemic injection of nimodipine was used to test the role of LTCCs in olfactory threat extinction. A subset of rats following extinction training with MK-801 injections were subjected to ex vivo LTD recording. Aged rats received the NMDAR agonist DCS during extinction training to investigate the role of NMDARs in the facilitation of olfactory threat extinction. To exclude the roles of BLA NMDARs in odor threat extinction, a cohort of rats underwent systemic s.c. DCS injection and BLA APV infusion during extinction training. The effects of DCS and training on PC LTD were tested with ex vivo electrophysiological recording.
Cannula Surgery.
Animals were anesthetized under isoflurane in an induction chamber and transferred to a stereotaxic frame. Twenty-three gauge metal guide cannulas were implanted bilaterally in the BLA (AP: −2.5 mm, ML: ±4.9 mm from bregma, DV: −7.8 mm from brain surface) (67) or in the PC (AP: −2.3 mm, ML: ±5.4 mm, DV: −8.7 mm) (38). The cannulas were affixed to the skull with dental cement. Incisions were sutured and rats were returned to their home cage after surgeries for recovery. Rats were allowed to recover for at least 1 wk before the onset of behavioral experiments. A subset of rats was perfused following the behavioral experiments to check cannula targeting (SI Appendix, Fig. S6).
Drug Administration.
All drugs were freshly prepared and administrated immediately following the olfactory threat recall test and 30 min before each extinction training session (Figs. 3A and 4A). For s.c. injections, nimodipine (Sigma-Aldrich) (5 or 15 mg/kg/1 mL) was dissolved in a vehicle consisting of 50% DMSO-PBS (5 mg/kg/1 mL) (38). DCS (Sigma-Aldrich) (15 mg/kg/1 mL) (40, 41) and MK-801 (Tocris) (0.01 or 0.05 mg/kg/1 mL) (36) were dissolved in saline.
For local infusions, APV (1 μg/0.5 μL/hemisphere, dissolved in saline) was infused into the PC. DCS was infused in the PC at two doses (1 or 10 μg/0.5 μL/hemisphere). Infusions took place at a rate of 0.2 μL/min through a stainless steel 33-gauge internal cannula connected to a 10-μL Hamilton syringe by polyethylene tubing and driven by an 11 Elite Syringe pump (Harvard Apparatus). The internal cannula extended 0.5 mm below the guide cannula. The infusion cannula was left in place for an additional 1 min following infusion. For intra-BLA APV infusions plus systemic administration of DCS, 1 µg/0.5 µL of APV and 15 mg/kg/1 mL of DCS were administered. Control rats received either PC saline infusions, or an intra-BLA saline infusion plus systemic injection of saline, and data were pooled.
Behavioral Studies.
Odorants.
Odorants were diluted with mineral oil to specific concentrations. Odorants used were terpinene (6.63%) or benzaldehyde (0.05%). Chosen odorants were affectively neutral to adult rats, and concentrations were chosen so that the odors would emit a vapor-phase partial pressure of 1 Pa (68) and based on previous publications (67, 69).
Apparatus.
All behavioral training and testing were conducted in a custom-made olfactometer for air and odorant delivery, attached to the shock chamber. The shock chamber consisted of a Plexiglas chamber sitting on top of an electrified grid, connected to a shock generator (Muromachi Kikai Model SGS-003DX, Japan or San Diego Instruments, United States). Each odorant was stored in a polyvinyl carbonate bottle, connected to the olfactometer by C-flex tubing, and clamped shut when not in use. A fan with evacuation tubing was attached to the top lid of the shock chamber to accelerate odor clearance.
Olfactory threat conditioning.
Rats were habituated to the shock chamber for 30 min on two consecutive days. On day 3, rats were trained individually with four separate exposures to either a pairing of an odor CS and a US foot shock, or odor only (control). The shock was delivered at 5, 15, 20, and 30 min during a 30-min training session. The odor was delivered for 1 min at each time point. Shocks coincided with the last second of odor delivery (0.5 mA for 1 s) in the threat-conditioned group. The control group did not receive shocks. On day 4 (recall day), rats were preexposed to the shock chamber with no odor delivery for 5 min to measure baseline activity before receiving a 5 min exposure to the CS. The percentage of time spent freezing in the presence of the CS was measured.
Olfactory threat extinction.
Twenty-four hours following the odor recall test, rats underwent olfactory threat extinction training in the shock chamber for six consecutive days, with 5 min preexposure to the shock chamber followed by 5 min CS exposure. All testing was videotaped and analyzed offline. Degree of extinction was calculated as (% recall freezing − % freezing on the last training day)/(% recall freezing).
Immunohistochemistry.
Rats were perfused 90 min following odor exposure on recall day or the last day of extinction training. The perfusion was conducted transcardially with ice-cold saline (0.9%), followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer. Brains were extracted and stored in 4% PFA for 24 h at 4 °C and then transferred to 0.1 M PBS. Coronal sections of 50 µm were cut using a Compresstome (VF-310-OZ, Greenville, NC) and transferred to 24-well plates containing a PVP solution. Free-floating sections were stored at 4 °C until further processing.
For SG-gray cFos staining, sections were washed for 3 × 5 min in Tris buffer (0.1 M, pH 7.6) and incubated for 30 min in 0.03% H2O2 in Tris buffer. This was followed by a 10-min wash in Tris A (0.1% Triton X in Tris buffer) and then Tris B (0.1% Triton X and 0.005% BSA in Tris buffer) before blocking with 10% normal goat serum (Sigma-Aldrich) in Tris B for 1.5 h. Sections were then transferred and washed in Tris A and Tris B for 10 min each and incubated in a primary monoclonal antibody prepared in Tris B (cFos, 1:10000, Cell Signaling) overnight. Following washes in Tris A and Tris B buffers, sections were incubated with a goat anti-rabbit biotinylated secondary antibody (1:1000; Thermofisher) in Tris B buffer for 45 min. This was followed by washes in Tris B and Tris D (0.1% Triton X and 0.005% BSA in 0.5 M Tris buffer) and incubation in avidin–biotin horseradish peroxidize complex (1:1000 in Tris D; Vector ABC kit, Vector Laboratories) for 1.5 h at room temperature (allowed the complex to form 30 min before using), and washed three times in Tris buffer. Immunostaining was performed with peroxidise substrate (Vector SG). cFos positive nuclei were stained blue-gray. Sections were washed in Tris buffer for 5 min, mounted on slides, air-dried, dehydrated in ethanol solutions and xylene, and coverslipped with Permount (Fisher).
Imaging and Analysis.
Images were obtained with a bright-field microscope (10x, BX53 Upright Microscope, Olympus) using cellsSens Standard software. Three sections of each anterior PC and pPC within the same rostral to caudal range were used from each animal and counts from the two hemispheres were averaged. The intensity of light and exposure parameters were standardized across all captured images. Image analysis was conducted using ImageJ software. In the PC, the numbers of c-Fos immunopositive nuclei in the layer II/III were counted and normalized to regions of interest (/mm2).
Ex Vivo Electrophysiology.
Following anesthesia by isofluorane, rats were decapitated for brain extraction. Sagittal slices of 400 µm containing the PC were obtained using a vibratome (Leica VT-1200 s) in ice-cold HEPES artificial cerebrospinal fluid, (aCSF; in mM, 92 NaCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 10 MgSO4, 0.5 CaCl2) bubbled with carbogen gas (95% O2/5% CO2). Slices were immediately incubated at 34 °C for 45 min before moving to recording aCSF (in mM, 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 24 NaHCO3, 5 HEPES, 12.5 glucose, 2 MgSO4, 2 CaCl2) at room temperature. Following at least an additional hour of recovery, slices were transferred to a continuously perfused open bath chamber maintained at 28–30 °C for electrophysiological field recordings.
Slices were visualized with an Olympus BX51WI microscope. fEPSPs of the associative layer of the PC were obtained by placing a concentric stimulation electrode in layer Ib of the pPC and a recording electrode (1–2 MΩ) filled with aCSF placed within 500 µm (35). Multiclamp 700B (Molecular Devices) was used for data acquisition, filtered at 2 kHz, and digitized at 10 kHz using pClamp 10.5 software (Molecular Devices).
With a 20-s interval, test stimulations of 0.2 ms were given for 20–30 min for slices to equilibrate. Test stimulations were chosen at intensities eliciting 70–80% of the maximum fEPSP slope. Following a stable baseline recording of 10 min, LTD was induced (1 Hz, 900 pulses) and the fEPSPs were recorded for 30 min postinduction. Nimodipine (10 µM, Sigma-Aldrich) was continuously applied during the 10-min baseline and during LTD induction. DCS (20 µM) was applied for 20 min before LTD induction, at a time point when the NMDAR component of fEPSPs (recorded in Mg2+ free aCSF in the presence of 50 µM NBQX) was elevated (SI Appendix, Fig. S6). Offline analysis of fEPSP slopes was conducted in ClampFit 10.5 (Molecular Devices). The slopes of fEPSPs were normalized to the baseline level and recorded as % fEPSP. The % fEPSPs at 25–30 min postinduction were compared among groups.
Statistics.
OriginPro 2022b software was used to analyze all data. Data were reported as the mean ± SEM. Two-way repeated ANOVAs were used in behavioral analysis for age or treatment groups interacting with training time (days), followed by post hoc Tukey tests. Student t-tests were used for two-group comparisons and one-way ANOVAs were used for multiple-group comparisons. Pearson correlation coefficient was used to measure the correlation between cFos expression levels and threat response (% freezing). Differences between groups were considered significant when P values were <0.05.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work was supported by a Natural Sciences and Engineering Research Council of Canada grant (RGPIN-2018-04401) to Q.Y. We wish to thank Ali T. Salman for technical assistance and Kyron D. Power for editing the manuscript.
Author contributions
Q.Y. designed research; T.S., N.N., T.Q., V.R., and Q.Y. performed research; T.S., N.N., T.Q., V.R., and Q.Y. analyzed data; and T.S., V.R., and Q.Y. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Buckner R. L., Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron 44, 195–208 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Hedden T., Gabrieli J. D., Insights into the ageing mind: A view from cognitive neuroscience. Nat. Rev. Neurosci. 5, 87–96 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Dunsmoor J. E., Niv Y., Daw N., Phelps E. A., Rethinking extinction. Neuron 88, 47–63 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouton M. E., Maren S., McNally G. P., Behavioural and neurobiological mechanisms of pavlovian and instrumental extinction learning. Physiol. Rev. 101, 611–681 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J., et al. , Amygdala depotentiation and fear extinction. Proc. Natl. Acad. Sci. U.S.A. 104, 20955–20960 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEchron M. D., McCabe P. M., Green E. J., Llabre M. M., Schneiderman N., Simultaneous single unit recording in the medial nucleus of the medial geniculate nucleus and amygdaloid central nucleus throughout habituation, acquisition, and extinction of the rabbit’s classically conditioned heart rate. Brain Res. 682, 157–166 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Hong I., et al. , Extinction of cued fear memory involves a distinct form of depotentiation at cortical input synapses onto the lateral amygdala. Eur. J. Neurosci. 30, 2089–2099 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Bauer E. P., LeDoux J. E., Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala. J. Neurosci. 24, 9507–9512 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polepalli J. S., Sullivan R. K., Yanagawa Y., Sah P., A specific class of interneuron mediates inhibitory plasticity in the lateral amygdala. J. Neurosci. 30, 14619–14629 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Royer S., Pare D., Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience 115, 455–462 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Lange M. D., Doengi M., Lesting J., Pape H. C., Jungling K., Heterosynaptic long-term potentiation at interneuron-principal neuron synapses in the amygdala requires nitric oxide signalling. J. Physiol. 590, 131–143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trouche S., Sasaki J. M., Tu T., Reijmers L. G., Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron 80, 1054–1065 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asede D., Bosch D., Luthi A., Ferraguti F., Ehrlich I., Sensory inputs to intercalated cells provide fear-learning modulated inhibition to the basolateral amygdala. Neuron 86, 541–554 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Bloodgood D. W., Sugam J. A., Holmes A., Kash T. L., Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Transl. Psychiatry 8, 60 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho J. H., Deisseroth K., Bolshakov V. Y., Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron 80, 1491–1507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pare D., Royer S., Smith Y., Lang E. J., Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann. N. Y. Acad. Sci. 985, 78–91 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Herry C., et al. , Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Hobin J. A., Goosens K. A., Maren S., Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J. Neurosci. 23, 8410–8416 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corcoran K. A., Maren S., Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J. Neurosci. 21, 1720–1726 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermann A., Stark R., Blecker C. R., Milad M. R., Merz C. J., Brain structural connectivity and context-dependent extinction memory. Hippocampus 27, 883–889 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Wang Q., et al. , Insular cortical circuits as an executive gateway to decipher threat or extinction memory via distinct subcortical pathways. Nat. Commun. 13, 5540 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin A., et al. , Exploration driven by a medial preoptic circuit facilitates fear extinction in mice. Commun. Biol. 6, 106 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Learning to smell danger: Acquired associative representation of threat in the olfactory cortex. Front. Behav. Neurosci. 8, 98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W., Wilson D. A., Threat memory in the sensory cortex: Insights from olfaction. Neuroscientist, 10.1177/10738584221148994 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Concina G., Renna A., Grosso A., Sacchetti B., The auditory cortex and the emotional valence of sounds. Neurosci. Biobehav. Rev. 98, 256–264 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Choi G. B., et al. , Driving opposing behaviours with ensembles of piriform neurons. Cell 146, 1004–1015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meissner-Bernard C., Dembitskaya Y., Venance L., Fleischmann A., Encoding of odor fear memories in the mouse olfactory cortex. Curr. Biol. 29, 367–380.e364 (2019). [DOI] [PubMed] [Google Scholar]
- 28.East B. S., et al. , Basolateral amygdala to posterior piriform cortex connectivity ensures precision in learned odor threat. Sci. Rep. 11, 21746 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouly A. M., et al. , PET metabolic imaging of time-dependent reorganization of olfactory cued fear memory networks in rats. Cereb. Cortex 32, 2717–2728 (2022). [DOI] [PubMed] [Google Scholar]
- 30.You Y., Novak L. R., Clancy K. J., Li W., Pattern differentiation and tuning shift in human sensory cortex underlie long-term threat memory. Curr. Biol. 32, 2067–2075.e2064 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson D. M., Illig K. R., Behan M., Haberly L. B., New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that "primary" olfactory cortex functions like "association" cortex in other sensory systems. J. Neurosci. 20, 6974–6982 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Z., et al. , Associations of unilateral whisker and olfactory signals induce synapse formation and memory cell recruitment in bilateral barrel cortices: Cellular mechanism for unilateral training toward bilateral memory. Front. Cell Neurosci. 10, 285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandairon N., et al. , Context-driven activation of odor representations in the absence of olfactory stimuli in the olfactory bulb and piriform cortex. Front. Behav. Neurosci. 8, 138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majak K., Ronkko S., Kemppainen S., Pitkanen A., Projections from the amygdaloid complex to the piriform cortex: A PHA-L study in the rat. J. Comp. Neurol. 476, 414–428 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Rajani V., Maziar A., Man K. N. M., Hell J. W., Yuan Q., Age-Dependent contributions of NMDA receptors and L-Type calcium channels to long-term depression in the piriform cortex. Int. J. Mol. Sci. 22, 13551 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svalbe B., et al. , Effects of the N-methyl-d-aspartate receptor antagonist, MK-801, on spatial memory and influence of the route of administration. Behav. Brain Res. 372, 112067 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Cain C. K., Blouin A. M., Barad M., L-type voltage-gated calcium channels are required for extinction, but not for acquisition or expression, of conditional fear in mice. J. Neurosci. 22, 9113–9121 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maziar A., et al. , Aging differentially affects LTCC function in hippocampal CA1 and piriform cortex pyramidal neurons. Cereb. Cortex 33, 1489–1503 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carew S. J., et al. , Pheromone-induced odor associative fear learning in rats. Sci. Rep.-UK 8, 17701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker D. L., Ressler K. J., Lu K. T., Davis M., Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J. Neurosci. 22, 2343–2351 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ledgerwood L., Richardson R., Cranney J., Effects of D-cycloserine on extinction of conditioned freezing. Behav. Neurosci. 117, 341–349 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Billard J. M., Rouaud E., Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by D-cycloserine. Eur. J. Neurosci. 25, 2260–2268 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Fanselow M. S., LeDoux J. E., Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23, 229–232 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Josselyn S. A., Tonegawa S., Memory engrams: Recalling the past and imagining the future. Science 367, eaaw4325 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberger N. M., Specific long-term memory traces in primary auditory cortex. Nat. Rev. Neurosci. 5, 279–290 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sacco T., Sacchetti B., Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science 329, 649–656 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Cambiaghi M., Grosso A., Renna A., Sacchetti B., Differential recruitment of auditory cortices in the consolidation of recent auditory fearful memories. J. Neurosci. 36, 8586–8597 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hegoburu C., Parrot S., Ferreira G., Mouly A. M., Differential involvement of amygdala and cortical NMDA receptors activation upon encoding in odor fear memory. Learn Mem. 21, 651–655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clem R. L., Schiller D., New learning and unlearning: Strangers or accomplices in threat memory attenuation? Trends Neurosci. 39, 340–351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luchkina N. V., Bolshakov V. Y., Mechanisms of fear learning and extinction: Synaptic plasticity-fear memory connection. Psychopharmacology (Berlin) 236, 163–182 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li G., Nair S. S., Quirk G. J., A biologically realistic network model of acquisition and extinction of conditioned fear associations in lateral amygdala neurons. J. Neurophysiol. 101, 1629–1646 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J. H., Richardson R., New findings on extinction of conditioned fear early in development: Theoretical and clinical implications. Biol. Psychiatry 67, 297–303 (2010). [DOI] [PubMed] [Google Scholar]
- 53.An B., et al. , Amount of fear extinction changes its underlying mechanisms. eLife 6, e25224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bekkers J. M., Suzuki N., Neurons and circuits for odor processing in the piriform cortex. Trends Neurosci. 36, 429–438 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Hernandez C. M., Jackson N. L., Hernandez A. R., McMahon L. L., Impairments in fear extinction memory and basolateral amygdala plasticity in the TgF344-AD rat model of Alzheimer’s disease are distinct from nonpathological aging. eNeuro 9, ENEURO.0181-22.2022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falls W. A., Miserendino M. J., Davis M., Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J. Neurosci. 12, 854–863 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sotres-Bayon F., Bush D. E., LeDoux J. E., Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology 32, 1929–1940 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Kaczorowski C. C., Davis S. J., Moyer J. R. Jr., Aging redistributes medial prefrontal neuronal excitability and impedes extinction of trace fear conditioning. Neurobiol. Aging 33, 1744–1757 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Lee H. K., Min S. S., Gallagher M., Kirkwood A., NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat. Neurosci. 8, 1657–1659 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Shankar S., Teyler T. J., Robbins N., Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J. Neurophysiol. 79, 334–341 (1998). [DOI] [PubMed] [Google Scholar]
- 61.Ressler K. J., et al. , Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch. Gen. Psychiatry 61, 1136–1144 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Davis M., Ressler K., Rothbaum B. O., Richardson R., Effects of D-cycloserine on extinction: Translation from preclinical to clinical work. Biol. Psychiatry 60, 369–375 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Bouton M. E., Vurbic D., Woods A. M., D-cycloserine facilitates context-specific fear extinction learning. Neurobiol. Learn. Mem. 90, 504–510 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ledgerwood L., Richardson R., Cranney J., D-cycloserine and the facilitation of extinction of conditioned fear: Consequences for reinstatement. Behav. Neurosci. 118, 505–513 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Yehuda R., Golier J. A., Tischler L., Stavitsky K., Harvey P. D., Learning and memory in aging combat veterans with PTSD. J. Clin. Exp. Neuropsychol. 27, 504–515 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Yaffe K., et al. , Posttraumatic stress disorder and risk of dementia among US veterans. Arch. Gen. Psychiatry 67, 608–613 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghosh A., et al. , Locus coeruleus activation patterns differentially modulate odor discrimination learning and odor valence in rats. Cereb. Cortex Commun. 2, tgab026 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Devore S., Lee J., Linster C., Odor preferences shape discrimination learning in rats. Behav. Neurosci. 127, 498–504 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carew S. J., et al. , Pheromone-induced odor associative fear learning in rats. Sci. Rep. 8, 17701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.