Abstract
The objective of this review was to provide an overview of the effects of dietary fiber (DF) on reproductive performance in gestating sows. Dietary fibers have been suggested to modulate microbiota in the intestine and the immune system of gestating sows and to improve gut health. Thus, DF may help alleviate the adverse effects of the stressful production cycle of gestating sows. These benefits may subsequently result in improved reproductive performance of sows. Previous studies have reported changes in microbiota by providing gestating sows with DF, and the responses of microbiota varied depending on the source of DF. The responses by providing DF to gestating sows were inconsistent for antioxidative capacity, hormonal response, and inflammatory response among the studies. The effects of DF on reproductive performance were also inconsistent among the previous studies. Potential reasons contributing to these inconsistent results would include variability in reproductive performance data, insufficient replication, influence of other nutrients contained in the DF diets, characteristics of DF, and experimental periods. The present meta-analysis suggests that increasing the total DF concentration by 10 percentage units (e.g., 12% to 22% as-fed basis) in gestating sow diets compared to the control group improves the litter born alive by 0.49 pigs per litter. However, based on the present review, questions remain regarding the benefits of fibers in gestating sow diets. Further research is warranted to clarify the mode of action of fibers and the association with subsequent reproductive performance in gestating sows.
Keywords: Dietary fiber, Microbiota, Reproductive Performance, Sows
INTRODUCTION
The life cycle of sows in modern swine industry can be intense and stressful, which may potentially affect their health and performance. The adoption of hyper-prolific sows to increase total litter size and piglet production per sow per year has become widespread in the industry, but this has led to side effects such as constipation, longer farrowing duration, and greater variation in litter birth weight, which may overburden sows [1,2]. Many researchers have observed reproductive performance problems, including reduced litter born alive, in gestating sows due to this stressful life cycle and the side effects associated with hyper-prolificacy [3–5].
Swine nutritionists are currently investigating alternative feed additives and feeding strategies to alleviate the reproductive performance problems without using antibiotics [6–8]. Among the alternative strategies, the addition of dietary fiber (DF) has been widely used as prebiotics in livestock to modulate the gut microbiota and immunological status, which can improve sow health and reproductive performance [9,10]. Previous meta-analyses have shown that the effects of DF on reproductive performance depend on the fiber source, neutral detergent fiber content, and parity, with a significant interaction between these factors and litter size born alive [11]. Updated reviews have suggested that more appropriate measures of fiber are soluble dietary fiber (SDF) and insoluble dietary fiber (IDF), and that the effect of supplemental DF is better when it is provided during one or more reproductive cycles [12]. Despite decades of research, questions remain about the effects of high-DF diets on gestating sows. Therefore, the objectives of the present review were to summarize the current data on the effects of DF on gestating sows and to provide an overview of the application of DF in gestating sow diets.
JUSTIFICATION FOR ADDING FIBER TO GESTATING SOW DIETS
Prolific sows often experience challenges related to farrowing duration, digestion, and digestive microbiota [1,13]. Prolonged farrowing duration can result in higher numbers of piglet deaths at birth, lower piglet survival rates, greater postpartum oxidative stress, and increased incidence of sow anorexia, ultimately leading to reduced productivity of litter piglets. These challenges can be intensified by factors such as constipation, oxidative stress, and insulin resistance, which can cause inadequate physical endurance during farrowing [5,14,15].
Digestive transit is also commonly affected in late gestation and plays a crucial role in the farrowing process and early lactation. Constipation can lead to dysbiosis and uncontrolled growth of undesired bacteria in the gut, causing digestive discomfort for the sow and potential health issues [16]. Therefore, maintaining a balanced digestive microbiota and optimal digestive function is a critical challenge that must be addressed with prolific sows.
Some prevention strategies include avoiding the abrupt change from high-fiber gestation diets to high energy with low-fiber lactation diets [17], adding the fiber source in a diet around farrowing period to optimize digestive transit [18], and using the prebiotics and probiotics to help balance the digestive microbiota [19].
POTENTIAL MODE OF ACTION OF DIETARY FIBER
The addition of DF to adult sow diets requires a clear understanding for their mode of action. Some researchers have proposed that the positive effect of adding DF to gestating sow diets on reproductive performance primarily arises from two factors: i) modulation of intestinal microbiota and ii) modulation of physiological status. These factors contribute to adequate physical endurance during farrowing, consequently, improving reproductive performance. Also, some researchers have proposed that positive modulation of microbiota and physiological status of sows can directly affect the fetus.
Effects on intestinal microbiota
The DF provides an essential fermentative substrate to the microbiome and is known to impact microbial composition, diversity, and metabolic capabilities [20,21]. The addition of DF to gestating sow diets resulted in a clear separation of the microbiota in gut or feces among the treatments (Table 1). It appears that separation of the microbiota depends on the different DF sources. In 10 experiments, 5 out of 19 DF-containing groups had a changed microbial diversity in the gut as compared with the control group. Three experiments reported a significant change in microbial diversity, while others failed to detect significance, which could be attributed to difference in feeding length. The studies that reported significant changes in microbial diversity fed the diet containing DF throughout the gestation period, whereas the other studies fed the diet from the late periods of gestation except one experiment. These findings indicate that a sufficient period for fiber consumption is needed to significantly change the microbial diversity of the gut [22,23].
Table 1.
The effects of dietary fiber on the fecal and gut microbiota of gestating sows1)
| Main source | Inclusion rate (%) | Fiber composition in diet2) (%) | Feed allowance3) (kg/d) | Feeding length | Microbial diversity | Changing relative abundance | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| Control | Treatment | Control | Treatment | Phylum level | Another taxon | ||||||||
|
|
|
|
|||||||||||
| SDF | IDF | SDF | IDF | ||||||||||
| Konjac flour | 2.20 | 2.30 | 18.16 | 4.00 | 45.93 | 2.42 | 2.41 | Gestating period | - | - | - | ○ | [19]5) |
| Guar gum plus Pregelatinized waxy maize starch | 2.00 | Not provided | Not provided | 2.42 | Gestating period | ↑ | ○ | F:B4)(↑) | ○ | [35] | |||
| Stevia residue | 20.0 | Not provided | Not provided | 2.76 | 3.70 | Gestating period | ↑ | ○ | F:B (↑) | ○ | [92]6) | ||
| 30.0 | |||||||||||||
| 40.0 | |||||||||||||
| Wheat bran | 18.0 | 1.57 | 10.91 | 2.02 | 15.33 | 2.40 | 2.56 | Gestating period | × | ○ | F:B (↓) | ○ | [93]6) |
| Wheat bran and fiber mix | 16.4 / 1.0 | 2.16 | 15.56 | ||||||||||
| (Wheat bran / fiber) | 14.8 / 2.0 | 2.29 | 15.79 | ||||||||||
| 13.2 / 3.0 | 2.42 | 16.01 | |||||||||||
| 11.6 / 4.0 | 2.56 | 16.24 | |||||||||||
| Inulin | 1.60 | Not provided | Not provided | 3.30 | d 80 of gestation to farrowing | × | ○ | F:B (↓) | ○ | [36] | |||
| Alfalfa meal | 10.0 | 2.12 | 15.9 | 2.23 | 22.74 | Recommendations of NRC (2012) | d 60 of gestation to farrowing | × | ○ | × | ○ | [37]5) | |
| Wheat bran | 30.0 | 1.39 | 9.98 | 1.86 | 19.95 | 3.00 | 3.31 | d 80 of gestation to weaning | × | ○ | × | ○ | [62]6),7) |
| Sugar beet pulp | 20.0 | 4.06 | 17.54 | 3.07 | × | ||||||||
| Fine wheat bran | 20.0 | 2.20 | 18.28 | 2.79 | 17.55 | 2.51 | Gestating period | ↑ | ○ | F:B (↓) | ○ | [48] | |
| Inulin | 0.83 | 0 | 0 | 0.83 | 20.00 | 2.40 | 2.90 | Insemination to d 106 of gestation | × | ○ | F:B (↓) | ○ | [90] |
| Cellulose | 20.0 | ||||||||||||
| Lignocellulose | 1.50 | 2.18 | 17.31 | 2.10 | 18.00 | 3.00 | 3.03 | d 85 of gestation to farrowing | × | ○ | × | ○ | [18]5),6) |
| Resistant starch | 2.00 | 4.10 | 16.50 | 3.04 | × | ||||||||
| Konjac flour | 2.00 | 2.17 | 14.84 | 3.04 | F:B (↓) | ||||||||
A circle sign (○) represents significant difference at p<0.05, a multiplication sign (×) represents no difference at p>0.05, an up-arrow sign (↑) and down arrow sign (↓) represents significant increase and decrease at p<0.05, respectively.
SDF, soluble dietary fiber; IDF, insoluble dietary fiber; IDF:SDF, insoluble dietary fiber to soluble dietary fiber ratio.
Weighted average value based on feeding length during gestating period.
F:B, Firmicutes to Bacteroidetes ratio.
The control and treatment groups were adjusted to have similar metabolizable or digestible energy intake through different feed intake.
Dietary fiber composition of lactation diet; the control, wheat bran, and sugar beet pulp diets contained 11.8%, 16.8%, and 16.9% total dietary fiber, 1.43%, 2.72%, and 1.70% soluble dietary fiber, and 10.4%, 14.1%, and 15.2% insoluble dietary fiber, respectively.
Microbiota are crucial for maintaining the nutrition status, physiology, and immune function of pigs [24,25]. Major or frequent changes in microbiota are often associated with ill health [26–28]. The Firmicutes and Bacteroidetes are generally the two dominant phyla, which make up about 90% of the fecal microbiota [29]. The Firmicutes bacteria are Gram-positive and play a key role in the nutrition and metabolism of the host through the synthesis of short-chain fatty acids (SCFA). Their metabolic products affect other tissues and organs, regulating hunger and satiety. In contrast, Bacteroidetes bacteria are Gram-negative and associated with immunomodulation. Their components, lipopolysaccharides and flagellin, interact with cell receptors and enhance immune reactions through cytokine synthesis [30]. The Firmicutes-to -Bacteroidetes ratio (F:B) is associated with maintaining homeostasis, and changes in this ratio can lead to various pathologies. For example, increased F:B is associated with the development of obesity, while decreased F:B is associated with the intestinal inflammation [31,32]. However, the interpretation of the F:B can vary depending on the viewpoint. For example, an increased F:B can be interpreted as the development of anti-inflammation, while a decreased F:B can be interpreted as anti-obesity. In 10 experiments, 9 out of 19 DF containing groups had a decreased F:B, while 4 DF containing groups had an increased F:B in the gut compared to the control group. The discrepancy observed among these studies can be attributed to the initial state of the gut microbiome in the sow groups before the start of the experiment. The effect of DF is likely dependent on the pre-existing status of the sow gut microbiota prior to the initiation of the experiment. The groups with a reduced F:B compared to the control group indicate that the sows fed DF deposited less energy under the same calorie intake. This not only prevents sow obesity but also allows undeposited energy to be allocated to the fetus or mammary glands. The prevention of sow obesity reduces farrowing duration [1], and this may be a potential mode of action of DF. Likewise, the groups with an increased F:B indicate that the sows fed DF had an improved ability to fight against inflammation, which may lead to energy deposition in the fetus or mammary gland. Therefore, the use of DF seems to balance the F:B, consequently treating obesity or intestinal inflammation.
At the phylum level, 14 out of 19 DF-containing groups showed a positive change in the relative abundance of microbiota compared to control groups. The increase in beneficial bacteria can enhance intestinal SCFA synthesis, which not only provides an important energy source contributing to gut health of host [33] but also downregulates the synthesis of hunger-suppressing hormones such as leptin, peptide YY, and glucagon-like peptide [34]. The SCFA-producing microorganisms, including Bacteroides, CF231, Eubacterium, Oscillospira, Parabacteroides, Prevotella, and Ruminococcacea, were increased. Xu et al [35] reported that sows fed diets supplemented with 2.0% guar gum and pregelatinized waxy maize starch during the gestating period increased the relative abundance of Eubacterium, Oscillospira, and Ruminococcacea. This result is consistent with the finding suggested by Li et al [36], who showed that 1.6% inulin addition increased SCFA-producing microorganisms such as CF231 and Prevotella, as well as reduced endotoxin production and thus reduced the intestinal inflammatory response [18,36]. The decrease in harmful bacteria can reduce potential damage to the gut microbial barrier and prevent endotoxin from permeating the blood [37]. The endotoxin-producing microorganism, including Cyanobacteria, Deuslfovibrio, and Oscillibacter, were decreased. Lu et al [18] reported that sows fed 2.0% resistant starch or konjaku flour from the day of 85 gestation to farrowing showed a decrease in the relative abundance of Deuslfovibrio and Oscillibacter. These positive changes in the relative abundance of microbiota may ultimately improve reproductivity by reducing metabolic syndrome. Although almost all DF-containing groups showed a positive change in the relative abundance of microbiota, some groups did not show significant differences compared to the control group. The limited information on the characteristics of DF makes it difficult to explain these contrasting results. Further research is required to verify the effects of the characteristics of DF, such as soluble or insoluble, on the gut microbiota.
Effects on endocrine and metabolic status
To confirm the effect of DF during gestation on the endocrine and metabolic status of sows, response criteria were classified into 4 categories including hormones, immune status, antioxidant index, and metabolite index (Table 2). Eleven studies out of 18 studies measured hormones related to feed intake, farrowing, lactation, and stress and showed a positive response in 7 studies. Although estrogen, insulin, leptin, lutropin, oxytocin, prolactin, serotonin, and stress hormones showed a positive response, not all studies have reported consistent results. Reproductive hormones, including estrogen, progesterone, and lutropin, play an important role in the regulation of female reproduction. Vallet et al [38] suggested a positive relationship between litter size and plasma estrogen on day 110 of gestation. A surge in lutropin triggers the production of progesterone by the corpus luteum, which contributes to pregnancy maintenance, embryo survival, and placental development and function [39,40]. In addition, Li et al [10] reported an increase in placental weight in sows fed a high-fiber diet over an extended period spanning the second and third parities, which was attributed to alterations in plasma concentrations of reproductive hormones.
Table 2.
The effects of dietary fiber on endocrine and metabolic status of gestating sows1)
| Main source | Inclusion rate (%) | Fiber composition in diet2) (%) | Feed allowance3) (kg/d) | Feeding length | The presence or absence of a positive response | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Control | Treatment | Control | Treatment | ||||||||||
|
|
|
|
|||||||||||
| SDF | IDF | SDF | IDF | Hormone | Immune | Antioxidant | Metabolite | ||||||
| Sunflower meal | 9.75 | Not provided | Not provided | 2.40 | 2.80 | Gestating period | ○ | - | - | × | [43]4),5) | ||
| Wheat bran | 9.75 | ||||||||||||
| Sugar beet pulp | 19.50 | ||||||||||||
| Soybean hulls | 9.75 | ||||||||||||
| Corn gluten feed | 3.00 | ||||||||||||
| Soybean hulls | 8.00 | 1.90 | 11.4 | 2.80 | 20.6 | Based on backfat thickness and body weight | d 90 of gestation to farrowing | × | - | - | ○ | [44] | |
| Wheat bran | 8.00 | ||||||||||||
| Sunflower meal | 8.00 | ||||||||||||
| Sugar beet pulp | 8.00 | ||||||||||||
| Soybean hulls | 12.2 | 5.00 | 10.6 | 5.40 | 16.9 | 2.26 | 2.36 | d 73 of gestation to farrowing | × | - | - | × | [45]5) |
| 24.4 | 7.50 | 20.7 | 2.56 | ||||||||||
| Oat straws | 10.0 | 2.70 | 17.4 | 2.55 | 23.0 | 2.40 | 2.40 | d 86 of gestation to farrowing | ○ | - | - | × | [52] |
| 2.50 | 23.2 | ||||||||||||
| Wheat straws | 2.60 | 23.4 | × | - | - | ○ | |||||||
| 2.50 | 23.0 | ||||||||||||
| Inulin | 2.50 | No control diet | 3.87 | 15.0 | No control diet | 2.44 | Gestating period | - | ○ | ○ | - | [71] | |
| Cellulose | 0.00 | ||||||||||||
| Inulin | 1.50 | 2.87 | 16.0 | ||||||||||
| Cellulose | 1.00 | ||||||||||||
| Inulin | 0.50 | 1.87 | 17.0 | - | × | × | - | ||||||
| Cellulose | 2.00 | ||||||||||||
| Inulin | 0.00 | 1.37 | 17.5 | ||||||||||
| Cellulose | 2.50 | ||||||||||||
| Resistant starch | 5.00 | 2.23 | 16.2 | 7.28 | 17.6 | 2.57 | Gestating period | ○ | - | ○ | - | [69]4) | |
| Fermented soybean fiber | 5.00 | Not provided | × | - | ○ | - | |||||||
| Inulin | 0.80 | 3.63 | 23.0 | 4.34 | 22.7 | 3.30 | d 80 of gestation to farrowing | × | - | ○ | - | [46] | |
| 1.60 | 5.05 | 22.5 | × | - | ○ | - | |||||||
| 2.40 | 5.77 | 22.2 | × | - | ○ | - | |||||||
| Wheat bran | 16.4 | 1.92 | 15.6 | 3.22 | 29.7 | 3.00 | 3.20 | d 90 of gestation to farrowing | - | ○ | ○ | × | [70]5) |
| Soybean hull | 16.4 | ||||||||||||
| Wheat bran | 12.0 | 5.06 | 28.4 | - | ○ | ○ | × | ||||||
| Soybean hull | 12.0 | ||||||||||||
| Sugar beet pulp | 11.4 | ||||||||||||
| Wheat bran | 20.0 | No control diet | Not provided | No control diet | 2.12 | d 30 of gestation to weaning | - | - | - | × | [94]4) | ||
| Soya hulls | 20.0 | 2.15 | - | - | - | ○ | |||||||
| Rice hulls | 20.0 | 2.20 | - | - | - | × | |||||||
| Guar gum plus pregelatinized waxy maize starch | 2.00 | Not provided | Not provided | 2.42 | Gestating period | - | ○ | - | ○ | [35] | |||
| Wheat bran | 18.0 | 1.57 | 10.9 | 2.02 | 15.3 | 2.40 | 2.56 | Gestating period | - | × | - | ○ | [93]5) |
| Wheat bran and fiber mix (Wheat bran / fiber) | 16.4 / 1.0 | 2.16 | 15.6 | ||||||||||
| 14.8 / 2.0 | 2.29 | 15.8 | |||||||||||
| 13.2 / 3.0 | 2.42 | 16.0 | |||||||||||
| 11.6 / 4.0 | 2.56 | 16.2 | |||||||||||
| Inulin | 2.60 | 1.10 | 9.14 | 2.77 | 30.3 | 2.27 | 2.73 | Gestating period | ○ | - | - | ○ | [10] |
| Cellulose | 18.2 | ||||||||||||
| Alfalfa meal | 10.0 | 2.12 | 15.9 | 2.23 | 22.7 | Recommendations of NRC (2012) | d 60 of gestation to farrowing | - | ○ | ○ | - | [37]4) | |
| Wheat bran | 30.0 | 1.39 | 9.98 | 1.86 | 20.0 | 3.00 | 3.31 | d 80 of gestation to weaning | - | ○ | - | × | [62]5),6) |
| Sugar beet pulp | 20.0 | 4.06 | 17.5 | 3.07 | - | ○ | - | ○ | |||||
| Wheat bran | 36.4 | 1.58 | 8.17 | 2.4 | 19.7 | 2.38 | Gestating period | × | - | × | × | [95]4) | |
| Chicory meal | 23.6 | Not provided | × | - | × | ○ | |||||||
| Soybean curd residue | 17.8 | × | - | × | × | ||||||||
| Corn gluten | 27.0 | × | - | × | ○ | ||||||||
| Rice bran meal | 46.5 | × | - | × | × | ||||||||
| Fine wheat bran | 20.0 | 2.20 | 18.3 | 2.79 | 17.6 | 2.51 | Gestating period | ○ | ○ | - | ○ | [48] | |
| Inulin | 0.83 | 0 | 0 | 0.83 | 20.0 | 2.40 | 2.90 | Insemination to d 106 of gestation | ○ | - | - | - | [90] |
| Cellulose | 20.0 | ||||||||||||
| Lignocellulose | 1.50 | 2.18 | 17.3 | 2.10 | 18.0 | 3.00 | 3.03 | d 85 of gestation to farrowing | × | ○ | - | - | [18]4),5) |
| Resistant starch | 2.00 | 4.10 | 16.5 | 3.04 | ○ | ○ | - | - | |||||
| Konjac flour | 2.00 | 2.17 | 14.8 | 3.04 | |||||||||
A circle sign (○) represents significant difference at p<0.05, a multiplication sign (×) represents no difference at p>0.05, no control diet; comparison between treatments.
SDF, soluble dietary fiber; IDF, insoluble dietary fiber; IDF:SDF, insoluble dietary fiber to soluble dietary fiber ratio.
Weighted average value based on feeding length during gestating period.
The control and treatment groups were adjusted to have similar metabolizable or digestible energy intake through different feed intake.
Dietary fiber composition of lactation diet; the control, wheat bran, and sugar beet pulp diets contained 11.8%, 16.8%, and 16.9% total dietary fiber, 1.43%, 2.72%, and 1.70% soluble dietary fiber, and 10.4%, 14.1%, and 15.2% insoluble dietary fiber, respectively.
Insulin regulates carbohydrates, fat, and protein metabolism by promoting the absorption of glucose from the blood into the liver, fat, and skeletal muscle cells. It has been proposed that greater insulin resistance may be the potential cause of longer than optimal farrowing durations in women [41], as well as greater than optimal production of reactive oxygen species (ROS) due to metabolic disorders. Additionally, Père and Etienne [42] reported that gestating sows become resistant to insulin towards the end of gestation. In a previous study conducted by Xu et al [35], SDF was found to alleviate insulin resistance in perinatal sows by increasing the level of circulating odd-chain fatty acids in plasma. However, while some studies have shown that DF delays postprandial peaks of insulin concentrations compared to sows fed a control diet [43], there have been no significant differences in insulin resistance between sows fed a DF-added diet and a control diet in other studies [44–46].
Leptin is an appetite-suppressing hormone that is secreted after consuming diets and acts on the brain to induce satiety [47]. Sows fed high DF during gestation experienced decreased plasma leptin concentrations before farrowing, which negatively correlated with the feed intake of sows during lactation [43,48]. However, recent research has focused on the effect of long-term DF consumption on serum leptin levels compared to a control diet through a meta-analysis in humans. These findings suggest that consuming DF over an extended period may lower serum leptin levels primarily in obese individuals [49]. A possible reason for increased lactation feed intake in sows fed high DF during gestation is that the increased size and capacity of the digestive tract may facilitate the adaptation of sows to the drastic increase in feeding intake required during lactation [50].
Prolactin is a crucial hormone for initiating and maintaining milk production [51]. Prolactin is involved in the cell proliferation, development of mammary glands, and secretion of milk. This hormone eventually helps provide nutrients to suckling piglets through sow milk and improving survival rates of offspring. High DF intake tended to increase prolactin concentrations in gestating sows [43,52], but other studies showed no effect on prolactin concentrations [44,46]. The potential reasons for these discrepancies among the studies may be associated with maternal obesity during the gestation period. According to the results of Lepe et al [53] who investigated the effect of maternal obesity on lactation, obese mothers had lower prolactin concentrations, which led to delayed lactogenesis. Therefore, preventing obesity in gestating sows through high DF intake may increase prolactin concentration in serum.
Oxytocin is a neurohypophysial hormone that plays a central role in the regulation of farrowing and lactation, such as the initiation of uterine contractions and milk secretion [54]. Li et al [10] reported that sows fed a diet supplemented with 2.26% inulin and 18.2% cellulose during the gestational period had increased plasma oxytocin levels, which were associated with postprandial satiety due to the high-DF diet consumption [55].
Among the hormones related to stress, cortisol in serum is a criterion that reflects stress intensity [56]. Several studies have reported that high-DF diets can influence welfare by decreasing stereotypical behavior that leads to cortisol stimulation [57,58]. A recent study suggested that a diet containing 5% resistant starch during the gestation period contributed to enhancing postprandial satiety, alleviating stress status, and reducing abnormal behaviors [59], but another study reported that stress hormones were not affected in sows fed a diet containing 40% soybean hulls as a fiber source [60]. These inconsistent results were likely due to differences in fiber source and type.
Eight out of 18 studies have measured the criteria of immune status and showed a positive response in 7 studies. Inflammation is a biological response of the immune system to harmful stimuli, including pathogens, damaged cells, toxic compounds, or irradiation [61]. Inflammatory stimuli activate intracellular signaling pathways that then activate the production of inflammatory mediators, including pro-inflammatory factors such as interleukin-6 and tumor necrosis factor-α. These factors have been used as primary criteria to determine the immune status. The studies reported a positive response in immune status, suggesting that sows fed high DF generate more microbiota-derived SCFA that enhances the barrier function in intestinal epithelial cells [18, 48,62]. Enhanced barrier function in the intestine decreases the gut permeability, which leads to the prevention of the inflow of endotoxins. Consequently, high DF fed to sows may reduce systemic inflammation. Another possible reason for the altered immune status is that DF could promote intestinal peristalsis and excretion of stool to reduce the incidence of gastrointestinal disorder such as constipation which may increase the absorption of harmful microbial endotoxins [18].
Six out of 18 studies measured antioxidant markers and showed positive responses in 4 studies. Oxidation is typically initiated by ROS produced by the metabolism of cells. Free radicals or ROS, in general, are known to play both detrimental and beneficial roles [63]. Low ROS levels interact with specific targets and play an essential role in redox signaling involved in stress adaptation, homeostasis, and health maintenance [64]. Conversely, high exposure to ROS affects non-specific targets and induces oxidative stress, such as lipid peroxidation, damaged DNA and cell death, leading to reduced immunity and resistance to various diseases [65]. Complex enzymatic systems containing catalase, superoxide dismutase, and glutathione peroxidase, as well as nonenzymatic systems containing glutathione, beta-carotene, and vitamin E, play vital roles in protecting organisms from oxidative damage [66]. The antioxidant enzymes and oxidative products such as malondialdehyde and protein carbonyl are usually used as biomarkers of oxidative stress. The increased demands of energy and oxygen for the placenta of sows in late gestation lead to excessive oxidative stress [67]. The oxidative stress in gestating sows fed a high-DF diet has been alleviated by increasing antioxidant capacity [68–70]. A study that supplemented gestating sow diets with 0.8%, 1.6%, or 2.4% inulin, resulting in a total dietary fiber (TDF) content of 27%, showed an increase in the antioxidant capacity of gestating sows [46]. This result is consistent with the result of Liu et al [70], who showed that wheat bran, soybean hulls, and sugar beet pulp addition, consequently containing 33% TDF, increased glutathione peroxidase or decreased malondialdehyde. However, another study reported that sows fed diets with the same TDF content of 19% but different IDF- to-SDF ratios (IDF:SDF) of 3.9, 5.6, 9.1, and 12.8 showed different oxidative statuses [71]. This study observed that the antioxidant capacity was improved when the IDF:SDF was less than 5.59, implying that the composition of DF in gestating diets played an important role in improving antioxidant capacity.
Twelve out of 18 studies have measured metabolites to verify energy status and nutrient metabolism, and 9 of these studies showed a positive response. However, the effects of DF on serum metabolites were inconsistent among the studies. Glucose concentration has been used as an indicator of energy status and diabetic tendencies [72], as sows can become glucose intolerant and have diabetic tendencies during pregnancy [73,74]. Supplying high DF to sows can be expected to prevent rapid increases in blood glucose due to delayed postprandial peaks [43]. However, there have been no studies demonstrating the effects of high DF on alleviating glucose tolerance in gestating sows. Because glucose intolerance is more pronounced in obese mothers [75], evaluating the effects of high DF on glucose tolerance in non-obese mothers may not be relevant or applicable to obese mothers, and may therefore lead to misinterpretation. This acknowledges that further research may be necessary to fully understand the effects of high DF on glucose tolerance in obese mothers during pregnancy.
Urea serves as a nitrogen source for gut microbes and can provide an estimate of the state of protein metabolism in pigs. While most studies suggest that feeding sows a high-DF diet does not significantly affect blood urea, some studies show a decrease in blood urea. The fermentation of DF in the gut can influence microbial mass and activity [76,77]. However, highly fermentable fibers tend to increase microbial mass, which results in a greater transfer of urea from the blood to the gut, thereby reducing plasma concentrations, as seen in rat studies [78]. Alternatively, lower plasma urea concentrations may indicate lower protein oxidation in the liver, which may be related to reduced intestinal protein absorption when sows are fed a high-DF diet [44].
It is well known that non-esterified fatty acids (NEFA) are a product of fat metabolism and a good indicator of catabolism of fat reserves [79]. Several studies have reported that feeding high DF to sows reduces NEFA in their serum during late gestation, suggesting that high DF might reduce fat catabolism and thus preserve body reserves [35,62]. Indeed, Shang et al [62] showed that sows fed sugar beet pulp had less body fat loss during lactation, but no significant difference in backfat loss was observed between treatments. In contrast, wheat bran supplementation did not affect serum NEFA concentrations compared to the control diet. Previous studies have shown a negative correlation between SCFA production and serum NEFA concentration, indicating that fermentable fiber can decrease serum NEFA concentration by increasing SCFA production [80]. Sugar beet pulp contains more SDF that is readily fermentable than wheat bran, leading to increased production of SCFA in sows fed sugar beet pulp as indicated by increased fecal concentration of total SCFA. The SCFA are composed of approximately 60% acetic acid, 25% propionic acid, and 15% butyric acid, respectively [81]. Acetic acid may modulate insulin sensitivity by reducing fatty acid flux [82], while butyrate is almost completely used by colonocytes as their preferred energy substrate [83]. Propionate is associated with positive effects on metabolic health, such as lowering serum cholesterol [84]. Therefore, increased total SCFA are beneficial in maintaining blood lipid levels and alleviating the inflammatory state of sows.
The results regarding the effect of high DF fed to sows on endocrine and metabolic status were inconsistent among the studies. The main reasons for the discrepancy in results could be attributed to the characteristics of DF used, the body condition scores of gestating sows during the experiment, and the experimental environment, particularly in high-temperature conditions. Further research is required to verify the interaction between the characteristics of the DF and the body condition scores of gestating sows.
Effects on reproductive performance
Balanced microbiota and reduced systemic inflammation due to DF may improve reproductive performance in gestating sows. Moreover, a previous meta-analysis of reproductive performance with DF supplementation showed that the number of pigs born alive increased by 0.4 piglets per litter when gestating sows were fed additional DF [11,85]. However, individual studies have reported inconsistent results regarding the effects of DF supplementation in the gestating diet on reproductive performance. Some studies showed no significant effect, while others showed positive or negative results. Similarly, the results of individual studies in the present meta-analysis showed no significant effects of a high-DF diet on any sow performance traits except for lactation feed intake and litter weaning weight (Table 3). One reason for the inconsistent results is the large variation often observed in reproductive data. Large replication per diet is needed to detect effects and to avoid drawing inaccurate conclusions. For instance, to detect a 10% difference with a statistical probability level of p<0.05 in litter size (about 1.0 pig/litter) among diets, at least 63 replications per diet would be required [86]. Another reason for the inconsistent results is that factors other than the elevated cell wall content of fibrous ingredients, such as differences in amino acids, vitamins, and trace mineral content, may also be important [85].
Table 3.
The effects of dietary fiber on reproductive performance of gestating sows1)
| Main source | n | Fiber composition in diet2) (%) | Feeding period | Δ Litter size3), No./Litter | Δ Litter weight3) (kg) | Δ FI3),4) (kg/d in lactation) | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Control | Treatment | |||||||||||||||
|
|
|
|
|
|||||||||||||
| TDF | SDF | IDF | TDF | SDF | IDF | Total born | Still born | Born alive | Weaned | At birth | Weaned | |||||
| Soybean hulls | 64 | 8.89 | 1.59 | 7.30 | 23.41 | 2.95 | 20.45 | Gestating period | 0.00 | - | 0.22 | 0.48 | −0.65 | 0.66 | 0.24 | [97]5) |
| Oat bran | 124 | 9.21 | 1.55 | 7.66 | 11.07 | 3.19 | 7.88 | Gestating period | −0.20 | - | 0.10 | −0.10 | −0.30 | −0.49 | −0.20 | [87] |
| Wheat straw | 119 | 16.75 | 1.40 | 15.35 | 0.10 | - | 0.10 | −0.20 | 0.15 | −1.12 | 0.00 | |||||
| Soybean hulls | 131 | 23.29 | 3.00 | 20.29 | −0.30 | - | 0.10 | 0.00 | −0.42 | −0.99 | 0.50* | |||||
| Wheat straw | 162 | 1.60 | 8.62 | 5.39 | 18.41 | 1.45 | 16.95 | Gestating period6) (3 reproductive cycle) | - | −0.05 | 0.31* | 0.25 | - | 1.48 | 0.34* | [98]5) |
| 111 | - | −0.13 | 0.61* | 0.92 | - | 8.48* | 0.35* | |||||||||
| 86 | - | −0.10 | 0.63* | 1.01 | - | 0.84 | 0.35* | |||||||||
| Soybean hulls | 15 | 13.3 | 1.90 | 11.4 | 23.44 | 2.80 | 20.60 | d 90 of gestation to farrowing | 0.10 | - | 0.30 | 0.50 | 0.50 | 1.10 | 0.10 | [44] |
| Konjac flour and Wheat bran | 28 | 20.5 | 2.30 | 18.2 | 49.93 | 4.00 | 45.93 | Gestating period | 0.25 | - | 0.26 | 0.18 | 0.66 | 6.12* | - | [19]5) |
| Konjac flour | 23 | 23.3 | 2.51 | 20.8 | 24.64 | 3.78 | 20.86 | Gestating period 6) (2 reproductive cycle) | 0.40 | - | 0.20 | 0.30 | 0.10 | 0.50 | 0.30* | [89]5) |
| Sugar beet pulp | 23 | 24.69 | 3.70 | 20.99 | 0.20 | - | −0.30 | 0.20 | −0.70 | 0.9 | 0.10 | |||||
| Konjac flour | 23 | 24.64 | 3.78 | 20.86 | −0.20 | - | 0.30 | 1.00* | 0.30 | 13.0* | 0.90* | |||||
| Sugar beet pulp | 23 | 24.69 | 3.70 | 20.99 | 0.30 | - | 0.88 | 0.40 | 0.60 | 3.40 | 0.20 | |||||
| Rice bran Soybean hulls | 11 | 15.6 | 5.00 | 10.6 | 22.30 | 5.40 | 16.90 | d 73 of gestation to farrowing | −0.50 | −0.40 | −1.00 | - | −1.40 | 3.50* | 0.20 | [45] |
| 11 | 28.20 | 7.50 | 20.70 | −1.20 | −0.40 | −0.20 | - | −0.30 | 3.80* | 0.00 | ||||||
| Oat straw | 30 | 20.1 | 2.70 | 17.4 | 25.58 | 2.55 | 23.03 | d 86 of gestation to farrowing | - | −0.39 | 0.70 | 0.50 | - | 5.26* | 0.45* | [52] |
| 30 | 25.73 | 2.50 | 23.23 | - | −0.83 | 0.50 | 0.70 | - | 10.9* | 0.45* | ||||||
| Wheat straw | 30 | 26.00 | 2.60 | 23.40 | - | −0.15 | 0.40 | 0.50 | - | 3.31 | −0.13 | |||||
| 30 | 25.54 | 2.50 | 23.04 | - | −0.70 | −0.10 | 0.40 | - | 2.26 | 0.11 | ||||||
| Wheat bran | 15 | 11.4 | 1.39 | 9.98 | 21.81 | 1.86 | 19.95 | d 107 gestating to weaning | −0.07 | −0.40 | 0.37 | 0.54 | 0.76 | 5.74 | 0.36* | [9]5),8) |
| Sugar beet pulp | 15 | 21.60 | 4.06 | 17.54 | −0.20 | −0.26 | 0.10 | 0.34 | 0.42 | 7.45* | 0.68* | |||||
| Inulin | 22 | 26.6 | 3.63 | 23.0 | 27.06 | 4.34 | 22.72 | d 80 of gestation to farrowing | 0.23 | 0.40 | 0.40 | 0.41 | 1.55 | 10.94* | 1.084* | [46] |
| 22 | 27.51 | 5.05 | 22.46 | −0.36 | 0.31 | 0.28 | 0.71 | 3.25 | 14.68* | 1.238* | ||||||
| 22 | 27.98 | 5.77 | 22.21 | −0.05 | 0.22 | 0.22 | 0.82* | 0.70 | 5.08 | 0.562 | ||||||
| Wheat bran | 20 | 17.5 | 1.92 | 15.6 | 32.90 | 3.22 | 29.68 | d 90 of gestation to farrowing | 0.82 | 0.06 | 0.5 | 0.66 | 1.11 | 3.86 | −0.19 | [70]5) |
| 20 | 33.50 | 5.06 | 28.44 | 0.08 | 0.47 | −0.53 | −0.67 | 0.22 | −8.35 | −0.15 | ||||||
| Wheat bran and fiber mix | 35 | 12.5 | 1.57 | 10.9 | 17.35 | 2.02 | 15.33 | Gestating period | 0.80 | - | 0.30 | 0.20 | 0.10 | 0.20 | 0.33 | [93] |
| 35 | 17.72 | 2.16 | 15.56 | 0.90 | - | 0.80 | 0.40 | 0.90 | 3.7 | 0.50* | ||||||
| 35 | 18.08 | 2.29 | 15.79 | 1.20 | - | 0.70 | −0.20 | 1.20 | 2.2 | 0.59* | ||||||
| 35 | 18.43 | 2.42 | 16.01 | 1.00 | - | 1.10 | −0.20 | 1.70 | 1.2 | 0.26 | ||||||
| 35 | 18.80 | 2.56 | 16.24 | 1.90 | - | 1.80 | −0.20 | 1.90 | −0.9 | 0.46* | ||||||
| Wheat bran | 12 | 9.75 | 1.58 | 8.17 | 22.15 | 2.40 | 19.75 | Gestating period | 0.54 | - | −0.79 | −0.37 | - | - | - | [95]5) |
| Inulin and Cellulose | 14 | 10.24 | 1.10 | 9.14 | 25.47 | 2.77 | 22.70 | Gestating period6) (3 reproductive cycle) | −0.02 | - | −0.07 | −0.53 | 0.62 | −1.61 | 0.55* | [10] |
| 13 | 2.11* | - | 2.00* | 0.27* | 4.11* | 7.38* | 0.51* | |||||||||
| 11 | 1.10* | - | 1.80* | 0.55* | 3.39* | 13.9* | 0.31* | |||||||||
| Alfalfa meal | 16 | 18.06 | 2.12 | 15.94 | 24.98 | 2.23 | 22.74 | d 60 of gestation to farrowing | −0.98 | - | −0.67 | −0.02 | −0.32 | 6.40 | 1.01* | [37] |
| Sugar beet pulp | 16 | 30.43 | 2.54 | 27.90 | −2.67 | - | −2.56 | −0.01 | −2.8 | 3.48 | 0.05 | |||||
| Soybean hulls | 16 | 34.15 | 2.59 | 31.86 | 0.00 | - | −0.11 | −0.01 | 0.91 | −0.23 | −0.09 | |||||
| Maximum | 26.6 | 5.00 | 23.0 | 49.9 | 7.50 | 45.9 | 2.11 | 0.47 | 2.00 | 1.01 | 4.11 | 14.7 | 1.24 | |||
| Minimum | 8.89 | 1.10 | 7.30 | 11.1 | 1.40 | 7.88 | −2.67 | −0.83 | −2.56 | −0.67 | −2.80 | −8.35 | −0.20 | |||
| Weighted average7) | 11.6 | 1.27 | 7.25 | 21.5 | 2.60 | 18.9 | 0.10 | −0.05 | 0.31 | 0.27 | 0.22 | 2.49 | 0.29 | |||
Asterisk sign (*) represent the significant difference at p<0.05.
TDF, total dietary fiber; SDF, soluble dietary fiber; IDF, insoluble dietary fiber.
The increase or decrease in litter size, litter weight and feed intake measured in dietary fiber groups relative to the control group.
FI, feed intake.
Each row represents reproductive performance of each reproductive cycle.
Weighted average according to replications per treatments.
Dietary fiber composition of lactation diet; the control, wheat bran, and sugar beet pulp diets contained 11.8%, 16.8%, and 16.9% total dietary fiber, 1.43%, 2.72%, and 1.70% soluble dietary fiber, and 10.4%, 14.1%, and 15.2% insoluble dietary fiber, respectively.
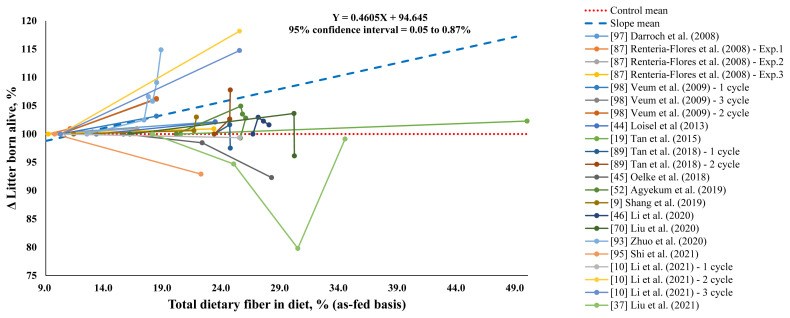
In this meta-analysis, we examined 15 published reports dating from 2008 to 2022 that reflect SDF and IDF as characteristic components of DF. For studies that did not provide information on SDF and IDF of ingredients, book or reference values were used to calculate these values. The concentration of TDF, SDF, and IDF in control diets ranged from 8.89% to 26.6%, 1.10% to 5.00%, and 7.30% to 23.0%, respectively, while the range for test diets was 11.1% to 49.9%, 1.40% to 7.50%, and 7.88% to 45.9%, respectively. The weighted averages of TDF, SDF, and IDF concentration in control and test diets according to number of observations were 11.7% and 21.5%, 1.27% and 2.60%, and 7.25% and 18.9%, respectively. The effects of DF addition on reproductive performance were inconsistent among individual studies. However, in this meta-analysis, a 1 percentage point increase in TDF concentration (as-fed basis) compared to the control group improved the litter born alive by 0.46% (95% confidence interval = 0.05% to 0.87%; Figure 1). Based on the weighted average of TDF concentrations and litter born alive in both the control diets (weighted average of TDF = 11.7%) and test diets (weighted average of TDF = 21.5%), a 10 percentage unit increase in TDF concentration compared to the control group improved the litter born alive by 0.49 pig per litter, which is in agreement with a previous meta-analysis [12].
Figure 1.
Effects of total dietary fiber (TDF) concentrations on changes (Δ) in litter born alive of the sows fed a fiber-supplemented diet compared with those fed a control diet. The slope mean (blue-colored dashed line) represents the mean of the linear slopes (n = 22). A linear slope was calculated for each experiment, and the slope data were pooled to calculate the mean slope and their 95% confidence interval using the UNIVARIATE procedure of SAS (SAS Inst. Inc., Cary, NC, USA). This linear slope indicates that a 1 percentage unit increase in dietary TDF concentration (as-fed basis) leads to a 0.46% increase in the number of piglets born alive per litter, as per the following equation: Y = 0.4605X+94.658.
The type of DF used in gestation diets may contribute to the discrepancies observed among studies. For instance, sows fed a high-SDF diet during gestation had higher numbers of live embryos and total embryo survival rates compared to sows fed a high-IDF diet [87]. However, Liu et al [70] showed that sows fed high-SDF or -IDF diets in the last gestation period could improve maternal immune function and redox status. Nonetheless, a high maternal SDF intake increased pre-weaning mortality and decreased the number of weaned piglets compared to sows fed an IDF diet. A recent study also revealed that IDF and SDF content, as well as the IDF:SDF, varied greatly among different fiber resources, leading to dramatic changes in fermentation kinetics parameters of gas production [88]. The IDF:SDF in a fiber resource could affect overall diet utilization and play an important role in improving the reproductive performance of sows [87]. In addition, recent studies found that the IDF:SDF had a significant effect on the health status of sows and their offsprings. Higher average piglet body weight and litter weight at weaning were observed when the IDF:SDF was 3.89 in the gestation diet [46,71]. Furthermore, a study using purified inulin and cellulose in gestation diets has been conducted to avoid the effect of fiber composition on reproductive performance [10]. This study investigated the effects of fiber addition with the same IDF:SDF and different TDF concentrations on sow and litter performance and reported that TDF addition to the gestation diet with an equal IDF:SDF during gestation promoted the physical status of sows and improved sow and litter performance. Some studies have reported that high-SDF diets can help improve reproductive performance by producing more SCFA [9,87,89]. However, it was challenging to distinguish the effect of a specific fiber type on sow reproductive performance through this meta-analysis. Recently, a study investigated the possible mechanism of DF improving sow reproductive performance [90]. They prepared two experimental diets: a semi-purified basal diet (non-fiber diet) and a fiber diet, which was the basal diet supplemented with 0.83% inulin and 20% cellulose. Then, they investigated fetal growth and placental development and function and reported that DF supplementation during gestation could increase maternal serum serotonin levels by promoting colonic serotonin synthesis, in which gut microbiota might be involved. In addition, DF supplementation during gestation promoted the transport of serotonin from the mother to the placenta in sows, improved placental development and function, and ultimately promoted fetal growth.
Sows that were fed high-DF diets for multiple reproductive cycles have shown greater benefits from the feeding of high-DF diets during gestation. In a previous meta-analysis, sows fed high DF during gestation in multiple-cycle studies produced 0.5 more pigs at weaning than those fed the control diet. However, in studies involving only one reproductive cycle, sows fed high DF produced 0.2 fewer pig at weaning than sows fed the control diet [12]. In this review, three out of 15 studies were conducted for multiple reproductive cycles and reported an improvement in litter born alive or the number piglet at weaning. A recent study reported that total born and born alive were similar among all treatments in the first parity, but they were greater for sows fed diets containing a 15%-point unit higher TDF content than the control diet in the second and third parity [10]. Unlike one-cycle studies, multiple-cycle studies have consistently shown that the addition of DF during gestation leads to an improvement in litter born alive or the number piglet at weaning. These results suggest that feeding long-term DF is required to modulate the microbiota in the intestine and reduce systemic inflammation, which may result in significant differences in reproductive performance.
Ten out of 15 studies reported that feeding DF during gestation increased feed intake in the lactating period. There are two potential reasons for the increase in feed intake. Firstly, the gestation diet containing high DF is more fermentable than the control diet, which can improve insulin sensitivity and increase feed intake [9,89]. Secondly, feeding a high-DF diet during gestation can increase the bulkiness of the diet, helping sows adapt to the sudden increase in feed intake required to meet the demands of lactation. [52,91]. The increased lactation feed intake can lead to increased milk production, resulting in a greater growth rate of piglets from sows fed high DF.
CONCLUSION
The effect of high-DF diets on intestinal microbiota, endocrine and metabolic status, as well as reproductive performance in gestating sows, remains inconsistent among individual studies. However, meta-analysis has demonstrated positive results, particularly in terms of litter born alive, indicating consistent improvement in reproductive performance when high-fiber diets are fed for multiple reproductive cycles. Based on this review, feeding gestating sows with diets containing around 21% TDF can be expected to increase litter born alive by 0.5 pigs per litter, and long-term feeding can further enhance the positive effects of high fiber diet. Nonetheless, the precise mechanisms underlying the benefits of DF in gestating sows remain unclear. Further research is needed to elucidate the mechanisms of action of DF and its association with subsequent reproductive performance in gestating sows.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
The authors received no financial support for this article.
REFERENCES
- 1.Oliviero C, Heinonen M, Valros A, Peltoniemi O. Environmental and sow-related factors affecting the duration of farrowing. Anim Reprod Sci. 2010;119:85–91. doi: 10.1016/j.anireprosci.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Oliviero C, Junnikkala S, Peltoniemi O. The challenge of large litters on the immune system of the sow and the piglets. Reprod Domest Anim. 2019;54:12–21. doi: 10.1111/rda.13463. [DOI] [PubMed] [Google Scholar]
- 3.Andersson E, Frössling J, Engblom L, Algers B, Gunnarsson S. Impact of litter size on sow stayability in Swedish commercial piglet producing herds. Acta Vet Scand. 2015;58:31. doi: 10.1186/s13028-016-0213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schild S-LA, Foldager L, Rangstrup-Christensen L, Pedersen LJ. Characteristics of piglets born by two highly prolific sow hybrids. Front Vet Sci. 2020;7:355. doi: 10.3389/fvets.2020.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju M, Wang X, Li X, et al. Effects of litter size and parity on farrowing duration of Landrace× Yorkshire sows. Animals. 2022;12:94. doi: 10.3390/ani12010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao M, Li Y, Wu QJ, et al. Effects of dietary Clostridium butyricum addition to sows in late gestation and lactation on reproductive performance and intestinal microbiota. J Anim Sci. 2019;97:3426–39. doi: 10.1093/jas/skz186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Wang S, Zhang D, et al. Effects of dietary supplementation with Pediococcus acidilactici ZPA017 on reproductive performance, fecal microbial flora and serum indices in sows during late gestation and lactation. Asian-Australas J Anim Sci. 2020;33:120–6. doi: 10.5713/ajas.18.0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu J, Kim YH, Kim IH. Effects of two bacillus strains probiotic supplement on reproduction performance, nutrient digestibility, blood profile, fecal score, excreta odor contents and fecal microflora in lactation sows, and growth performance in sucking piglets. Livest Sci. 2021;244:104293. doi: 10.1016/j.livsci.2020.104293. [DOI] [Google Scholar]
- 9.Shang Q, Liu H, Liu S, He T, Piao X. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets. J Anim Sci. 2019;97:4922–33. doi: 10.1093/jas/skz278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, He J, Zhang L, et al. Effects of dietary fiber supplementation in gestation diets on sow performance, physiology and milk composition for successive three parities. Anim Feed Sci Technol. 2021;276:114945. doi: 10.1016/j.anifeedsci.2021.114945. [DOI] [Google Scholar]
- 11.Reese D. Dietary fiber in sow gestation diets-a review. Lincoln, NE, USA: University of Nebraska; 1997. Nebraska Swine Reports 229. [Google Scholar]
- 12.Reese D, Prosch A, Travnicek DA, Eskridge KM. Dietary fiber in sow gestation diets-an updated review. Lincoln, NE, USA: University of Nebraska; 2008. Nebraska Swine Reports 45. [Google Scholar]
- 13.Berchieri-Ronchi C, Kim S, Zhao Y, Correa CR, Yeum KJ, Ferreira ALA. Oxidative stress status of highly prolific sows during gestation and lactation. Animal. 2011;5:1774–9. doi: 10.1017/S1751731111000772. [DOI] [PubMed] [Google Scholar]
- 14.Tummaruk P, Sang-Gassanee K. Effect of farrowing duration, parity number and the type of anti-inflammatory drug on postparturient disorders in sows: a clinical study. Trop Anim Health Prod. 2013;45:1071–7. doi: 10.1007/s11250-012-0315-x. [DOI] [PubMed] [Google Scholar]
- 15.Oliviero C, Kothe S, Heinonen M, Valros A, Peltoniemi O. Prolonged duration of farrowing is associated with subsequent decreased fertility in sows. Theriogenology. 2013;79:1095–9. doi: 10.1016/j.theriogenology.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Pearodwong P, Muns R, Tummaruk P. Prevalence of constipation and its influence on post-parturient disorders in tropical sows. Trop Anim Health Prod. 2016;48:525–31. doi: 10.1007/s11250-015-0984-3. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen TF, Van Vliet S, Bruun TS, Theil PK. Feeding sows during the transition period—is a gestation diet, a simple transition diet, or a lactation diet the best choice? Transl Anim Sci. 2020;4:34–48. doi: 10.1093/tas/txz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu D, Pi Y, Ye H, et al. Consumption of dietary fiber with different physicochemical properties during late pregnancy alters the gut microbiota and relieves constipation in sow model. Nutrients. 2022;14:2511. doi: 10.3390/nu14122511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan CQ, Wei HK, Sun HQ, et al. Effects of supplementing sow diets during two gestations with konjac flour and Saccharomyces boulardii on constipation in peripartal period, lactation feed intake and piglet performance. Anim Feed Sci Technol. 2015;210:254–62. doi: 10.1016/j.anifeedsci.2015.10.013. [DOI] [Google Scholar]
- 20.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20:779–86. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jha R, Fouhse JM, Tiwari UP, Li L, Willing BP. Dietary fiber and intestinal health of monogastric animals. Front Vet Sci. 2019;6:48. doi: 10.3389/fvets.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–85. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 23.Nowland TL, Plush KJ, Barton M, Kirkwood RN. Development and function of the intestinal microbiome and potential implications for pig production. Animals. 2019;9:76. doi: 10.3390/ani9030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–73. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676–84. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 27.Handelsman J. Microbial symbiosis: in sickness and in health. DNA Cell Biol. 2009;28:359–60. doi: 10.1089/dna.2009.1507. [DOI] [PubMed] [Google Scholar]
- 28.Chow J, Lee SM, Shen Y, Khosravi A, Mazmanian SK. Host–bacterial symbiosis in health and disease. Adv Immunol. 2010;107:243–74. doi: 10.1016/B978-0-12-381300-8.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8:1715. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abenavoli L, Scarpellini E, Colica C, et al. Gut microbiota and obesity: a role for probiotics. Nutrients. 2019;11:2690. doi: 10.3390/nu11112690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen ZH, Zhu CX, Quan YS, et al. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24:5–14. doi: 10.3748/wjg.v24.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jha R, Berrocoso JFD. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim Feed Sci Technol. 2016;212:18–26. doi: 10.1016/j.anifeedsci.2015.12.002. [DOI] [Google Scholar]
- 34.Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu C, Cheng C, Zhang X, Peng J. Inclusion of soluble fiber in the gestation diet changes the gut microbiota, affects plasma propionate and odd-chain fatty acids levels, and Improves insulin sensitivity in sows. Int J Mol Sci. 2020;21:635. doi: 10.3390/ijms21020635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Ma L, Zhang L, et al. Dietary inulin regulated gut microbiota and improved neonatal health in a pregnant sow model. Front Nutr. 2021;8:716723. doi: 10.3389/fnut.2021.716723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B, Zhu X, Cui Y, et al. Consumption of dietary fiber from different sources during pregnancy alters sow gut microbiota and improves performance and reduces inflammation in sows and piglets. mSystems. 2021;6:e00591–20. doi: 10.1128/mSystems.00591-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallet JL, Miles JR, Brown-Brandl TM, Nienaber JA. Proportion of the litter farrowed, litter size, and progesterone and estradiol effects on piglet birth intervals and stillbirths. Anim Reprod Sci. 2010;119:68–75. doi: 10.1016/j.anireprosci.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Levasseur MC. Utero-ovarian relationships in placental mammals: role of uterus and embryo in the regulation of progesterone secretion by the corpus luteum. a review. Reprod Nutr Dev. 1983;23:793–816. doi: 10.1051/rnd:19830601. [DOI] [PubMed] [Google Scholar]
- 40.Ferguson EM, Slevin J, Hunter MG, Edwards SA, Ashworth CJ. Beneficial effects of a high fibre diet on oocyte maturity and embryo survival in gilts. Reproduction. 2007;133:433–9. doi: 10.1530/REP-06-0018. [DOI] [PubMed] [Google Scholar]
- 41.Catalano PM. Obesity, insulin resistance and pregnancy outcome. Reproduction (Cambridge, England) 2010;140:365–71. doi: 10.1530/REP-10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Père MC, Etienne M. Insulin sensitivity during pregnancy, lactation, and postweaning in primiparous gilts. J Anim Sci. 2007;85:101–10. doi: 10.2527/jas.2006-130. [DOI] [PubMed] [Google Scholar]
- 43.Quesnel H, Meunier-Salaun MC, Hamard A, et al. Dietary fiber for pregnant sows: influence on sow physiology and performance during lactation. J Anim Sci. 2009;87:532–43. doi: 10.2527/jas.2008-1231. [DOI] [PubMed] [Google Scholar]
- 44.Loisel F, Farmer C, Ramaekers P, Quesnel H. Effects of high fiber intake during late pregnancy on sow physiology, colostrum production, and piglet performance. J Anim Sci. 2013;91:5269–79. doi: 10.2527/jas.2013-6526. [DOI] [PubMed] [Google Scholar]
- 45.Oelke CA, Ribeiro AML, Noro M, et al. Effect of different levels of total dietary fiber on the performance of sows in gestation and lactation. Rev Bras Zootecn. 2018;47:e20170299. doi: 10.1590/rbz4720170299. [DOI] [Google Scholar]
- 46.Li H, Liu Z, Lyu H, et al. Effects of dietary inulin during late gestation on sow physiology, farrowing duration and piglet performance. Anim Reprod Sci. 2020;219:106531. doi: 10.1016/j.anireprosci.2020.106531. [DOI] [PubMed] [Google Scholar]
- 47.Blundell JE, Goodson S, Halford JCG. Regulation of appetite: role of leptin in signalling systems for drive and satiety. Int J Obes. 2001;25(Suppl 1):S29–S34. doi: 10.1038/sj.ijo.0801693. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Chen Y, Wang W, et al. Dietary supplementation with fine-grinding wheat bran improves lipid metabolism and inflammatory response via modulating the gut microbiota structure in pregnant sow. Front Microbiol. 2022;13:835950. doi: 10.3389/fmicb.2022.835950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassanzadeh-Rostami Z, Faghih S. Effect of dietary fiber on serum leptin level: a systematic review and meta-analysis of randomized controlled trials. Exp Clin Endocrinol Diabetes. 2021;129:322–33. doi: 10.1055/a-0998-3883. [DOI] [PubMed] [Google Scholar]
- 50.Farmer C, Robert S, Matte JJ. Lactation performance of sows fed a bulky diet during gestation and receiving growth hormone-releasing factor during lactation. J Anim Sci. 1996;74:1298–306. doi: 10.2527/1996.7461298x. [DOI] [PubMed] [Google Scholar]
- 51.Farmer C, Robert S, Rushen J. Bromocriptine given orally to periparturient of lactating sows inhibits milk production. J Anim Sci. 1998;76:750–7. doi: 10.2527/1998.763750x. [DOI] [PubMed] [Google Scholar]
- 52.Agyekum AK, Columbus DA, Farmer C, Beaulieu AD. Effects of supplementing processed straw during late gestation on sow physiology, lactation feed intake, and offspring body weight and carcass quality. J Anim Sci. 2019;97:3958–71. doi: 10.1093/jas/skz242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lepe M, Gascón MB, Castañeda-González L, Morales MEP, Cruz AJ. Effect of maternal obesity on lactation: systematic review. Nutr Hosp. 2011;26:1266–9. doi: 10.3305/nh.2011.26.6.5388. [DOI] [PubMed] [Google Scholar]
- 54.Kim SH, Bennett PR, Terzidou V. Advances in the role of oxytocin receptors in human parturition. Mol Cell Endocrinol. 2017;449:56–63. doi: 10.1016/j.mce.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 55.Aulinas A, Pulumo RL, Asanza E, et al. Endogenous oxytocin levels in relation to food intake, menstrual phase, and age in females. J Clin Endocrinol Metab. 2019;104:1348–56. doi: 10.1210/jc.2018-02036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Webel DM, Finck BN, Baker DH, Johnson RW. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J Anim Sci. 1997;75:1514–20. doi: 10.2527/1997.7561514x. [DOI] [PubMed] [Google Scholar]
- 57.Meunier-Salaün MC, Edwards SA, Robert S. Effect of dietary fibre on the behaviour and health of the restricted fed sow. Anim Feed Sci Technol. 2001;90:53–69. doi: 10.1016/S0377-8401(01)00196-1. [DOI] [Google Scholar]
- 58.Sapkota A, Marchant-Forde JN, Richert BT, Lay DC., Jr Including dietary fiber and resistant starch to increase satiety and reduce aggression in gestating sows. J Anim Sci. 2016;94:2117–27. doi: 10.2527/jas.2015-0013. [DOI] [PubMed] [Google Scholar]
- 59.Huang S, Wei J, Yu H, et al. Effects of dietary fiber sources during gestation on stress status, abnormal behaviors and reproductive performance of sows. Animals. 2020;10:141. doi: 10.3390/ani10010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holt JP, Johnston LJ, Baidoo SK, Shurson GC. Effects of a high-fiber diet and frequent feeding on behavior, reproductive performance, and nutrient digestibility in gestating sows. J Anim Sci. 2006;84:946–55. doi: 10.2527/2006.844946x. [DOI] [PubMed] [Google Scholar]
- 61.Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–18. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shang Q, Liu S, Liu H, Mahfuz S, Piao X. Impact of sugar beet pulp and wheat bran on serum biochemical profile, inflammatory responses and gut microbiota in sows during late gestation and lactation. J Anim Sci Biotechnol. 2021;12:54. doi: 10.1186/s40104-021-00573-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:PR453–62. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci. 2007;52:2015–21. doi: 10.1007/s10620-006-9622-2. [DOI] [PubMed] [Google Scholar]
- 66.Thérond P, Bonnefont-Rousselot D, Davit-Spraul A, Conti M, Legrand A. Biomarkers of oxidative stress: an analytical approach. Curr Opin Clin Nutr Metab Care. 2000;3:373–84. doi: 10.1097/00075197-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Mueller A, Koebnick C, Binder H, et al. Placental defence is considered sufficient to control lipid peroxidation in pregnancy. Med Hypotheses. 2005;64:553–7. doi: 10.1016/j.mehy.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Liu H, Zhang L, et al. Maternal dietary fiber composition during gestation induces changes in offspring antioxidative capacity, inflammatory response, and gut microbiota in a sow model. Int J Mol Sci. 2020;21:31. doi: 10.3390/ijms21010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang S, Wei J, Yu H, et al. Effects of dietary fiber sources during gestation on stress status, abnormal behaviors and reproductive performance of sows. Animals (Basel) 2020;10:141. doi: 10.3390/ani10010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Chen N, Li D, et al. Effects of dietary soluble or insoluble fiber intake in late gestation on litter performance, milk composition, immune function, and redox status of sows around parturition. J Anim Sci. 2020;98:skaa303. doi: 10.1093/jas/skaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, Zhang L, Liu H, et al. Effects of the ratio of insoluble fiber to soluble fiber in gestation diets on sow performance and offspring intestinal development. Animals. 2019;9:422. doi: 10.3390/ani9070422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Camp Montoro J, Solà-Oriol D, Muns R, Gasa J, Llanes N, Manzanilla EG. Blood and faecal biomarkers to assess dietary energy, protein and amino acid efficiency of utilization by growing and finishing pigs. Porc Health Manag. 2022;8:32. doi: 10.1186/s40813-022-00273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.George PB, England DC, Siers DG, Stanton HC. Diabetogenic effects of pregnancy in sows on plasma glucose and insulin release. J Anim Sci. 1978;46:1694–700. doi: 10.2527/jas1978.4661694x. [DOI] [PubMed] [Google Scholar]
- 74.Schaefer AL, Tong AKW, Sather AP, Beltranena E, Pharazyn A, Aherne FX. Preparturient diabetogenesis in primiparous gilts. Can J Anim Sci. 1991;71:69–77. doi: 10.4141/cjas91-008. [DOI] [Google Scholar]
- 75.Lin LH, Lin J, Yan JY. Interactive affection of pre-pregnancy overweight or obesity, excessive gestational weight gain and glucose tolerance test characteristics on adverse pregnancy outcomes among women with gestational diabetes mellitus. Front Endocrinol. 2022;13:942271. doi: 10.3389/fendo.2022.942271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jha R, Berrocoso JD. Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal. 2015;9:1441–52. doi: 10.1017/s1751731115000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agyekum AK, Nyachoti CM. Nutritional and metabolic consequences of feeding high-fiber diets to swine: a review. Engineering. 2017;3:716–25. doi: 10.1016/j.Eng.2017.03.010. [DOI] [Google Scholar]
- 78.Younes H, Garleb K, Behr S, Rémésy C, Demigné C. Fermentable fibers or oligosaccharides reduce urinary nitrogen excretion by increasing urea disposal in the rat cecum. J Nutr. 1995;125:1010–6. doi: 10.1093/jn/125.4.1010. [DOI] [PubMed] [Google Scholar]
- 79.Laws J, Juniper DT, Lean IJ, et al. Supplementing sow diets with palm oil during late gestation and lactation: effects on milk production, sow hormonal profiles and growth and development of her offspring. Animal. 2018;12:2578–86. doi: 10.1017/S1751731118000885. [DOI] [PubMed] [Google Scholar]
- 80.Tarini J, Wolever TMS. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab. 2010;35:9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- 81.LeBlanc JG, Chain F, Martín R, et al. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact. 2017;16:79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernandes J, Vogt J, Wolever TMS. Inulin increases short-term markers for colonic fermentation similarly in healthy and hyperinsulinaemic humans. Eur J Clin Nutr. 2011;65:1279–86. doi: 10.1038/ejcn.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–9. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 84.Genda T, Kondo T, Sugiura S, et al. Bacterial fermentation of water-soluble cellulose acetate raises large-bowel acetate and propionate and decreases plasma cholesterol concentrations in rats. J Agric Food Chem. 2018;66:11909–16. doi: 10.1021/acs.jafc.8b04093. [DOI] [PubMed] [Google Scholar]
- 85.Grieshop CM, Reese DE, Fahey GF. swine nutrition. 2 ed. New York, NY, USA: CRC Press; 2001. Nonstarch polysaccharides and oligosaccharides; pp. 107–30. [Google Scholar]
- 86.Aaron DK, Hays VW. How many pigs? Statistical power considerations in swine nutrition experiments. J Anim Sci. 2004;82:E245–E54. doi: 10.2527/2004.8213_supplE245x. [DOI] [PubMed] [Google Scholar]
- 87.Renteria-Flores JA, Johnston LJ, Shurson GC, Moser RL, Webel SK. Effect of soluble and insoluble dietary fiber on embryo survival and sow performance. J Anim Sci. 2008;86:2576–84. doi: 10.2527/jas.2007-0376. [DOI] [PubMed] [Google Scholar]
- 88.Mou D, Li S, Yan C, et al. Dietary fiber sources for gestation sows: Evaluations based on combined in vitro and in vivo methodology. Anim Feed Sci Technol. 2020;269:114636. doi: 10.1016/j.anifeedsci.2020.114636. [DOI] [Google Scholar]
- 89.Tan CQ, Sun HQ, Wei HK, et al. Effects of soluble fiber inclusion in gestation diets with varying fermentation characteristics on lactational feed intake of sows over two successive parities. Animal. 2018;12:1388–95. doi: 10.1017/S1751731117003019. [DOI] [PubMed] [Google Scholar]
- 90.Li Y, Yang M, Zhang L, et al. Dietary fiber supplementation in gestating sow diet improved fetal growth and placental development and function through serotonin signaling pathway. Front Vet Sci. 2022;9:831703. doi: 10.3389/fvets.2022.831703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matte JJ, Robert S, Girard CL, Farmer C, Martineau GP. Effect of bulky diets based on wheat bran or oat hulls on reproductive performance of sows during their first two parities. J Anim Sci. 1994;72:1754–60. doi: 10.2527/1994.7271754x. [DOI] [PubMed] [Google Scholar]
- 92.Yu M, Gao T, Liu Z, Diao X. Effects of dietary supplementation with high fiber (stevia residue) on the fecal flora of pregnant sows. Animals. 2020;10:2247. doi: 10.3390/ani10122247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhuo Y, Feng B, Xuan Y, et al. Inclusion of purified dietary fiber during gestation improved the reproductive performance of sows. J Anim Sci Biotechnol. 2020;11:47. doi: 10.1186/s40104-020-00450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weng RC. Dietary supplementation with different types of fiber in gestation and lactation: effects on sow serum biochemical values and performance. Asian-Australas J Anim Sci. 2020;33:1323–31. doi: 10.5713/ajas.19.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi B, He W, Su G, Xu X, Shan A. The effect of increasing neutral detergent fiber level through different fiber feed ingredients throughout the gestation of sows. Animals. 2021;11:415. doi: 10.3390/ani11020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jaworski NW, Lærke HN, Bach Knudsen KE, Stein HH. Carbohydrate composition and in vitro digestibility of dry matter and nonstarch polysaccharides in corn, sorghum, and wheat and coproducts from these grains. J Anim Sci. 2015;93:1103–13. doi: 10.2527/jas.2014-8147. [DOI] [PubMed] [Google Scholar]
- 97.Darroch CS, Dove CR, Maxwell CV, et al. A regional evaluation of the effect of fiber type in gestation diets on sow reproductive performance. J Anim Sci. 2008;86:1573–8. doi: 10.2527/jas.2007-0662. [DOI] [PubMed] [Google Scholar]
- 98.Veum TL, Crenshaw JD, Crenshaw TD, et al. The addition of ground wheat straw as a fiber source in the gestation diet of sows and the effect on sow and litter performance for three successive parities. J Anim Sci. 2009;87:1003–12. doi: 10.2527/jas.2008-1119. [DOI] [PubMed] [Google Scholar]