Abstract
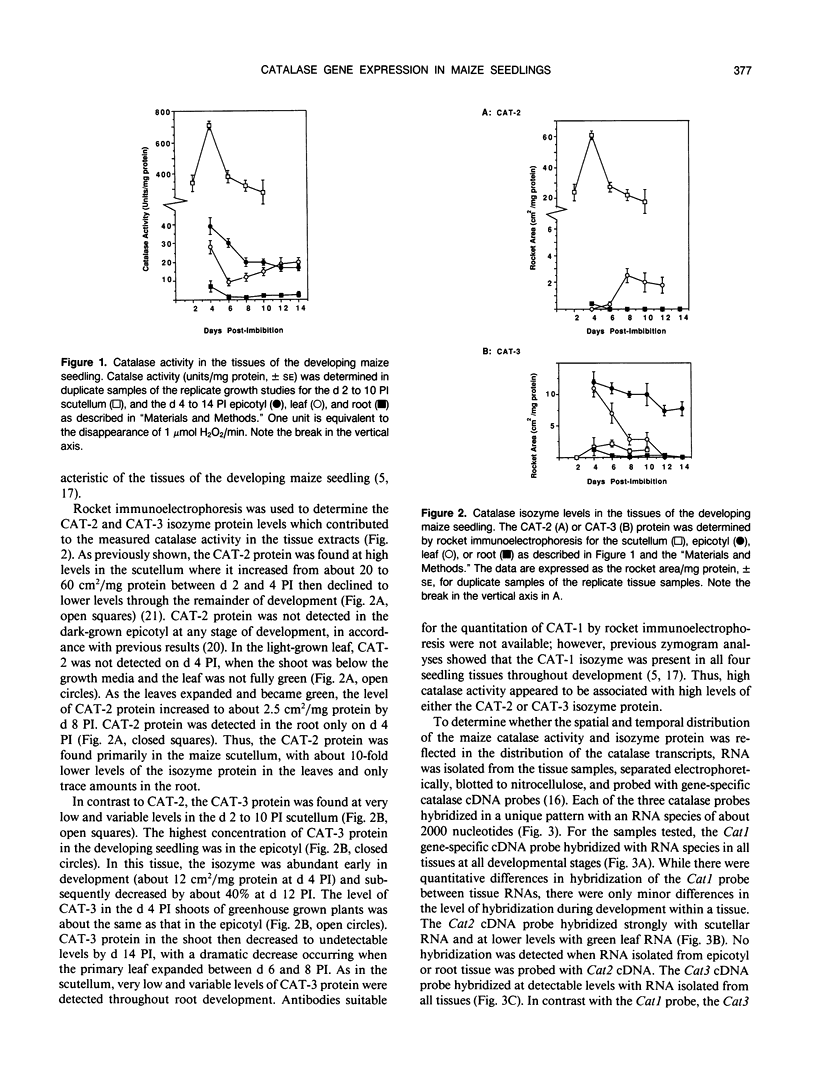
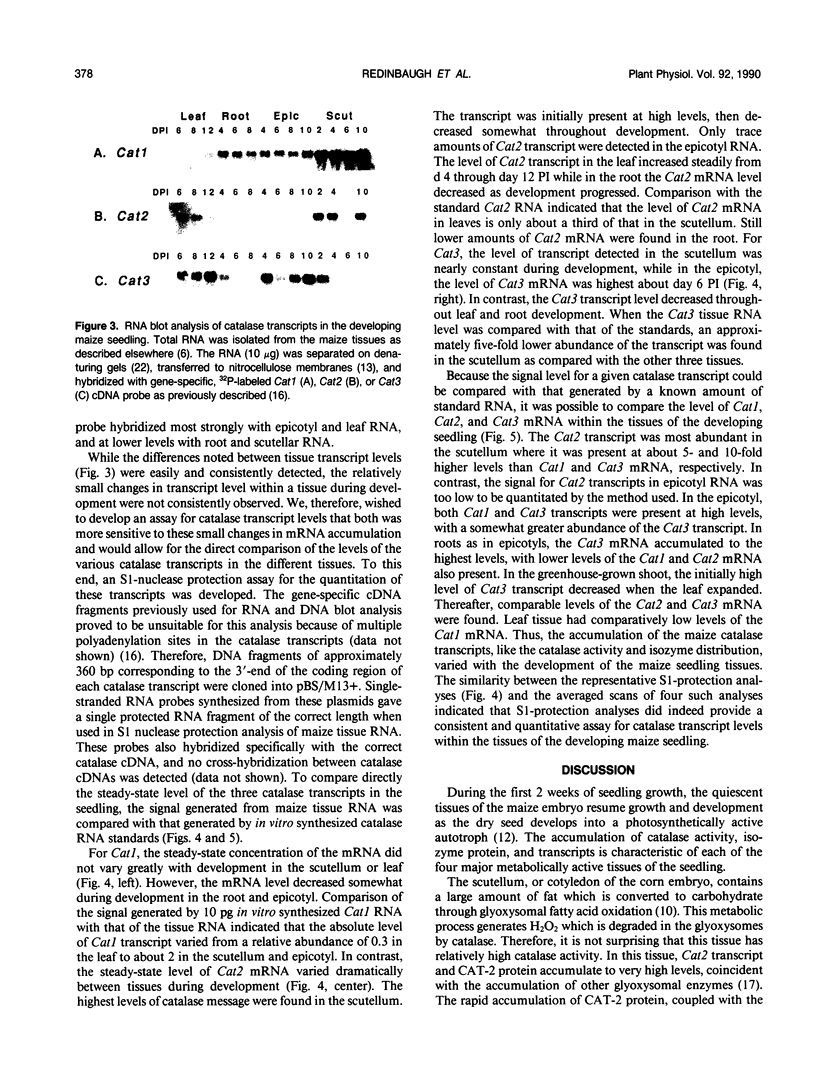
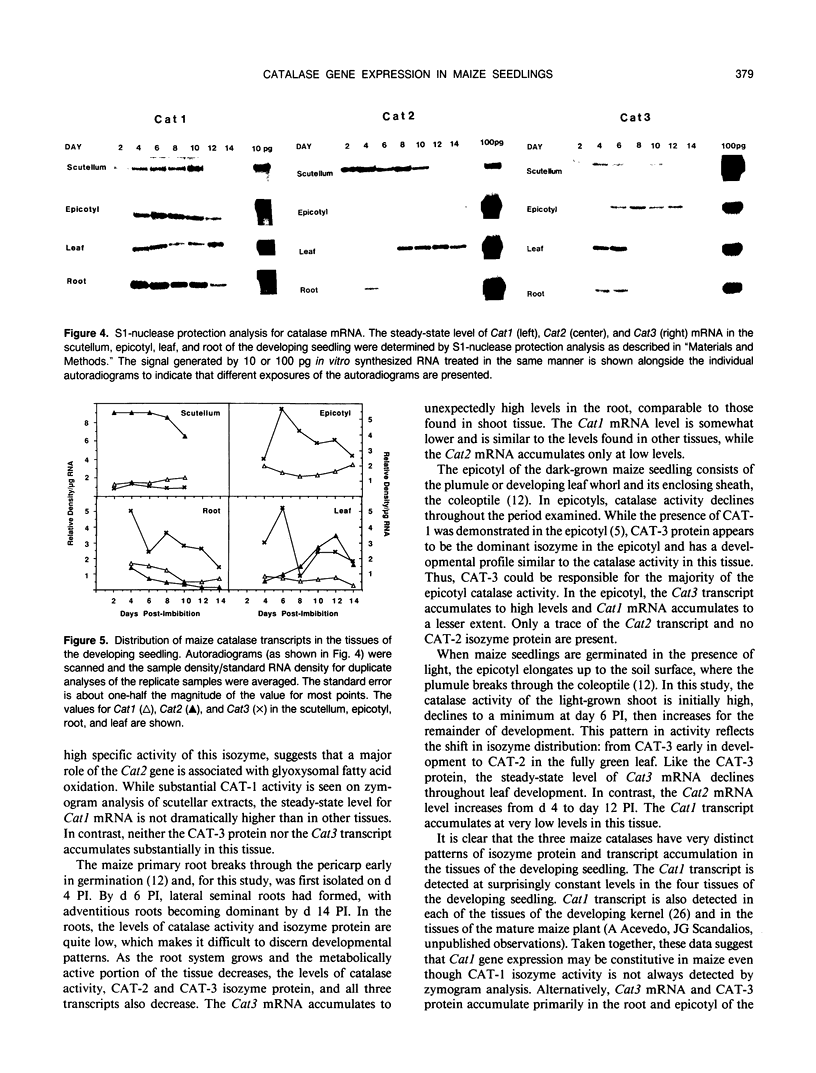
The catalase activity, CAT-2 and CAT-3 isozyme protein levels, and the steady-state mRNA levels for each of the three catalase genes were determined in the scutellum, root, epicotyl, and leaf of the developing maize (Zea mays L.) seedling. Catalase activity was highest in the scutellum, with 10-fold lower enzyme activity in the leaf and epicotyl. Very low levels of catalase activity were found in the root. The highest levels of CAT-2 protein were found in the scutellum, with about 10-fold lower levels in the green leaf. CAT-2 protein was present in trace amounts early in root development and no CAT-2 protein was detected in the epicotyl. Shortly after germination, CAT-3 protein was present at high levels in both the epicotyl and green leaf. With development, the amount of CAT-3 protein decreased slowly in the epicotyl and rapidly in the green leaf. Low levels of this isozyme were detected in the scutellum and root. The Cat1 transcript accumulated to low levels in all four tissues during the 14 day developmental period. High levels of the Cat2 transcript were found in the scutellum, with moderate levels of the mRNA in the green leaf. The Cat2 transcript levels were very low in the root and epicotyl. While the Cat3 mRNA level in the scutellum was low, high levels of the Cat3 transcript were detected in the root, epicotyl, and leaf. There was a positive correlation between the accumulation of a catalase isozyme and its transcript, indicating that the tissue specificity of maize catalase gene expression was regulated pretranslationally.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bethards L. A., Scandalios J. G. Molecular basis for the CAT-2 null phenotype in maize. Genetics. 1988 Jan;118(1):149–153. doi: 10.1093/genetics/118.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethards L. A., Skadsen R. W., Scandalios J. G. Isolation and characterization of a cDNA clone for the Cat2 gene in maize and its homology with other catalases. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6830–6834. doi: 10.1073/pnas.84.19.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann H., Martinez P., Quigley F., Martin W., Cerff R. Endosymbiotic origin and codon bias of the nuclear gene for chloroplast glyceraldehyde-3-phosphate dehydrogenase from maize. J Mol Evol. 1987;26(4):320–328. doi: 10.1007/BF02101150. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Forde B. G., Day H. M., Turton J. F., Shen W. J., Cullimore J. V., Oliver J. E. Two glutamine synthetase genes from Phaseolus vulgaris L. display contrasting developmental and spatial patterns of expression in transgenic Lotus corniculatus plants. Plant Cell. 1989 Apr;1(4):391–401. doi: 10.1105/tpc.1.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Turley R. B. Adaptation of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal Biochem. 1986 Mar;153(2):267–271. doi: 10.1016/0003-2697(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Redinbaugh M. G., Wadsworth G. J., Scandalios J. G. Characterization of catalase transcripts and their differential expression in maize. Biochim Biophys Acta. 1988 Nov 10;951(1):104–116. doi: 10.1016/0167-4781(88)90030-9. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G., Chang D. Y., McMillin D. E., Tsaftaris A., Moll R. H. Genetic regulation of the catalase developmental program in maize scutellum: Identification of a temporal regulatory gene. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5360–5364. doi: 10.1073/pnas.77.9.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios J. G. The antioxidant enzyme genes Cat and Sod of maize: regulation, functional significance, and molecular biology. Isozymes Curr Top Biol Med Res. 1987;14:19–44. [PubMed] [Google Scholar]
- Skadsen R. W., Scandalios J. G. Evidence for processing of maize catalase 2 and purification of its messenger RNA aided by translation of antibody-bound polysomes. Biochemistry. 1986 Apr 22;25(8):2027–2032. doi: 10.1021/bi00356a029. [DOI] [PubMed] [Google Scholar]
- Skadsen R. W., Scandalios J. G. Pretranslational control of the levels of glyoxysomal protein gene expression by the embryonic axis in maize. Dev Genet. 1989;10(1):1–10. doi: 10.1002/dvg.1020100102. [DOI] [PubMed] [Google Scholar]
- Skadsen R. W., Scandalios J. G. Translational control of photo-induced expression of the Cat2 catalase gene during leaf development in maize. Proc Natl Acad Sci U S A. 1987 May;84(9):2785–2789. doi: 10.1073/pnas.84.9.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaftaris A. S., Bosabalidis A. M., Scandalios J. G. Cell-type-specific gene expression and acatalasemic peroxisomes in a null Cat2 catalase mutant of maize. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4455–4459. doi: 10.1073/pnas.80.14.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi K., Tsutsumi R., Daimon M., Numazaki M., Ishikawa K. Tissue-specific and developmentally specific controls involved in rat aldolase B gene expression. Isozymes Curr Top Biol Med Res. 1987;14:177–193. [PubMed] [Google Scholar]
- Wadsworth G. J., Redinbaugh M. G., Scandalios J. G. A procedure for the small-scale isolation of plant RNA suitable for RNA blot analysis. Anal Biochem. 1988 Jul;172(1):279–283. doi: 10.1016/0003-2697(88)90443-5. [DOI] [PubMed] [Google Scholar]
- Wadsworth G. J., Scandalios J. G. Differential expression of the maize catalase genes during kernel development: the role of steady-state mRNA levels. Dev Genet. 1989;10(4):304–310. doi: 10.1002/dvg.1020100405. [DOI] [PubMed] [Google Scholar]




